Abstract
The gut microbiome (GM) has emerged as a key factor in the genesis and progression of many diseases. The intestinal bacterial composition also influences treatment-related side effects and even the efficacy of oncological therapies. Acute leukemia (AL) is the most common cancer among children and the most frequent cause of cancer-related death during childhood. Outcomes have improved considerably over the past 4 decades, with the current long-term survival for acute lymphoblastic leukemia being ∼90%. However, several acute toxicities and long-term sequelae are associated with the multimodal therapy protocols applied in these patients. Specific GM configurations could contribute to the multistep developmental hypothesis for leukemogenesis. Moreover, GM alterations occur during the AL therapeutic course and are associated with treatment-related complications, especially during hematopoietic stem cell transplantation. The GM perturbation could last even after the removal of microbiome-modifying factors, like antibiotics, chemotherapeutic drugs, or alloimmune reactions, contributing to several health-related issues in AL survivors. The purpose of this article is to provide a comprehensive review of the chronological changes of GM in children with AL, from predisposition to cure. The underpinning biological processes and the potential interventions to modulate the GM toward a potentially health-promoting configuration are also highlighted.
Introduction
The gut microbiome (GM) has emerged in the past 20 years as a key player in a variety of physiological and pathological processes.1 This advance has been made possible by next-generation sequencing technologies (ie, marker 16S rRNA gene sequencing and shotgun metagenomics), which have revolutionized the field, allowing for the profiling of multiple microbial communities simultaneously, in a relatively short time and at costs that are progressively declining.2 It should be noted that, while 16S gene sequencing is the gold standard for bacterial taxonomic profiling, metagenomics provides a higher taxonomic and functional resolution and the possibility of characterizing other GM components (ie, fungi and viruses), thus enabling an unparalleled view of the ecosystem, albeit at the price of greater computational power and time. Over the years, other functional approaches have been developed, providing extensive knowledge of microbiome-derived metabolites thanks to metabolomics, while promising advances have been made in the field of metatranscriptomics, studies of animal models, or, more recently, culturomics. The implementation of these techniques combined with the improvement in next-generation sequencing technologies have provided several insights into the molecular crosstalk between the human organism and our lifelong symbiotic microbial partners, which we now know shapes a wide range of host responses.
Pediatric patients present with a peculiar GM that is still in its developmental phase, moving toward the typical adult-like profile, and is characterized by high variability between individuals (ie, beta diversity) and over time, with intraindividual diversity (ie, alpha diversity) and functional complexity gradually increasing with age.3,4 In particular, alpha diversity (to be understood as richness, evenness, or both, depending on the metrics used) is recognized as an index of stability and functioning of microbial ecosystems, with higher values generally indicative of a healthier GM (and therefore host) and potentially predictive of better health outcomes in several contexts.5 While in adult cancer patients the role of GM has been widely studied, providing new, exciting perspectives to understand and interfere with the biological processes on-going in these patients, much less evidence is available in children.3,6
Acute leukemia (AL) is the leading cause of childhood cancer-related deaths worldwide. It accounts for one-third of all cases of cancer, with a incidence rate of 10 to 50 cases per 100 000 per year.7 Acute lymphoblastic leukemia (ALL) represents the majority of newly diagnosed leukemia, accounting for ∼80%.8 The current long-term survival or cure rate is ∼90%, but treatment is aggressive, and the long-term sequelae strongly impair the quality of life.9 The study of GM in these patients has revealed new strategies for improving general outcomes and reducing treatment-related complications. In this review, we focus on the role of GM in pediatric AL, from cancer predisposition to cure, in an attempt to underline areas of poor knowledge and inspire future studies.
