Key Points
The survival of patients with aggressive ATL has improved by increased application of allo-HCT after response to initial chemotherapy.
The use of cord blood or HLA-haploidentical donors may be feasible for aggressive ATL when HLA-matched related donors are unavailable.
Abstract
Aggressive adult T-cell leukemia/lymphoma (ATL) is a hematological malignancy that is difficult to treat with chemotherapy alone, and allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy. We conducted a multicenter, prospective, observational study to clarify the treatment outcomes of aggressive ATL in the current era. Between 2015 and 2018, 113 patients aged 70 years or younger with newly diagnosed aggressive ATL were enrolled. The median age at diagnosis was 61 years. Treatment outcomes were compared with those of 1792 ATL patients diagnosed between 2000 and 2013 in our previous retrospective study. The inclusion criteria were the same in both studies. The prospective cohort demonstrated better overall survival (OS) than the retrospective cohort (2-year OS, 45% vs 29%, respectively; P < .001), with a much higher proportion of patients receiving allo-HCT (80% vs 34%, respectively; P < .001) and a shorter interval from diagnosis to allo-HCT (median, 128 vs 170 days, respectively; P < .001). Among the 90 patients who received allo-HCT (cord blood, n = 30; HLA-haploidentical related donors, n = 20; other related donors, n = 14; other unrelated donors, n = 26), the 2-year probabilities of OS, non-relapse mortality (NRM), and disease progression were 44%, 23%, and 46%, respectively. OS and NRM did not differ statistically according to donor type. Our results suggest that increased application of allo-HCT improved the survival of patients with aggressive ATL. The use of cord blood or HLA-haploidentical donors may be feasible for aggressive ATL when HLA-matched related donors are unavailable. This study was registered at the UMIN Clinical Trials Registry as #000017672.
Introduction
Aggressive adult T-cell leukemia/lymphoma (ATL) is a hematological malignancy associated with human T-cell leukemia virus type 1 (HTLV-1). The prognosis of aggressive ATL treated by conventional chemotherapy alone is poor. Following the administration of vincristine, cyclophosphamide, doxorubicin, and prednisone (VCAP), doxorubicin, ranimustine, and prednisone (AMP), and vindesine, etoposide, carboplatin, and prednisone (VECP) (modified LSG15), which is a standard intensive regimen for aggressive ATL in Japan, the median survival time and 3-year overall survival (OS) were reported to be 12.7 months and 24%, respectively.1 Outcomes in the real world were found to be worse than those in clinical trials, with a 4-year OS of 12% reported in a previous nationwide retrospective study in Japan.2 Two novel agents, mogamulizumab3-5 and lenalidomide,6,7 showed high response rates for relapsed or refractory ATL, but the response was not durable in most patients.
Current Japanese guidelines recommend that transplant-eligible patients undergo upfront allogeneic hematopoietic cell transplantation (allo-HCT) from HLA-matched related donors (MRDs) or unrelated donors after response to initial chemotherapy.8 The long-term OS probability after allo-HCT from MRDs or HLA-matched unrelated donors is ∼40%.9,10 However, as the median age of ATL patients at diagnosis is 68 years,11 only a small proportion have an MRD. Moreover, many ATL patients experience disease progression during donor coordination for unrelated bone marrow transplantation (uBMT) or unrelated peripheral blood stem cell transplantation (uPBSCT), because unrelated donor coordination usually takes 4 to 5 months in Japan.
When a suitable MRD is unavailable, cord blood transplantation (CBT) or haploidentical HCT (haplo-HCT) can be an alternative to uBMT/uPBSCT.12 We previously conducted a multicenter retrospective cohort study. A total of 1792 patients with aggressive ATL diagnosed at age ≤70 years between 2000 and 2013 were analyzed.13 In that cohort, the proportion of patients who received allo-HCT was only 34%. The OS after CBT was inferior to that after uBMT by ∼20%. Previous studies based on Japanese registry data also reported inferior outcomes of CBT9,10 and conventional haplo-HCT without posttransplant cyclophosphamide (PTCy)14 compared with uBMT. However, some investigators showed that the outcome of CBT was comparable to that of uBMT when disease was held in remission.10,15,16 Further, haplo-HCT with PTCy is increasingly being conducted for aggressive ATL. In current clinical practice, when MRD is unavailable, CBT and haplo-HCT are commonly performed after response to initial chemotherapy, as opposed to waiting for months of unrelated donor coordination.
Here, we conducted a multicenter, prospective, observational study of aggressive ATL. The first aim was to clarify whether the survival of patients with aggressive ATL improved by increasing the application of allo-HCT. The second was to evaluate the feasibility of CBT and haplo-HCT compared with uBMT/uPBSCT for aggressive ATL.
Patients and methods
Patients
We prospectively enrolled patients with newly diagnosed aggressive ATL. The inclusion criteria were age ≤70 years and acute- or lymphoma-type ATL classified by Shimoyama’s criteria.17 Physicians prospectively reported standardized information on the treatment and outcomes of ATL patients at their centers. The therapeutic strategy of ATL was not specified in this study, although compliance with the current Japanese guidelines was encouraged.8 Informed consent was obtained from all participating patients in accordance with the Declaration of Helsinki. All participating institutions obtained institutional review board approval for this study. This study was registered with the UMIN Clinical Trials Registry as #UMIN 000017672.
Definitions
The modified prognostic index for ATL (mATL-PI) was defined as previously reported.13 Conditioning regimens were categorized as either myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC) by the Center for International Blood and Marrow Transplant Research criteria.18,19 Acute and chronic graft-versus-host disease (GVHD) was diagnosed and graded according to the modified Glucksberg-Seattle criteria20 and National Institutes of Health criteria,21 respectively. Response to treatment was assessed according to the revised response evaluation criteria in solid tumor guideline.22 OS was measured from diagnosis or allo-HCT to death from any cause. Progression-free survival (PFS) was measured from diagnosis or allo-HCT until disease progression or death. Disease progression was diagnosed by morphological or imaging tests. Non-relapse mortality (NRM) was defined as death without disease progression.