Predisposition to pediatric AL
For most of the AL forms, a multistep developing model has been proposed.10 The first step in the pathogenesis is represented by an intrinsic genetic susceptibility to development of B-cell precursor ALL (pB-ALL), followed by additional chromosomal aberrations and a broad range of secondary mutational events driven by environmental stimuli.11 Common pathogens, such as viruses responsible for upper respiratory tract or gastrointestinal infections, may represent a progression-enhancing stimulus in predisposed subjects.10 For instance, Rodríguez-Hernández et al demonstrated that pB-ALL–predisposed mice exposed to infections (murine norovirus, murine hepatitis virus, Helicobacter species, and Trichomonas muris) showed a higher incidence of leukemia compared with those raised in a pathogen-free environment. Interestingly, the pB-ALL- predisposed mice did not show a significantly higher cancer incidence than the wild-type mice,12 suggesting a major role for infective stimuli in leukemia progression. Recently, some researchers have suggested a wider hypothesis, considering the whole GM as a player in the genesis of AL. During childhood, the GM profile is highly variable and sensitive to stressors, and early microbial exposures can produce lasting changes. In turn, GM is well known for shaping immune responses through structural components or metabolites of its constituent bacteria.13 Alterations of this delicate balance can enhance disease progression. This hypothesis was specifically addressed by Vicente-Dueñas et al in a mouse model of pB-ALL.14 The authors used mice harboring a genetic predisposition to cancer, namely Pax5+/− and ETV6-RUNX1, and found that the GM was shaped according to these mutations. Moreover, they analyzed ALL incidence in genetically predisposed mice raised in a specific pathogen-free condition vs those raised in a conventional facility. Notably, none of the mice raised in pathogen-free conditions developed ALL, suggesting that an “intact” GM protects genetically predisposed mice from developing ALL. Therefore, GM alterations may trigger ALL development in the context of genetic predisposition14 (Figure 1). These intriguing data were confirmed in patients with ALL showing distinctive GM features since the time of diagnosis. Liu et al analyzed GM composition in 58 pediatric patients at the time of ALL diagnosis and found that the microbial configuration clustered apart from healthy matched controls in the beta-diversity analysis.15 In particular, patients with ALL had a higher prevalence of Bacteroides clarus, Roseburia faecis, Edwardsiella tarda, and Fusobacterium naviforme. These data were confirmed by Chua et al in a single-center study comparing the GM of 7 patients with ALL at the time of diagnosis with those of the same number of healthy controls. Patients who developed ALL had lower median alpha diversity, higher average relative abundance of Bacteroidetes and lower abundance of Firmicutes and Actinobacteria compared with that of the controls.16 These data were biased, however, by the use of wide-spectrum antibiotics in all the patients diagnosed with ALL before sampling, as opposed to controls, because of their common presentation with fever of unknown origin. Rajagopala et al analyzed the GM configuration in 28 pediatric patients at the time of ALL diagnosis compared with healthy sibling controls.17 Interestingly, they stratified patients with ALL into those who received and did not receive antibiotics before the GM analysis. In both groups, they found that alpha diversity was lower than in the control group and that this was enriched in Anaerostipes, Coprococcus, Roseburia, and Ruminococcus. Similar results, provided by Bai et al, were also underlying a higher prevalence of Megamonas and a lower prevalence of Blautia in the ALL group at diagnosis.18 Of note, an imbalance of the oral microbiome was also found in patients with ALL at the time of diagnosis, suggesting a possible role of other human-associated microbiomes from different anatomic localizations.19
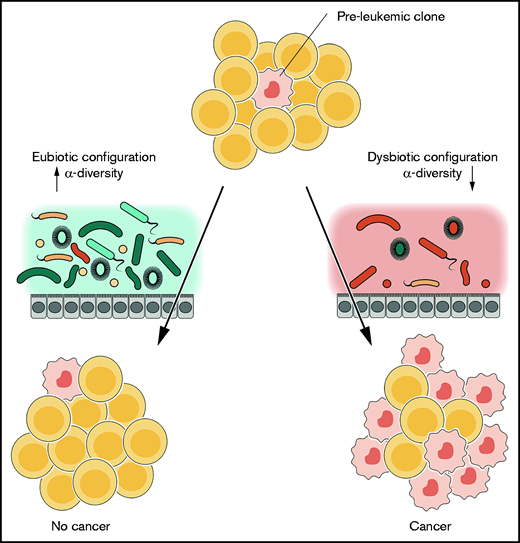
The contribution of the GM to the multistep developmental hypothesis for leukemogenesis. In intrinsically predisposed subjects, preleukemic clones may arise and, according to the hypothesis, their development may be influenced by environmental stimuli. The GM can represent one of these, serving as an additional hit in the multistep process, as was demonstrated in patients at the time of diagnosis (right). Conversely, an “intact” GM and/or the absence of interaction with common pathogens may protect genetically predisposed subjects (left).
The contribution of the GM to the multistep developmental hypothesis for leukemogenesis. In intrinsically predisposed subjects, preleukemic clones may arise and, according to the hypothesis, their development may be influenced by environmental stimuli. The GM can represent one of these, serving as an additional hit in the multistep process, as was demonstrated in patients at the time of diagnosis (right). Conversely, an “intact” GM and/or the absence of interaction with common pathogens may protect genetically predisposed subjects (left).
Gut microbiome modifications during chemotherapy
The studies that addressed GM modifications during chemotherapy for childhood AL have identified antibiotics, immunosuppression, dietary changes, and direct toxicity as being among the factors that contribute to alterations in the intestinal ecosystem20-22 (Table 1). GM diversity decreases significantly after intensive induction and reinduction protocols, with a slight rebound in the period after recovery from induction.23 The GM diversity and richness in patients at any time during treatment are significantly lower compared with those in healthy controls,24 as are the total number of bacteria in fecal samples. A decrease primarily in anaerobic bacteria and a slight increase in potentially pathogenic enterococci generally occur.25 The restitution of GM diversity takes place after the cessation of chemotherapy, unlike its composition, which may remain altered in the long term.16
Summary of studies evaluating the GM during chemotherapy in pediatric patients with AL
| Study . | Year . | Patients, n . | Leukemia type . | Type of analysis . | Sample site . | Main results . |
|---|---|---|---|---|---|---|
| Van Vliet et al25 | 2009 | 9 | AML | PCR denaturing gradient gel electrophoresis fingerprinting. FISH with specific bacterial oligonucleotide probes in order to quantify fecal bacteria. | Fecal samples | Reduction in the total number of bacteria; mainly anaerobic bacteria decreased, with a slight increase in enterococci. |
| Tunyapanit et al40 | 2018 | 87 | ALL/lymphoma | MICs according to the E-test. | Rectal swabs | Increased rate of E coli and K pneumoniae resistant to ciprofloxacin in patients receiving ciprofloxacin prophylaxis, with an increase in the MIC50 of ceftazidime. |
| Hakim et al23 | 2018 | 199 | ALL | V1-V3 16S rRNA gene sequencing | Fecal samples | Reduction of GM diversity (according to the Chao1, Shannon, and Simpson indices). Relative abundances of Bacteroidetes decreased, whereas Clostridiaceae and Streptococcaceae increased. Baseline GM with greater relative abundance of Proteobacteria predicted the development of febrile neutropenia. Enterococcaceae dominance during chemotherapy predicted febrile neutropenia and diarrheal illness, whereas Streptococcaceae dominance predicted diarrheal illness. |
| Nearing et al30 | 2019 | 16 | ALL | V4-V5 16S rRNA gene sequencing and metagenomic shotgun sequencing | Fecal samples | Differences in alpha (according to the Shannon index, evenness, number of observed amplicon sequence variants, and Faith’s phylogenetic diversity) and beta diversity (according to weighted UniFrac distances) between samples from patients with infectious complications and those without. Increased relative abundances of Proteobacteria and opportunistic pathogens, decrease in Bacteroidetes and F prausnitzii in patients with infectious complications, and increase in aerobic respiration pathways. |
| Chua et al16 | 2020 | 7 (+7 healthy controls) | ALL | V4 16S rRNA gene sequencing | Anal swabs | Relative abundance of Bacteroides and alpha diversity (according to the Chao 1 and Shannon indices) decreased. Restitution of diversity, but not composition, occurred after the cessation of chemotherapy. |
| Rajagopala et al24 | 2020 | 32 (+25 healthy siblings) | ALL | V4 16S rRNA gene sequencing | Fecal samples | GM richness (based on the Chao 1 index) and evenness (based on the Shannon index) during treatment were lower than those of healthy controls. Increased relative abundances of the mucolytic bacteria R gnavus and R torques. |
| De Pietri et al32 | 2020 | 51 | ALL | V3-V4 16S rRNA gene sequencing | Fecal samples | During induction, a low number of alpha-diversity variants (according to the number of observed amplicon sequence variants and the Shannon index) and an increased abundance of Enterococcus spp. associated with high levels of CRP and low plasma citrulline. High abundance of Lachnospiraceae spp. associated with increased citrulline and reduced CRP levels. |
| Study . | Year . | Patients, n . | Leukemia type . | Type of analysis . | Sample site . | Main results . |
|---|---|---|---|---|---|---|
| Van Vliet et al25 | 2009 | 9 | AML | PCR denaturing gradient gel electrophoresis fingerprinting. FISH with specific bacterial oligonucleotide probes in order to quantify fecal bacteria. | Fecal samples | Reduction in the total number of bacteria; mainly anaerobic bacteria decreased, with a slight increase in enterococci. |
| Tunyapanit et al40 | 2018 | 87 | ALL/lymphoma | MICs according to the E-test. | Rectal swabs | Increased rate of E coli and K pneumoniae resistant to ciprofloxacin in patients receiving ciprofloxacin prophylaxis, with an increase in the MIC50 of ceftazidime. |
| Hakim et al23 | 2018 | 199 | ALL | V1-V3 16S rRNA gene sequencing | Fecal samples | Reduction of GM diversity (according to the Chao1, Shannon, and Simpson indices). Relative abundances of Bacteroidetes decreased, whereas Clostridiaceae and Streptococcaceae increased. Baseline GM with greater relative abundance of Proteobacteria predicted the development of febrile neutropenia. Enterococcaceae dominance during chemotherapy predicted febrile neutropenia and diarrheal illness, whereas Streptococcaceae dominance predicted diarrheal illness. |
| Nearing et al30 | 2019 | 16 | ALL | V4-V5 16S rRNA gene sequencing and metagenomic shotgun sequencing | Fecal samples | Differences in alpha (according to the Shannon index, evenness, number of observed amplicon sequence variants, and Faith’s phylogenetic diversity) and beta diversity (according to weighted UniFrac distances) between samples from patients with infectious complications and those without. Increased relative abundances of Proteobacteria and opportunistic pathogens, decrease in Bacteroidetes and F prausnitzii in patients with infectious complications, and increase in aerobic respiration pathways. |
| Chua et al16 | 2020 | 7 (+7 healthy controls) | ALL | V4 16S rRNA gene sequencing | Anal swabs | Relative abundance of Bacteroides and alpha diversity (according to the Chao 1 and Shannon indices) decreased. Restitution of diversity, but not composition, occurred after the cessation of chemotherapy. |