Statistical analysis
The primary end point was the probability of OS at 2 years after diagnosis. The secondary end points included the application rate of allo-HCT and other survival outcomes (PFS, relapse/progressive disease [Rel/PD], and NRM). Differences between groups were compared using the Mann-Whitney U test or Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. The probability of OS and PFS were calculated with the Kaplan-Meier method, and the difference between groups was compared by the log-rank test. The frequencies of GVHD, NRM, and Rel/PD were calculated with the cumulative incidence method to accommodate competing risks, and the difference between groups was compared with Gray’s test. Univariate and multivariate analysis was conducted to identify potential risk factors for transplant outcomes. Hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were calculated with the Cox proportional hazard model for OS and PFS and with the Fine-Gray proportional hazard model for NRM and Rel/PD. The following variables were analyzed: age at allo-HCT (<60 years vs ≥60 years), gender, ATL type (acute vs lymphoma type), mATL-PI (low vs intermediate vs high), induction chemotherapy (modified LSG15 [mLSG15: vincristine, cyclophosphamide, doxorubicin, and prednisone (VCAP), doxorubicin, ranimustine, and prednisone (AMP), and vindesine, etoposide, carboplatin, and prednisone (VECP)] vs CHOP [cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate (Oncovin), and prednisone]-like regimens), the number of cycles of chemotherapy before allo-HCT (≤3 vs >3), the interval between diagnosis and allo-HCT (<128 days vs ≥128 days), disease status at allo-HCT (complete response [CR] vs partial response [PR] vs stable disease [SD] or PD), donor type (5/6 or 6/6 HLA-matched related donor vs unrelated donor vs cord blood vs HLA-haploidentical related donor), conditioning therapy (MAC vs fludarabine [Flu]/busulphan [Bu]-based RIC vs Flu/melphalan [Mel]-based RIC). To clarify the prognostic significance, we simultaneously entered the donor types into the multivariate model. All P values were 2 sided, and P values ≤ .05 were considered significant. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).23
Results
Patient characteristics
Between 2015 March and 2018 March, 113 patients with newly diagnosed aggressive ATL were prospectively enrolled from 30 centers. In 27 of the 30 (90%) centers, the patients were consecutively enrolled. The median interval between diagnosis and enrolment was 51 days (range, 0-187 days).
Patient characteristics at diagnosis are shown in Table 1. The median age was 61 years (range, 27-70 years). The Eastern Cooperative Oncology Group Performance Status (ECOG-PS) was 2 to 4 in 24 (21%) patients. The soluble interleukin-2 receptor was >5000 U/mL in 83 (73%) patients. The modified ATL-PI was low in 33 (29%) patients, intermediate in 71 (63%), and high in 9 (8%). Ten of 113 patients presented with central nervous system (CNS) involvement at diagnosis (cerebrospinal fluid in 7, brain/spinal mass lesion in 3, negative in 42, and not tested in 61). Ninety (80%) patients received allo-HCT after initial chemotherapy. Patients in the HCT group were significantly younger than those in the non-HCT group (median, 60 vs 65 years, respectively; P < .001) and had a better ECOG-PS (2 to 4, 17% vs 39%, respectively; P = .046). Further, in the HCT group, there was a nonsignificantly lower proportion of males (56% vs 78%, respectively; P = .058) and a mATL-PI of intermediate or high (67% vs 87%, respectively; P = .079). The other characteristics at diagnosis were similarly distributed between the 2 groups.
Patient characteristics at diagnosis (n = 113)
| . | Total (n = 113) . | HCT (n = 90) . | non-HCT (n = 23) . | P . |
|---|---|---|---|---|
| Age at diagnosis (y), median (range) | 61 (27-70) | 60 (27-69) | 65 (55-70) | <.001 |
| Gender | .058 | |||
| Male | 68 (60) | 50 (56) | 18 (78) | |
| Female | 45 (40) | 40 (44) | 5 (22) | |
| ATL subtype | .32 | |||
| Acute type | 79 (70) | 65 (72) | 14 (61) | |
| Lymphoma type | 34 (30) | 25 (28) | 9 (39) | |
| ECOG-PS | .046 | |||
| 0-1 | 83 (73) | 69 (77) | 14 (61) | |
| 2-4 | 24 (21) | 15 (17) | 9 (39) | |
| Unknown | 6 (5) | 6 (7) | 0 | |
| Compensated calcium concentration (mg/dL), median (range) | 9.7 (8.3-18.3) | 9.7 (8.3-18.3) | 9.7 (7.4-17.0) | .95 |
| ≥12 mg/dL | 14 (12) | 11 (12) | 3 (13) | 1.00 |
| CRP (mg/dL), median (range) | 0.36 (0.02-19.50) | 0.35 (0.02-19.50) | 0.38 (0.02-13.35) | .85 |
| ≥2.5 mg/dL | 23 (20) | 18 (20) | 5 (22) | 1.00 |
| sIL-2R (U/mL), median (range) | 12 476 (278-178 682) | 11 121 (278-178 682) | 17 042 (1,050-77 900) | .24 |
| >5000 U/mL | 83 (73) | 65 (72) | 18 (78) | .39 |
| mATL-PI | .079 | |||
| Low risk | 33 (29) | 30 (33) | 3 (13) | |
| Intermediate risk | 71 (63) | 52 (58) | 19 (83) | |
| High risk | 9 (8) | 8 (9) | 1 (4) |
| . | Total (n = 113) . | HCT (n = 90) . | non-HCT (n = 23) . | P . |
|---|---|---|---|---|
| Age at diagnosis (y), median (range) | 61 (27-70) | 60 (27-69) | 65 (55-70) | <.001 |
| Gender | .058 | |||
| Male | 68 (60) | 50 (56) | 18 (78) | |
| Female | 45 (40) | 40 (44) | 5 (22) | |
| ATL subtype | .32 | |||
| Acute type | 79 (70) | 65 (72) | 14 (61) | |
| Lymphoma type | 34 (30) | 25 (28) | 9 (39) | |
| ECOG-PS | .046 | |||
| 0-1 | 83 (73) | 69 (77) | 14 (61) | |
| 2-4 | 24 (21) | 15 (17) | 9 (39) | |
| Unknown | 6 (5) | 6 (7) | 0 | |
| Compensated calcium concentration (mg/dL), median (range) | 9.7 (8.3-18.3) | 9.7 (8.3-18.3) | 9.7 (7.4-17.0) | .95 |
| ≥12 mg/dL | 14 (12) | 11 (12) | 3 (13) | 1.00 |
| CRP (mg/dL), median (range) | 0.36 (0.02-19.50) | 0.35 (0.02-19.50) | 0.38 (0.02-13.35) | .85 |
| ≥2.5 mg/dL | 23 (20) | 18 (20) | 5 (22) | 1.00 |
| sIL-2R (U/mL), median (range) | 12 476 (278-178 682) | 11 121 (278-178 682) | 17 042 (1,050-77 900) | .24 |
| >5000 U/mL | 83 (73) | 65 (72) | 18 (78) | .39 |
| mATL-PI | .079 | |||
| Low risk | 33 (29) | 30 (33) | 3 (13) | |
| Intermediate risk | 71 (63) | 52 (58) | 19 (83) | |
| High risk | 9 (8) | 8 (9) | 1 (4) |
Patient characteristics at diagnosis were compared between the HCT group (n = 90) and the non-HCT group (n = 23).
CRP, C-reactive protein; sIL-2R, soluble interleukin-2 receptor.