| Rajagopala et al24 | 2020 | 32 (+25 healthy siblings) | ALL | V4 16S rRNA gene sequencing | Fecal samples | GM richness (based on the Chao 1 index) and evenness (based on the Shannon index) during treatment were lower than those of healthy controls. Increased relative abundances of the mucolytic bacteria R gnavus and R torques. |
| De Pietri et al32 | 2020 | 51 | ALL | V3-V4 16S rRNA gene sequencing | Fecal samples | During induction, a low number of alpha-diversity variants (according to the number of observed amplicon sequence variants and the Shannon index) and an increased abundance of Enterococcus spp. associated with high levels of CRP and low plasma citrulline. High abundance of Lachnospiraceae spp. associated with increased citrulline and reduced CRP levels. |
As for alpha diversity, the following metrics were used: the Shannon index (accounting for both abundance and evenness of the species present), the Chao 1 index (taking into account, besides species richness, the ratio of singletons to doubletons, giving more weight to rare species), the number of observed amplicon sequence variants (estimating the total species richness by counting the number of exact sequence variants down to the level of single-nucleotide differences), evenness (quantifying how numerically the community is equal), the Simpson index (accounting for both abundance and evenness of the species present but giving more weight to evenness and common or dominant species), and Faith’s phylogenetic diversity (the sum of the branch lengths of a phylogenetic tree connecting all species, thus reflecting the overall evolutionary history of divergence of a set of taxa).
AML, acute myeloid leukemia; FISH, fluorescence in situ hybridization; PCR, polymerase chain reaction.
During treatment, the relative abundance of some taxa is significantly altered. Members of the Bacteroidetes phylum decrease, whereas other taxa, such as those belonging to the Clostridiaceae and Streptococcaceae families, increase.23 Moreover, the proportion of mucolytic gram-positive anaerobic bacteria, such as Ruminococcus gnavus and Ruminococcus torques, increases and continues to be elevated 1 year later.24 These bacterial species modulate the glycosylation pattern of glycoconjugated molecules and mucus in goblet cells, influencing the production of intestinal mucus.26 This process could lead to increased mucolytic activity and reduced thickness of the intestinal mucus layer, potentially predisposing patients to bloodstream infection (BSI).24
Chemotherapy causes the disruption of the structural integrity of the epithelial barrier and immunosuppression, allowing the translocation of bacteria into the bloodstream and throughout the organism. Therefore, infections play a pivotal role in the morbidity and mortality of childhood leukemia. One of the main functions of the commensal microbiota is the protection against colonization by pathogens and overgrowth of indigenous pathobionts.27 The mechanism that regulates the interplay between potential pathogens, protective commensals, and the host immune system is complex. It involves competitive metabolic interactions, quorum sensing, release of antimicrobial and immunomodulatory molecules, and the regulation of mucus layer thickness.27-29
Hakim et al examined 199 children with ALL who had undergone chemotherapy and observed that GM composition, but not diversity, was an independent predictor of infections during chemotherapy. A baseline GM before initiation of chemotherapy characterized by greater relative abundance of Proteobacteria predicted the development of febrile neutropenia. GM dominance, defined as ≥30% relative abundance, by Enterococcaceae or Streptococcaceae at any time during chemotherapy, predicted infection in subsequent phases of chemotherapy. In particular, Enterococcaceae dominance predicted a significantly greater risk of subsequent febrile neutropenia and diarrheal illness, whereas Streptococcaceae dominance predicted a greater risk of subsequent diarrheal illness.23
Another study reported distinctive differences in alpha- and -diversity between samples from patients with and without infections, mainly BSI, in the first 6 months of therapy. Patients without infectious complications had increased relative abundance of the phylum Bacteroidetes and the species Faecalibacterium prausnitzii, whereas the other group had increased levels of Proteobacteria. The researchers also observed a significantly greater relative abundance of Burkholderiales, a bacterial order belonging to the Proteobacteria phylum, in prechemotherapy samples of patients who went on to develop infections. Moreover, enrichment in many aerobic bacterial species described as opportunistic pathogens occurred after treatment. Consistently, through shotgun metagenomic sequencing, they found increased representation of aerobic respiration pathways. They finally speculated that patients with infections experienced greater impairment of gut barrier integrity and low-grade intestinal blood loss, thereby increasing oxygen availability in the gut and favoring aerobic rather than anaerobic species.30
Intestinal mucositis is one of the most common side effects of chemotherapy, resulting from damage to the gut mucosal barrier that could lead to diarrhea, abdominal pain, and translocation of bacteria into the bloodstream. Mucositis severity can be estimated by measuring the plasma level of citrulline, an amino acid produced almost exclusively by enterocytes, and that is therefore a reliable marker of intestinal epithelium integrity.31 De Pietri et al observed that low alpha diversity and increased abundance of Enterococcusspp. were associated with high levels of C-reactive protein (CRP) and low plasma citrulline, whereas high abundance of Lachnospiraceae spp. was associated with increased citrulline and reduced CRP levels.32 Members of the Lachnospiraceae family are known producers of short-chain fatty acids, especially butyrate. Butyrate has a pivotal trophic effect on the intestinal epithelial barrier, acting as an energy source for enterocytes and increasing the expression of mucin and tight-junction proteins. Moreover, it possesses anti-inflammatory properties.33,34 Chemotherapy induces the loss of enterocytes and Paneth cells, which produce antimicrobial peptides, such as α-defensin and REGIIIγ,35,36 thus possibly leading to enterococcal overgrowth. The loss of enterocyte-produced lactase as a result of chemotherapy-induced damage allows undigested lactose to reach the lower intestinal tract and serve as a nutrient source for enterococci, whose growth is dependent on the presence of lactose.37 Enterococci could, in turn, exacerbate intestinal injury and inflammatory responses by compromising gut mucosal integrity, stimulating antigen-presenting cell and macrophage activation, and inducing CD4+RORγ+ T-cell infiltration.32,37
The interaction between drugs given during treatment of childhood leukemia and the GM is one of the key components of the complex scenario of chemotherapy-induced modifications of the intestinal ecosystem. Antibiotics, either prophylactic or therapeutic, play a major role in the genesis of GM dysbiosis, but data on this topic are controversial.3,6,38 Routine antibacterial prophylaxis for pediatric patients with AL is not recommended, although in some centers, fluoroquinolones or amoxicillin-clavulanate are still used.39 Monotherapy with an antipseudomonal noncarbapenem β-lactam and β-lactamase inhibitor combination or with fourth-generation cephalosporin is commonly used as empirical therapy in children with fever and neutropenia. Carbapenems are reserved for clinically unstable patients or in centers with a high rate of resistant pathogens.39 In 2 studies, pediatric patients exposed to antibiotics during chemotherapy presented lower diversity than nonexposed children,24,32 but others failed to observe a difference in diversity and composition.23,25,30 The use of ciprofloxacin prophylaxis in patients with neutropenia increased the rate of ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae isolates, and discontinuation of ciprofloxacin for at least 1 month made intestinal bacteria susceptible to ciprofloxacin again. The ceftazidime resistance rate after ciprofloxacin prophylaxis did not rise, although an increase in the minimum inhibitory concentration (MIC50) of ceftazidime over time was observed, suggesting that ciprofloxacin prophylaxis may induce ceftazidime resistance in the GM.40
Chemotherapy drugs may also have a direct effect on the GM. For example, in vitro studies have revealed that both daunorubicin and etoposide have a negative effect on the growth of anaerobic and aerobic bacteria, with only etoposide showing a dose-dependent correlation.25 In mouse models, cyclophosphamide significantly alters the composition of the microbiota in the small intestine and induces the translocation of gram-positive bacteria into secondary lymphoid organs.41 Methotrexate has also been show to broadly alter the GM composition and the expression and activity of metabolic pathways.42 On the other hand, the GM is known to interact with drug metabolism, activating prodrugs, inactivating biologically active drugs, or interfering with detoxification processes, thus possibly modulating toxicity and efficacy.43-45
It is therefore not surprising that modulation of the GM structure toward a eubiotic configuration could lead to improved clinical outcomes in different clinical settings.46 This goal is achievable through several strategies, ranging from antibiotic stewardship to nutritional or probiotics-based approaches.47 In the context of pediatric patients undergoing chemotherapy for AL, probiotics have been used to reduce chemotherapy-related side effects.22 Probiotics, defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”48 could remodel dysbiotic microbial communities to regain a healthy intestinal ecosystem. In a randomized, single-blind study, 60 children during induction or reinduction chemotherapy were enrolled. Thirty patients received Lactobacillus rhamnosus GG during chemotherapy, whereas the others did not. During hospitalization, gastrointestinal side effects were less frequent in the probiotic group, in particular diarrhea, nausea, vomiting, and abdominal distension.49 After a 30-day follow-up after hospital discharge, the probiotic group still presented fewer gastrointestinal manifestations, in addition to lower use of antibiotics.50
Gut microbiome modifications during hematopoietic stem cell transplantation
Despite the substantial improvement in AL prognosis in children, there are still several patients who have low overall survival with chemotherapy alone. These patients exhibit clinical, biological, and treatment response characteristics that have been associated with a higher probability of treatment failure. In these very-high-risk conditions, as well as in disease relapse, allogeneic hematopoietic stem cell transplantation (HSCT) is considered the gold-standard consolidation treatment in many international treating protocols.51 Therefore, HSCT represents the treatment of choice for 20% to 30% of children diagnosed with AL.51-53 During the procedure, many treatment-related upsetting events deeply modify the GM layout. It takes a variable period, up to a few months, for the GM to approximate its pre-HSCT state.54-56 These modifications cause significant dysbiosis, with loss of alpha diversity and changes in beta diversity. In particular, there is a reduction in health-promoting taxa, such as Faecalibacterium and Ruminococcus,55 and a higher presence of genera usually subdominant in the GM, such as Enterococcus, Staphylococcus, and Enterobacter.56 These compositional modifications are also accompanied by functional ones, with a reduction in the levels of numerous microbe-derived molecules, such as short-chain fatty acids.3,33,57,58 However, it should be noted that most studies of GM modifications during HSCT focus on transplantation for both malignant and nonmalignant diseases, and data regarding the differences between these 2 groups in terms of GM composition are still not available. Certainly, as previously mentioned, HSCT results in profound GM injury that may abolish differences between different conditions. In any case, considering the presence of patients with leukemia in all these studies and the relevance of the microbiological data presented, we decided to include them in this review.