Patient characteristics at allo-HCT are shown in Table 2. The median age at allo-HCT was 60 years (range, 27-70 years). The number of regimens before allo-HCT was 1 in 69 (77%) patients, 2 in 20 (22%), and 4 in 1 (1%). Six (7%) patients received mogamulizumab-containing therapy before allo-HCT, and the median number of cycles was 3 (range, 1-7). The median interval between the last administration of mogamulizumab and allo-HCT (3 uBMT, 2 CBT, and 1 related PBSCT) was 74 days (range, 45-154 days). The median time from diagnosis to allo-HCT was 128 days (range, 42-471 days). Nine patients had history of CNS involvement before allo-HCT (7 at diagnosis and 2 during induction chemotherapy). The disease status at allo-HCT was CR in 41 (46%) patients, PR in 28 (31%), SD in 6 (7%), and PD in 15 (17%). The donor type was 5/6 or 6/6 HLA-matched related BM/PBSC in 14 (16%) patients, unrelated BM/PBSC in 26 (29%), CB in 30 (33%), and HLA-haploidentical related PBSC in 20 (22%). Four (4%) patients received HCT from an HTLV-1–seropositive donor. The conditioning regimen was MAC in 14 (16%) patients, Flu/Bu-based RIC in 16 (18%), Flu/Mel-based RIC in 58 (64%), and Flu/Bu/Mel in 2 (2%). For GVHD prophylaxis, PTCy was used in 19 patients who received haplo-HCT. The median time from diagnosis to HCT was shorter in CBT and haplo-HCT than in uBMT/uPBSCT (124 days in CB vs 120 days in haplo-HCT vs 163 days in uBMT/uPBSCT; P < .001). Flu-Bu-based RIC was most common in uBMT/uPBSCT, while Flu/Mel-based RIC was used most frequently in CBT and haplo-HCT (P < .001).
Patient characteristics at allo-HCT (n = 90)
| . | Total (n = 90) . | 5-6/6 HLA-matched related (n = 14) . | Unrelated (n = 26) . | CB (n = 30) . | HLA-haploidentical related (n = 20) . | P . |
|---|---|---|---|---|---|---|
| Age at allo-HCT (y) | .63 | |||||
| Median (range) | 60 (27-70) | 60 (40-69) | 60 (35-67) | 61 (36-70) | 60 (27-67) | |
| Gender | .29 | |||||
| Male | 50 (56) | 6 (43) | 12 (46) | 18 (60) | 14 (70) | |
| Female | 40 (44) | 8 (57) | 14 (54) | 12 (40) | 6 (30) | |
| ATL subtype | .60 | |||||
| Acute type | 65 (72) | 11 (79) | 20 (77) | 22 (73) | 12 (60) | |
| Lymphoma type | 25 (28) | 3 (21) | 6 (23) | 8 (27) | 8 (40) | |
| mATL-PI at diagnosis | .25 | |||||
| Low risk | 30 (33) | 1 (7) | 10 (38) | 11 (37) | 8 (40) | |
| Intermediate risk | 52 (58) | 12 (86) | 14 (54) | 17 (57) | 9 (45) | |
| High risk | 8 (9) | 1 (7) | 2 (8) | 2 (7) | 3 (15) | |
| Induction chemotherapy | .46 | |||||
| mLSG15 regimen | 79 (88) | 13 (93) | 22 (85) | 28 (93) | 16 (80) | |
| CHOP-like regimen | 11 (12) | 1 (7) | 4 (15) | 2 (7) | 4 (20) | |
| Response to induction therapy | .98 | |||||
| CR | 36 (40) | 6 (43) | 9 (35) | 12 (40) | 9 (45) | |
| PR | 34 (38) | 4 (29) | 10 (38) | 12 (40) | 8 (40) | |
| SD | 4 (4) | 1 (7) | 1 (4) | 1 (3) | 1 (5) | |
| PD | 16 (18) | 3 (21) | 6 (23) | 5 (17) | 2 (10) | |
| Number of regimens before allo-HCT | .32 | |||||
| 1 | 69 (77) | 8 (57) | 20 (77) | 25 (83) | 16 (80) | |
| 2 | 20 (22) | 6 (43) | 5 (19) | 5 (17) | 4 (20) | |
| 4 | 1 (1) | 0 | 1 (4) | 0 | 0 | |
| Mogamulizumab use before allo-HCT | 6 (7) | 1 (7) | 3 (12) | 2 (7) | 0 | .52 |
| Number of cycles, median (range) | 3 (1-7) | 6 | 4 (1-7) | 1.5 (1-2) | — | — |
| Interval between the last dose to allo-HCT (days), median (range) | 74 (45-154) | 78 | 70 (49-154) | 67 (45-89) | — | — |
| Interval between the diagnosis and allo-HCT (days), median (range) | 128 (42-471) | 107 (58-278) | 163 (109-382) | 124 (83-282) | 120 (42-471) | <.001 |
| Disease status at allo-HCT | .69 | |||||
| CR | 41 (46) | 6 (43) | 12 (46) | 12 (40) | 11 (55) | |
| PR | 28 (31) | 4 (29) | 11 (42) | 10 (33) | 3 (15) | |
| SD | 6 (7) | 1 (7) | 1 (4) | 2 (7) | 2 (10) | |
| PD | 15 (17) | 3 (21) | 2 (8) | 6 (20) | 4 (20) | |
| Stem cell source | — | |||||
| BM | 27 (30) | 6 (43) | 21 (81) | 0 | 0 | |
| PBSC | 33 (37) | 8 (57) | 5 (19) | 0 | 20 (100) | |
| CB | 30 (33) | 0 | 0 | 30 (100) | 0 | |
| HTLV-1 seropositive donor | 4 (4) | 3 (21) | 0 | 0 | 1 (5) | — |
| Conditioning therapy | <.001 | |||||
| Cy/TBI-based MAC | 7 (8) | 4 (29) | 2 (8) | 1 (3) | 0 | |
| Flu/Bu-based MAC | 7 (8) | 3 (21) | 1 (4) | 1 (3) | 2 (10) | |
| Flu/Mel-based RIC | 58 (64) | 6 (43) | 10 (38) | 26 (87) | 16 (80) | |
| Flu-Bu-based RIC | 16 (18) | 1 (7) | 13 (50) | 0 | 2 (10) | |
| Flu/Bu2/Mel80 | 2 (2) | 0 | 0 | 2 (7) | 0 | |
| GVHD prophylaxis | ||||||
| ATG use | 10 (11) | 2 (14) | 8 (31) | 0 | 0 | — |
| ATG dose (mg/kg), median (range) | 2.0 (1.0-2.5) | 1.8 (1.5-2.0) | 2.0 (1.0-2.5) | — | — | — |
| PTCy use | 19 (21) | 0 | 0 | 0 | 19 (95) | — |
| . | Total (n = 90) . | 5-6/6 HLA-matched related (n = 14) . | Unrelated (n = 26) . | CB (n = 30) . | HLA-haploidentical related (n = 20) . | P . |
|---|---|---|---|---|---|---|
| Age at allo-HCT (y) | .63 | |||||
| Median (range) | 60 (27-70) | 60 (40-69) | 60 (35-67) | 61 (36-70) | 60 (27-67) | |
| Gender | .29 | |||||
| Male | 50 (56) | 6 (43) | 12 (46) | 18 (60) | 14 (70) | |
| Female | 40 (44) | 8 (57) | 14 (54) | 12 (40) | 6 (30) | |
| ATL subtype | .60 | |||||
| Acute type | 65 (72) | 11 (79) | 20 (77) | 22 (73) | 12 (60) | |
| Lymphoma type | 25 (28) | 3 (21) | 6 (23) | 8 (27) | 8 (40) | |
| mATL-PI at diagnosis | .25 | |||||
| Low risk | 30 (33) | 1 (7) | 10 (38) | 11 (37) | 8 (40) | |
| Intermediate risk | 52 (58) | 12 (86) | 14 (54) | 17 (57) | 9 (45) | |
| High risk | 8 (9) | 1 (7) | 2 (8) | 2 (7) | 3 (15) | |
| Induction chemotherapy | .46 | |||||
| mLSG15 regimen | 79 (88) | 13 (93) | 22 (85) | 28 (93) | 16 (80) | |
| CHOP-like regimen | 11 (12) | 1 (7) | 4 (15) | 2 (7) | 4 (20) | |
| Response to induction therapy | .98 | |||||
| CR | 36 (40) | 6 (43) | 9 (35) | 12 (40) | 9 (45) | |
| PR | 34 (38) | 4 (29) | 10 (38) | 12 (40) | 8 (40) | |
| SD | 4 (4) | 1 (7) | 1 (4) | 1 (3) | 1 (5) | |
| PD | 16 (18) | 3 (21) | 6 (23) | 5 (17) | 2 (10) | |
| Number of regimens before allo-HCT | .32 | |||||
| 1 | 69 (77) | 8 (57) | 20 (77) | 25 (83) | 16 (80) | |
| 2 | 20 (22) | 6 (43) | 5 (19) | 5 (17) | 4 (20) | |
| 4 | 1 (1) | 0 | 1 (4) | 0 | 0 | |
| Mogamulizumab use before allo-HCT | 6 (7) | 1 (7) | 3 (12) | 2 (7) | 0 | .52 |
| Number of cycles, median (range) | 3 (1-7) | 6 | 4 (1-7) | 1.5 (1-2) | — | — |
| Interval between the last dose to allo-HCT (days), median (range) | 74 (45-154) | 78 | 70 (49-154) | 67 (45-89) | — | — |
| Interval between the diagnosis and allo-HCT (days), median (range) | 128 (42-471) | 107 (58-278) | 163 (109-382) | 124 (83-282) | 120 (42-471) | <.001 |
| Disease status at allo-HCT | .69 | |||||
| CR | 41 (46) | 6 (43) | 12 (46) | 12 (40) | 11 (55) | |
| PR | 28 (31) | 4 (29) | 11 (42) | 10 (33) | 3 (15) | |
| SD | 6 (7) | 1 (7) | 1 (4) | 2 (7) | 2 (10) | |
| PD | 15 (17) | 3 (21) | 2 (8) | 6 (20) | 4 (20) | |
| Stem cell source | — | |||||
| BM | 27 (30) | 6 (43) | 21 (81) | 0 | 0 | |
| PBSC | 33 (37) | 8 (57) | 5 (19) | 0 | 20 (100) | |
| CB | 30 (33) | 0 | 0 | 30 (100) | 0 | |
| HTLV-1 seropositive donor | 4 (4) | 3 (21) | 0 | 0 | 1 (5) | — |
| Conditioning therapy | <.001 | |||||
| Cy/TBI-based MAC | 7 (8) | 4 (29) | 2 (8) | 1 (3) | 0 | |
| Flu/Bu-based MAC | 7 (8) | 3 (21) | 1 (4) | 1 (3) | 2 (10) | |
| Flu/Mel-based RIC | 58 (64) | 6 (43) | 10 (38) | 26 (87) | 16 (80) | |
| Flu-Bu-based RIC | 16 (18) | 1 (7) | 13 (50) | 0 | 2 (10) | |
| Flu/Bu2/Mel80 | 2 (2) | 0 | 0 | 2 (7) | 0 | |
| GVHD prophylaxis | ||||||
| ATG use | 10 (11) | 2 (14) | 8 (31) | 0 | 0 | — |
| ATG dose (mg/kg), median (range) | 2.0 (1.0-2.5) | 1.8 (1.5-2.0) | 2.0 (1.0-2.5) | — | — | — |
| PTCy use | 19 (21) | 0 | 0 | 0 | 19 (95) | — |
Patient characteristics were compared among the 4 groups according to donor type.
ATG, anti-thymocyte globulin; BM, bone marrow; CB, cord blood; Cy, cyclophosphamide; PBSC, peripheral blood stem cell; TBI, total body irradiation.
The patient characteristics at allo-HCT were compared between the current prospective cohort and the retrospective cohort in our previous study (supplemental Table 1).13 The 2 cohorts showed a similar distribution in terms of age at diagnosis, gender, ATL subtype (acute vs lymphoma), and mATL-PI. More patients in the prospective cohort than in the retrospective cohort received the mLSG15 regimen as induction therapy (81% vs 46%, respectively; P < .001), and these patients had a higher overall response to induction therapy (72% vs 65%, respectively; P < .001). Further, in the prospective cohort, a higher proportion of patients received allo-HCT (80% vs 34%, respectively; P < .001), and there was a shorter median interval between diagnosis and allo-HCT (128 days vs 170 days, respectively; P < .001). There was no significant difference in disease status at allo-HCT between the 2 cohorts (P = .28). More patients received CBT or haplo-HCT in the prospective cohort than in the retrospective cohort (CBT, 33% vs 23%; haplo-HCT, 22% vs 8%; P < .001).
OS of the entire cohort
The median follow-up period of survivors was 1192 days (range, 729-1912) from the diagnosis of aggressive ATL. The probability of OS at 2 years after diagnosis was 45.1% (95% CI, 35.8-54.0; Figure 1A). The OS probability was nonsignificantly higher in transplanted patients than nontransplanted patients (at 2 years, 47.8% [95% CI, 37.2-57.6] vs 34.8% [95% CI, 16.6-53.7], respectively; P = .12; Figure 1B).
Comparison of OS in transplanted and nontransplanted patients between the present prospective study and our previous retrospective study. Entire cohort (A) and transplanted and nontransplanted cohorts (B) of each study. Kaplan-Meier curves are plotted from the day of diagnosis.
Comparison of OS in transplanted and nontransplanted patients between the present prospective study and our previous retrospective study. Entire cohort (A) and transplanted and nontransplanted cohorts (B) of each study. Kaplan-Meier curves are plotted from the day of diagnosis.