In adults, a lower diversity of the GM at engraftment has been clearly associated with various clinical outcomes, such as overall survival, transplant-related mortality and graft-versus-host disease (GVHD).59 The pediatric population presents some peculiar features that justify specific studies.60 The GM configuration before HSCT is age dependent, as is the post-HSCT reconstitution.61 Compared with adults, in pediatric patients there is greater interindividual variability.62 On the one hand, conclusive data on the relationship between the development of GM dysbiosis and overall survival are missing, because the available evidence is from small cohorts of patients and the results are somewhat discordant.60,63,64 On the other hand, important data have been published on other transplant-related complications, mainly GVHD and infectious sequelae.61,65
Simms-Waldrip et al analyzed the GM of 15 pediatric patients who underwent HSCT for both malignant and nonmalignant diseases and developed acute GVHD (aGVHD) and found that these patients showed a decline in anti-inflammatory Clostridia and an increase in proinflammatory Enterobacteriaceae.66 Subsequently, Biagi et al found, in a multicenter cohort of 36 patients who underwent HSCT, a GM signature before transplantation that was potentially predictive of aGVHD. In particular, the GM of patients who went on to develop gut aGVHD was characterized by reduced diversity, higher abundance of Fusobacterium and decreased abundance of Blautia, an anti-inflammatory member of the Clostridia class.61 These data suggest that the pre-HSCT configuration could exert a trigger for alloimmune manifestation and may be considered to be a risk factor.
Bacterial BSIs represent a life-threatening complication of HSCT as it has been described in children undergoing chemotherapy. Pediatric studies are particularly lacking on this matter. Kelly et al analyzed the GM composition in 9 children undergoing HSCT for both malignant and nonmalignant diseases who developed mucosal barrier injury-related BSIs.67 They found that, in 6 patients, the fecal strains were identical with the blood culture isolates, and in 4 cases, GM dominance by a specific strain preceded the BSI episode by a median of 17 days. In the same study, the authors found that the increasing abundance of the resulting BSI strains in the GM was associated with specific antibiotic exposures.67 In fact, the GM has also been demonstrated to act as a reservoir for antibiotic-resistant bacteria. We directly addressed this issue in 8 pediatric patients who underwent HSCT, half of whom developed aGVHD, by analyzing their gut resistome by shotgun metagenomics, defined as the overall pattern of antimicrobial resistance genes present in the GM.38 We found that the patients developing aGVHD showed expansion of their gut resistome, involving the acquisition of new resistances, as well as the consolidation of those already present before HSCT. This expansion involved genes conferring resistance even to antibiotics that were not administered during the therapeutic course.38
The GM also exerts a role in modulating immune system reconstitution after HSCT. The conditioning regimen, comprising chemotherapy, radiotherapy, and immunotherapy, represents a severe perturbation of the immune system of the child: this could offer a unique opportunity to understand the link between GM and the immune system in vivo. Ingham et al analyzed 37 pediatric patients who underwent HSCT for malignant and nonmalignant diseases.68 They found that children with rapid NK- and B-cell reconstitution had a higher abundance of anaerobic bacteria, such as Clostridiales, and lower aGVHD severity and mortality. Conversely, patients with slow NK- and B-cell reconstitution presented higher relative abundances of facultative anaerobic bacteria (Enterobacteriaceae, Staphylococcus spp, and Streptococcus spp).