These outcomes were compared with those of the retrospective cohort in our previous study.13 The OS probability was significantly higher in the prospective cohort than in the retrospective cohort (at 2 years, 45.1% [95% CI, 35.8-54.0] vs 28.9% [95% CI, 26.7-31.1], respectively; P < .001; Figure 1A). When comparing the transplanted and nontransplanted patients in the 2 cohorts, the OS probability was similar in the transplanted patients (at 2 years, 47.8% [95% CI, 37.2-57.6] vs 45.0% [95% CI, 40.9-49.1], respectively; P = .78), but it was slightly but nonsignificantly higher in the nontransplanted patients in the prospective cohort (34.8% [95% CI, 16.6-53.7] vs 20.2% [95% CI, 17.8-22.7], respectively; P = .14; Figure 1B).
Transplant outcomes
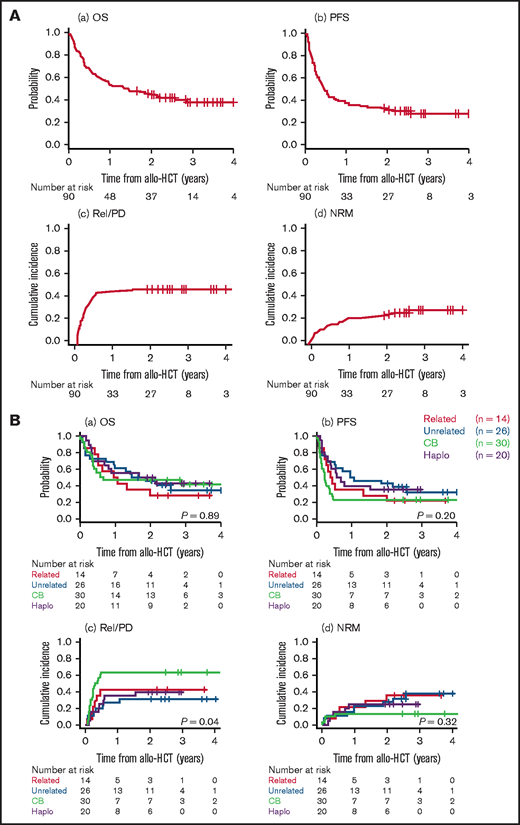
The median follow-up period of survivors was 728 days (range, 308-1488 days) from allo-HCT. The probabilities of OS and PFS at 2 years were 44.3% (95% CI, 33.9-54.3; Figure 2Aa) and 31.1% (95% CI, 21.8-40.7; Figure 2Ab), respectively. The cumulative incidences of Rel/PD and NRM at 2 years were 45.6% (95% CI, 35.0-55.5; Figure 2Ac) and 23.4% (95% CI, 15.2-32.6; Figure 2Ad), respectively. The cumulative incidences of grade II to IV and grade III to IV acute GVHD at day 100 and moderate-to-severe chronic GVHD at 2 years were 27.8% (95% CI, 18.9-37.3), 6.7% (95% CI, 2.7-13.1), and 14.4% (95% CI, 8.1-22.6), respectively. Forty-one of the 90 patients had Rel/PD after allo-HCT. One of the 4 patients who received HCT from an HTLV-1–seropositive donor had relapsed ATL early (day 77) after HCT, which was clinically considered recipient-derived ATL. The median interval between allo-HCT and Rel/PD was 86 days (range, 23-561 days). Salvage therapy was administered to 35 of the 41 patients, either systemically or focally (radiotherapy in 4 and intravitreal methotrexate in 1). The median number of salvage regimens was 2 (range, 1-9). For salvage therapy, mogamulizumab monotherapy was used in 23 (66%) patients, mogamulizumab combined with CHOP in 1 (3%), lenalidomide monotherapy in 15 (43%).
Transplant outcomes. Transplant outcomes of the entire cohort (A) and according to donor type (B) showing OS (a), PFS (b), relapse/progression (c), and NRM (d). Outcomes are plotted from the day of allo-HCT.
Transplant outcomes. Transplant outcomes of the entire cohort (A) and according to donor type (B) showing OS (a), PFS (b), relapse/progression (c), and NRM (d). Outcomes are plotted from the day of allo-HCT.
When stratified by donor type, there was no significant difference in the probabilities of OS (at 2 years, 28.6% [95% CI, 8.8-52.4] in 5-6/6 HLA-matched related donor vs 45.8% [95% CI, 26.3-63.4] in unrelated vs 46.7% [95% CI, 28.4-63.0] in CB vs 50.0% [95% CI, 27.1-69.2] in HLA-haploidentical related; P = .89; Figure 2Ba), PFS (P = .20; Figure 2Bb), or NRM (P = .32; Figure 2Bd). However, the Rel/PD rates were significantly higher with CBT than with the other types of HCT (at 2 years, 63.3% [95% CI, 42.8-78.2] in CB vs 42.9% [95% CI, 16.5-67.2] in 5-6/6 HLA-matched related donor vs 30.8% [95% CI, 14.2-49.0] in unrelated vs 40.0% [95% CI, 18.5-60.8] in HLA-haploidentical related; P = .027; Figure 2Bc). When stratified by disease status at allo-HCT, the probabilities of OS and PFS were significantly lower (OS, P = .003; PFS, P = .002) and the cumulative incidence of Rel/PD was significantly higher (P = .007) in patients in SD/PD than in those in CR or PR (supplemental Figure 1A). When stratified by the mATL-PI at diagnosis (supplemental Figure 1B) or the conditioning regimen (supplemental Figure 1C), there were no significant differences in OS, PFS, Rel/PD, or NRM. All 6 patients who received mogamulizumab before allo-HCT died. The cause of death was ATL progression early after allo-HCT in 4 patients and NRM in the other 2 (bacterial bloodstream infection and interstitial pneumonia, respectively). Six (7%) of 90 transplanted patients, including 2 with a history of CNS involvement before allo-HCT, had refractory or relapsed CNS disease after allo-HCT (cerebrospinal fluid in 3, intraocular in 2, and unknown in 1). Both patients with intraocular disease were salvaged with intraocular chemotherapy or radiotherapy and are alive in CR at the last follow-up; the other 4 patients died of ATL progression.
The results of univariate and multivariate analyses for survival outcomes are shown in Table 3. In multivariate analysis, the mLSG15 regimen was significantly associated with superior OS (HR, 0.31; 95% CI, 0.14-0.69; P = .004), while SD/PD at allo-HCT was significantly associated with inferior OS (HR, 3.97; 95% CI, 1.95-8.09; P <.001). Donor type was not a significant risk factor. An mATL-PI risk group of “high” at diagnosis (HR, 3.20; 95% CI, 1.14-8.96; P = .027) and SD/PD at allo-HCT (HR, 2.63; 95% CI, 1.08-6.44; P = .034) were significantly associated with a higher risk of Rel/PD in multivariate analysis. CB was nonsignificantly associated with a higher risk of Rel/PD (HR, 2.37; 95% CI, 0.93-6.03; P = .071). No variables were significantly associated with NRM in the multivariate analysis.
Univariate and multivariate analysis for transplant outcomes
| Outcomes . | Relapse/progression . | NRM . | PFS . | OS . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis . | Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | ||||||||||
| Variables . | n . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | |
| Age at allo-HCT (years) | <60 | 41 | 1 | .61 | 1 | .48 | 1 | .50 | 1 | .25 | 1 | .22 | 1 | .16 | 1 | .09 | ||
| ≥60 | 49 | 1.17 (0.64-2.15) | 1.35 (0.59-3.09) | 1.38 (0.54-3.50) | 1.34 (0.82-2.21) | 1.39 (0.82-2.37) | 1.49 (0.86-2.57) | 1.68 (0.92-3.07) | ||||||||||
| Gender | Female | 40 | 1 | .88 | 1 | .43 | 1 | .23 | 1 | .40 | ||||||||
| Male | 50 | 1.04 (0.57-1.92) | 1.39 (0.62-3.15) | 1.36 (0.83-2.23) | 1.26 (0.73-2.16) | |||||||||||||
| ATL type | Lymphoma | 25 | 1 | .16 | 1 | .052 | 1 | .92 | 1 | .51 | ||||||||
| Acute | 65 | 1.76 (0.81-3.85) | 0.44 (0.20-1.01) | 1.03 (0.60-1.78) | 0.82 (0.46-1.47) | |||||||||||||
| mATL-PI at diagnosis | Low | 30 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| Intermediate | 52 | 1.65 (0.80-3.41) | .18 | 1.84 (0.78-4.33) | .16 | 0.80 (0.34-1.84) | .59 | 1.31 (0.75-2.26) | .34 | 1.34 (0.73-2.47) | .35 | |||||||
| High | 8 | 2.50 (0.83-7.48) | .10 | 3.20 (1.14-8.96) | .027 | 0.38 (0.05-3.15) | .37 | 1.61 (0.64-4.03) | .31 | 2.14 (0.83-5.54) | .12 | |||||||
| Induction chemotherapy | CHOP-like regimens | 11 | 1 | .5 | 1 | .3 | 1 | .03 | 1 | 0.006 | 1 | .003 | 1 | .004 | ||||
| mLSG15 | 79 | 0.74 (0.32-1.75) | 0.55 (0.18-1.69) | 0.46 (0.23-0.91) | 0.35 (0.16-0.74) | 0.34 (0.17-0.69) | 0.31 (0.14-0.69) | |||||||||||
| Number of cycles of chemotherapy before allo-HCT | ≤3 | 61 | 1 | .64 | 1 | .85 | 1 | .67 | 1 | .64 | ||||||||
| >3 | 29 | 1.17 (0.61-2.22) | 0.92 (0.38-2.22) | 1.12 (0.66-1.89) | 1.14 (0.65-2.02) | |||||||||||||
| Time to allo- HCT (day) | <128 | 43 | 1 | .087 | 1 | .72 | 1 | .073 | 1 | .33 | ||||||||
| ≥128 | 47 | 0.58 (0.31-1.08) | 1.16 (0.51-2.63) | 0.64 (0.39-1.04) | 0.76 (0.45-1.31) | |||||||||||||
| Status at allo-HCT | CR | 41 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| PR | 28 | 1.10 (0.53-2.30) | .80 | 1.07 (0.50-2.26) | .87 | 1.71 (0.72-4.12) | .22 | 1.71 (0.73-4.03) | .22 | 1.45 (0.81-2.59) | .21 | 1.48 (0.81-2.67) | .2 | 1.40 (0.73-2.67) | .31 | 1.37 (0.71-2.65) | .35 | |
| SD/PD | 21 | 2.88 (1.36-6.12) | .006 | 2.63 (1.08-6.44) | .034 | 0.89 (0.27-2.99) | .85 | 1.03 (0.29-3.71) | .96 | 2.87 (1.56-5.29) | <.001 | 3.05 (1.60-5.81) | <.001 | 2.97 (1.55-5.71) | .001 | 3.97 (1.95-8.09) | <.001 | |
| Donor | Unrelated | 26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Related | 14 | 1.54 (0.54-4.40) | .42 | 0.95 (0.24-3.78) | .94 | 1.05 (0.38-2.96) | .92 | 1.15 (0.44-3.03) | .78 | 1.43 (0.67-3.06) | .36 | 1.32 (0.60-2.91) | .50 | 1.23 (0.56-2.72) | .60 | 1.21 (0.53-2.78) | .65 | |
| CB | 30 | 2.92 (1.26-6.75) | .013 | 2.37 (0.93-6.03) | .071 | 0.37 (0.11-1.22) | .10 | 0.37 (0.11-1.29) | .12 | 1.83 (1.97-3.45) | .061 | 1.68 (0.85-3.33) | .14 | 1.00 (0.50-1.98) | 1.00 | 0.82 (0.39-1.73) | .61 | |
| Haplo | 20 | 1.30 (0.51-3.32) | .59 | 0.88 (0.30-2.59) | .82 | 0.73 (0.25-2.13) | .57 | 0.83 (0.27-2.58) | .75 | 1.03 (0.50-2.12) | .94 | 0.75 (0.35-1.62) | .47 | 0.88 (0.41-1.90) | .74 | 0.63 (0.28-1.40) | .26 | |
| Conditioning therapy | MAC | 14 | 1 | 1 | 1 | 1 | ||||||||||||
| Flu/Bu- based RIC | 16 | 0.57 (0.19-1.71) | .32 | 1.44 (0.45-4.61) | .54 | 0.74 (0.32-1.67) | .46 | 1.14 (0.46-2.78) | .78 | |||||||||
| Flu/Mel- based RIC | 58 | 0.98 (0.45-2.16) | .97 | 0.70 (0.22-2.19) | .54 | 0.86 (0.44-1.69) | .66 | 0.91 (0.42-1.99) | .82 | |||||||||
| Outcomes . | Relapse/progression . | NRM . | PFS . | OS . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis . | Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | Univariate . | Multivariate . | ||||||||||
| Variables . | n . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | HR (95%CI) . | P . | |
| Age at allo-HCT (years) | <60 | 41 | 1 | .61 | 1 | .48 | 1 | .50 | 1 | .25 | 1 | .22 | 1 | .16 | 1 | .09 | ||
| ≥60 | 49 | 1.17 (0.64-2.15) | 1.35 (0.59-3.09) | 1.38 (0.54-3.50) | 1.34 (0.82-2.21) | 1.39 (0.82-2.37) | 1.49 (0.86-2.57) | 1.68 (0.92-3.07) | ||||||||||
| Gender | Female | 40 | 1 | .88 | 1 | .43 | 1 | .23 | 1 | .40 | ||||||||
| Male | 50 | 1.04 (0.57-1.92) | 1.39 (0.62-3.15) | 1.36 (0.83-2.23) | 1.26 (0.73-2.16) | |||||||||||||
| ATL type | Lymphoma | 25 | 1 | .16 | 1 | .052 | 1 | .92 | 1 | .51 | ||||||||
| Acute | 65 | 1.76 (0.81-3.85) | 0.44 (0.20-1.01) | 1.03 (0.60-1.78) | 0.82 (0.46-1.47) | |||||||||||||