Antibiotics, as explained above, certainly represent a GM-modifying factor.57 However, rather than a direct intervention, an "antibiotic-sparing" approach should be considered. Antibiotics that are effective against anaerobic bacteria, such as vancomycin and ciprofloxacin, can have a detrimental effect on GM, especially on commensal Clostridiales.66 Clinicians should always balance the risk of infection and of GM dysbiosis in defining the optimal antibiotic strategy. Nutritional support has also emerged in the past several years as a crucial participant in modulating the GM. In this regard, we performed a longitudinal study of 20 pediatric patients who underwent HSCT, comparing parenteral and enteral nutrition (EN).58 Patients receiving EN presented the recovery of a rich and diverse GM earlier after HSCT and the restoration of health-promoting taxa, such as Faecalibacterium, Dorea, Blautia, Bacteroides, Parabacteroides, and Oscillospira. Clinical data seem to be in line with the microbiological findings, indicating that EN could be associated with improved clinical outcomes regarding aGVHD and infections. These observations were confirmed by the results of our recent meta-analysis in which EN was found to be protective against aGVHD, severe aGVHD, and gut GVHD.69-71 Data should be further confirmed in larger multicenter cohorts and randomized trials. Fecal microbiota transplantation (FMT) represents an alternative, more direct modulatory intervention using live microorganisms. The infusion of donor fecal matter is aimed at restoring GM eubiosis by replacing the bacterial communities harbored by the gut mucosa.72 Bluestone et al reported the use of FMT to treat refractory Clostridioides difficile in 3 pediatric patients who underwent HSCT with no adverse effects, even if they had only 1 remission.73 Merli et al treated 5 pediatric patients who underwent HSCT with FMT, to decolonize the gut of multidrug-resistant bacteria. No adverse effects were observed; however, long-term colonization was successful in only 1 patient.74 FMT was also performed in a child to treat steroid refractory aGVHD, resulting in the remission of symptoms. Taxonomic analysis after FMT showed the restoration of GM diversity, with gradual reduction in Proteobacteria and increase in Firmicutes.75 However, solid data on pediatric patients are still lacking and some concerns regarding safety and feasibility remain.76-78
Long-term gut microbiome modifications
The GM perturbation induced by AL treatment could last even after the removal of microbiota-modifying factors, such as antibiotics, chemotherapeutic drugs or alloimmune reactions. These long-term GM alterations could have an impact on the health profile of childhood AL survivors, potentially predisposing them to conditions related to an altered GM. GM diversity is usually restored 1 year after chemotherapy, but the relative abundances of some species remain significantly altered.24 In a study conducted by Thomas et al,79 survivors of pediatric ALL did not show differences compared with their siblings in alpha- or beta-diversity in samples taken at least 1 year after completion of therapy, although members of the Ruminococcaceae and Lachnospiraceae families, and, notably, Faecalibacterium, were still significantly depleted in survivors.79 In adult survivors, a decrease in the abundance of Faecalibacterium and alpha diversity in the anal microbiome has been reported, and correlated with increased plasma concentrations of interleukin-6 and CRP and higher levels of HLA-DR+CD4+ and HLA-DR+CD8+ T cells, markers of increased inflammation and T-cell activation.80
Adult survivors of childhood ALL are at increased risk of obesity, metabolic syndrome, and cardiovascular disease,81 and, notably, a lower abundance of Faecalibacterium has been linked to obesity in other studies,82 suggesting that GM alterations in pediatric ALL survivors established in childhood may play a role in predisposition to chronic illness in later years of survivorship.
Conclusions and future directions
Our understanding is growing of how gut bacteria influence the genesis, treatment course, and related complications of childhood AL (Figure 2). Pediatric leukemias are peculiar entities, different from the adult diseases, as regards pathogenesis, molecular biology, treatment toxicity, and response to therapy.8 Furthermore, the GM in pediatric patients is not yet “mature” but rapidly and continuously evolves from birth to adolescence. These considerations make the study of the relationship between AL and the GM in pediatric patients a crucial and distinct field of research.
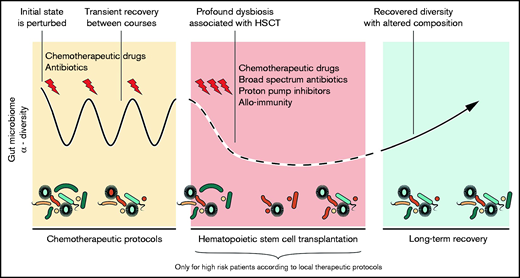
The trajectory of the GM during the therapeutic course of AL. The initial microbial state is perturbed by chemotherapeutic cycles with partial recovery between them. HSCT exerts a strong dysbiotic effect on the GM. Reconstitution after HSCT resembles the pre-HSCT state, but dysbiotic features often persist.
The trajectory of the GM during the therapeutic course of AL. The initial microbial state is perturbed by chemotherapeutic cycles with partial recovery between them. HSCT exerts a strong dysbiotic effect on the GM. Reconstitution after HSCT resembles the pre-HSCT state, but dysbiotic features often persist.