| mATL-PI at diagnosis | Low | 30 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| Intermediate | 52 | 1.65 (0.80-3.41) | .18 | 1.84 (0.78-4.33) | .16 | 0.80 (0.34-1.84) | .59 | 1.31 (0.75-2.26) | .34 | 1.34 (0.73-2.47) | .35 | |||||||
| High | 8 | 2.50 (0.83-7.48) | .10 | 3.20 (1.14-8.96) | .027 | 0.38 (0.05-3.15) | .37 | 1.61 (0.64-4.03) | .31 | 2.14 (0.83-5.54) | .12 | |||||||
| Induction chemotherapy | CHOP-like regimens | 11 | 1 | .5 | 1 | .3 | 1 | .03 | 1 | 0.006 | 1 | .003 | 1 | .004 | ||||
| mLSG15 | 79 | 0.74 (0.32-1.75) | 0.55 (0.18-1.69) | 0.46 (0.23-0.91) | 0.35 (0.16-0.74) | 0.34 (0.17-0.69) | 0.31 (0.14-0.69) | |||||||||||
| Number of cycles of chemotherapy before allo-HCT | ≤3 | 61 | 1 | .64 | 1 | .85 | 1 | .67 | 1 | .64 | ||||||||
| >3 | 29 | 1.17 (0.61-2.22) | 0.92 (0.38-2.22) | 1.12 (0.66-1.89) | 1.14 (0.65-2.02) | |||||||||||||
| Time to allo- HCT (day) | <128 | 43 | 1 | .087 | 1 | .72 | 1 | .073 | 1 | .33 | ||||||||
| ≥128 | 47 | 0.58 (0.31-1.08) | 1.16 (0.51-2.63) | 0.64 (0.39-1.04) | 0.76 (0.45-1.31) | |||||||||||||
| Status at allo-HCT | CR | 41 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| PR | 28 | 1.10 (0.53-2.30) | .80 | 1.07 (0.50-2.26) | .87 | 1.71 (0.72-4.12) | .22 | 1.71 (0.73-4.03) | .22 | 1.45 (0.81-2.59) | .21 | 1.48 (0.81-2.67) | .2 | 1.40 (0.73-2.67) | .31 | 1.37 (0.71-2.65) | .35 | |
| SD/PD | 21 | 2.88 (1.36-6.12) | .006 | 2.63 (1.08-6.44) | .034 | 0.89 (0.27-2.99) | .85 | 1.03 (0.29-3.71) | .96 | 2.87 (1.56-5.29) | <.001 | 3.05 (1.60-5.81) | <.001 | 2.97 (1.55-5.71) | .001 | 3.97 (1.95-8.09) | <.001 | |
| Donor | Unrelated | 26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Related | 14 | 1.54 (0.54-4.40) | .42 | 0.95 (0.24-3.78) | .94 | 1.05 (0.38-2.96) | .92 | 1.15 (0.44-3.03) | .78 | 1.43 (0.67-3.06) | .36 | 1.32 (0.60-2.91) | .50 | 1.23 (0.56-2.72) | .60 | 1.21 (0.53-2.78) | .65 | |
| CB | 30 | 2.92 (1.26-6.75) | .013 | 2.37 (0.93-6.03) | .071 | 0.37 (0.11-1.22) | .10 | 0.37 (0.11-1.29) | .12 | 1.83 (1.97-3.45) | .061 | 1.68 (0.85-3.33) | .14 | 1.00 (0.50-1.98) | 1.00 | 0.82 (0.39-1.73) | .61 | |
| Haplo | 20 | 1.30 (0.51-3.32) | .59 | 0.88 (0.30-2.59) | .82 | 0.73 (0.25-2.13) | .57 | 0.83 (0.27-2.58) | .75 | 1.03 (0.50-2.12) | .94 | 0.75 (0.35-1.62) | .47 | 0.88 (0.41-1.90) | .74 | 0.63 (0.28-1.40) | .26 | |
| Conditioning therapy | MAC | 14 | 1 | 1 | 1 | 1 | ||||||||||||
| Flu/Bu- based RIC | 16 | 0.57 (0.19-1.71) | .32 | 1.44 (0.45-4.61) | .54 | 0.74 (0.32-1.67) | .46 | 1.14 (0.46-2.78) | .78 | |||||||||
| Flu/Mel- based RIC | 58 | 0.98 (0.45-2.16) | .97 | 0.70 (0.22-2.19) | .54 | 0.86 (0.44-1.69) | .66 | 0.91 (0.42-1.99) | .82 | |||||||||
Outcomes of nontransplanted patients
The median follow-up period of survivors was 1267 days (range, 797-1668 days) after diagnosis. The initial chemotherapy was mLSG15 in 13 (57%) patients and CHOP-like regimens in 9 (39%). Mogamulizumab was used as monotherapy or combination therapy in 6 (26%) patients. The overall response rate to induction chemotherapy was 52%, with a CR rate of 26%. Among the 18 patients who were refractory to (n = 11) or relapsed after (n = 7) induction chemotherapy, 12 received salvage chemotherapy. The median number of salvage regimens was 2 (range, 0-7). For salvage chemotherapy, mogamulizumab was used in 10 patients, and lenalidomide was used in 1 patient. The probabilities of OS, PFS, Rel/PD, and NRM at 2 years after diagnosis were 34.8% (95% CI, 16.6-53.7), 13.0% (95% CI, 3.3-29.7), 78.3% (95% CI, 53.2-90.9), and 8.7% (95% CI, 1.3-25.6), respectively. At the last follow-up, 7 patients were alive, with 4 patients in CR, 1 in PR, and 2 in PD. Four patients in CR had long-term, disease-free survival without allo-HCT (median, 1,151 days; range, 797-1,668). Among them, 3 responded dramatically to mogamulizumab-containing therapy. Fourteen patients died of disease progression at a median of 68 days (range, 2-685) after diagnosis of Rel/PD. Two patients had NRM. One died of fulminant hepatitis at day 195 and the other of acute respiratory distress syndrome on day 529.
Discussion
This multicenter, prospective, observational study investigated the treatment outcomes of aggressive ATL in the current era. The survival of patients with aggressive ATL has improved with increased application of allo-HCT. Further, CBT and haplo-HCT shorten the interval from diagnosis to allo-HCT and may increase the chance of receiving allo-HCT. The survival outcomes of CBT and haplo-HCT in this study were comparable to those of uBMT/uPBSCT. These results suggest that CBT and haplo-HCT are feasible for aggressive ATL.