Groundbreaking advancements have been made in the past few years regarding the role of GM dysbiosis in the genesis of ALL. Microbes may directly affect the preleukemic cell clone in terms of its proliferation and acquisition of successive mutations, and the GM could interact with the process of leukemogenesis through the modulation of the immune system.14 These findings, if confirmed in larger scale studies, could open up new opportunities in the development of preventive strategies for childhood AL.
The strongest evidence relating the GM to clinical outcomes has been produced in adult allo-HSCT. In particular, lower alpha diversity at the time of neutrophil engraftment was associated with higher mortality in a multicenter study by Peled et al.45 These data remain to be confirmed in pediatric patients, and this validation should be a major focus of research in the upcoming years.
These studies must take into account the specific characteristics of the pediatric setting. Children differ in indications for transplantation, complication rates, and microbiome. In particular, their GM is heterogeneous and changes continuously in an age-related manner, while interlinked in tight connection with the developing immune system.3
Moreover, the comprehension of the mechanistic relationship between the GM and outcomes is lacking. The application of so-called omics approaches, including metagenomics, metatranscriptomics, metaproteomics, metabolomics, and, not least, culturomics, could help in understanding the biological processes underpinning the observed clinical findings. In particular, in our opinion, 2 key areas seem to be extremely promising: bacterial drug metabolism and the relationship between nutrition and the GM metabolome.
The use of potential microbiome-targeted interventions to improve clinical outcomes during chemotherapy and allo-HSCT is already feasible in clinical practice. Different approaches could be applied in children to modulate the GM toward a protective configuration, ranging from nutritional intervention to FMT. Each one has its unique safety issues and hypothesis of efficacy, derived mainly from studies of adult cohorts.83,84 Therefore, further studies focused on the pediatric population are warranted to extend these interventions to patients with childhood AL. Promoting EN could be considered one of the most cost-effective strategies.85 The use of prebiotics could also be implemented in clinical trials to investigate whether they could exert an effect on the intestinal ecosystem so that they could significantly reduce treatment-related toxicities. Prebiotics, defined as “a substrate that is selectively utilized by host microorganisms, conferring a health benefit,” are a heterogeneous group of substances.86 Among them, fibers and oligosaccharides, but also glutamine and lactoferrin are promising molecules that have an effect on the GM and are relatively safe and potential beneficial during treatment of AL. Interestingly, an ongoing randomized trial (registered on www.clinical trials.gov as NCT03414775) is examining the influence of a high-fiber, organic, whole-food diet on the GM of critically ill children, and among them patients with ALL.
More data are needed on the safety of probiotics and FMT in the pediatric AL population. In particular, the risk of infection resulting from the delivery of live microorganisms to immunocompromised children with impaired gut permeability is a major concern. Some studies have shown that probiotic strains can cause bacteremia in critically ill pediatric patients and in adolescents receiving immunosuppressive treatment of ulcerative colitis.87,88 Moreover, the potential transmission of disease-related GM configurations from the FMT donor and the long-term effects of GM manipulation are not fully understood.57 Therefore, despite the strong scientific rationale and the emerging clinical utility, the administration of single probiotics or microbial consortia still requires great caution.
One of the most effective ways to interact with the GM is through the modulation of antibiotic use. Different classes of antibiotics have diverse impacts on the GM structure, and these effects could be reflected in clinical outcomes.47 For example, the use of antibiotics sparing anaerobic bacteria has been shown to reduce the risk of developing gut GVHD in adults.89,90 Different strategies, including administering narrow-spectrum antibiotics and improving timing and duration of treatment, should be implemented in future pediatric clinical studies. A randomized pediatric trial, currently recruiting (registered on www.clinical trials.gov as NCT04637464), is addressing early discontinuation of empirical antibiotics for febrile neutropenia in patients with ALL and pediatric cancer, with the secondary end point of studying the modifications in GM composition associated with this strategy.
The GM has been shown to influence immune system dynamics in allo-HSCT recipients13 and also to modulate the anticancer response to immunotherapy.91 Therefore, it is possible to speculate that it could influence the response rates and toxicities of the most recent and innovative treatments for childhood AL: CAR-T cells and monoclonal antibodies.
In conclusion, the disruption of the symbiotic relationship between the GM and the human organism could interact with the natural history of childhood AL at several key time points, from AL genesis to treatment-related complications. The maintenance of a eubiotic layout of the intestinal ecosystem could instead have a positive effect on outcomes, potentially reducing morbidity and mortality.
Authorship
Contribution: R.M., D.Z., E.M., and D.L. conceived of the study; D.L. designed the images; E.M. designed the table; E.M. and D.L. prepared the original draft; R.M., D.Z., and S.T. reviewed and edited the manuscript; A.P., S.E., and P.B. supervised the study; and all authors read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edoardo Muratore, Pediatric Oncology and Hematology Unit “Lalla Seràgnoli,” Pediatric Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; e-mail: edoardo.muratore@studio.unibo.it.