The treatment outcomes of the current prospective cohort were compared with those of the retrospective cohort in our previous study, which had the same inclusion criteria as the present study.13 The OS probability of the entire cohort was significantly better in the prospective cohort than in the retrospective cohort (at 2 years, 45.1% vs 28.9%, respectively; P < .001). Transplanted patients in the prospective cohort accounted for the majority of that cohort and showed a similar OS probability to the transplanted patients in the retrospective cohort (at 2 years, 47.8% vs 45.0%, respectively; P = .78). The OS probability of the nontransplanted patients was higher in the prospective cohort than in the retrospective cohort, although there was no significant difference (at 2 years, 34.8% vs 20.2%, respectively; P = .14). While NRM after allo-HCT has decreased with improved supportive care over the last decade,24 the better survival in the entire prospective cohort was primarily due to the increased application rate of allo-HCT compared with the entire retrospective cohort (80% vs 34%, respectively; P < .001).
The application rate of allo-HCT for aggressive ATL has markedly increased in the prospective cohort than in the retrospective cohort (80% vs 34%, respectively; P < .001). Further, the median interval between diagnosis and allo-HCT was considerably shorter in the prospective cohort (128 days vs 170 days, respectively; P < .001). Application of allo-HCT after response to initial chemotherapy was recently recommended in the current Japanese guidelines.8 One possible factor contributing to earlier allo-HCT is the improved overall response rate to initial chemotherapy with the advent of the mLSG15 regimen, which was associated with superior OS compared with CHOP-like regimens.1 Another factor that is probably more important is the increased use of alternative donors. In the prospective cohort, CBT and haplo-HCT accounted for 55% of the donor types, and the median interval between diagnosis and allo-HCT was significantly shorter in CBT and haplo-HCT than in uBMT/uPBSCT (Table 2). The median time to Rel/PD was found to be shorter than 6 months after diagnosis, especially in patients with intermediate- and high-risk disease according to the mATL-PI.13 It is speculated that increasing numbers of patients are receiving CBT or haplo-HCT instead of waiting months for unrelated donor coordination.
Our results suggest that CBT and haplo-HCT are feasible for aggressive ATL when MRD is unavailable. Previous studies showed that outcomes of CBT9,10 and haplo-HCT14 for aggressive ATL were inferior to those of other types of HCT, but this was not the case in the present study. In the multivariate analysis, donor type was not a significant adverse prognostic factor for OS probability or NRM. Early application of CBT in remission was previously shown to achieve comparable outcomes to uBMT/uPBSCT.10,15,16 Enhanced supportive care, such as management of pre-engraftment immune response and prevention of infectious diseases, including human herpesvirus 6, may contribute to decreasing NRM after CBT. In addition, increasingly widespread haplo-HCT with PTCy might be safe for elderly or otherwise frail ATL patients and improve outcomes compared with those in reports in the pre-PTCy era.14 In this study, however, early relapse occurred significantly more frequently after CBT than after other types of HCT. Adopting novel drugs as salvage chemotherapy might prolong the survival of relapsed patients, but further follow-up is needed.
The survival of nontransplanted patients was slightly better in the prospective cohort than in the retrospective cohort. As the patient number was small, and half of the survivors were in non-CR at the last follow-up, longer follow-up is needed. In the present study, 3 patients who were treated with mogamulizumab had long-term, disease-free survival without allo-HCT. A recent study reported that patients with CCR4 gene mutations, which are detected in one-third of ATL patients, achieved durable PFS after mogamulizumab-containing therapy.25 Such genetic biomarkers might identify the subset of ATL patients who will demonstrate a profound response to mogamulizumab and achieve cure without allo-HCT. On the other hand, all 6 patients who received allo-HCT after mogamulizumab therapy died in the present study. Administering mogamulizumab to possibly transplant-eligible patients should be avoided, if possible, due to the increased risk of severe GVHD and NRM after allo-HCT.26 Anti-thymocyte globulin was added for GVHD prophylaxis in 2 of those patients, but both died soon after HCT, one due to severe GVHD and the other due to ATL recurrence. Further research is needed to clarify the optimal strategy for GVHD prevention and management in mogamulizumab-pretreated patients.
The relapse rate of aggressive ATL after allo-HCT is still high in the current era and was ∼45% at 2 years in the present study. One of the greatest challenges to improving the survival of patients with aggressive ATL is identifying effective measures to detect Rel/PD after allo-HCT.27 There is a need for posttransplant therapies to reduce relapse of aggressive ATL. The safety and efficacy of mogamulizumab and lenalidomide for relapsed ATL after allo-HCT have not been established.28-30 Considering the risk of severe immune-related complications31 or hematological toxicities associated with mogamulizumab or lenalidomide, one practical approach for aggressive ATL might be preemptive therapy triggered by detection of measurable residual disease, as exemplified in Philadelphia chromosome–positive acute lymphoblastic leukemia.32 However, there is no established marker for predicting ATL relapse after allo-HCT. Cytometry-based monitoring of measurable residual disease, including the HTLV-1 analysis system (HAS-Flow) that we previously described,33 might be promising.33,34 In addition, 6 (7%) of 90 transplanted patients had refractory or relapsed CNS disease after allo-HCT. Novel strategies to enhance CNS prophylaxis might be explored.
There are several limitations to our study. First, the patient number was small. As ATL is a rare hematological malignancy, we prospectively recruited 113 patients through a multicenter study. At most centers (90%), the patients were consecutively enrolled. Second, the follow-up period of 3 years was insufficient to draw a definitive conclusion on the long-term outcomes of both transplanted and nontransplanted patients. Third, this was not an interventional study. Allo-HCT was not a mandatory therapeutic option, and transplant timing, donor selection, and conditioning regimen were heterogeneous. However, this study yielded real-world data that reflect the current clinical practice of aggressive ATL in Japan. Fourth, we compared the survival outcomes of the prospective cohort with those of the retrospective cohort that included patients diagnosed between 2000 and 2013, when the standard of care was different from the current era. Although the inclusion and exclusion criteria were the same, and the participating centers and patient characteristics were similar between the 2 studies, we could not exclude the influence of increased use of novel agents and improved supportive care on differences in survival outcomes between the 2 studies.
In conclusion, the survival of patients with aggressive ATL has recently improved with increased application of allo-HCT after response to initial chemotherapy. Further, CBT and haplo-HCT, which are commonly performed for aggressive ATL in current clinical practice, shorten the interval from diagnosis to allo-HCT and afford comparable outcomes to uBMT/uPBSCT. For aggressive ATL, which frequently results in Rel/PD after initial response to chemotherapy, CBT and haplo-HCT can be a standard alternative option for early application of allo-HCT when MRD is not available. Although further studies are needed, our data contribute to clarifying the current status and unresolved issues in the treatment of aggressive ATL.
Acknowledgments
The authors thank all the patients who participated in this study. They are also indebted to the medical, nursing, and clinical staff at all participating institutions for their patient care. This work was supported by a grant from the Practical Research for Innovative Cancer Control program of the Japan Agency for Medical Research and Development (19ck0106342h0003) T.F.
Authorship
Contribution: T.F. conceived, designed, and supervised the study; S.F. and Y.I. designed the study; A.I. analyzed and interpreted the data and wrote the paper; and all other authors were involved in the acquisition of the data and the review and revision of the manuscript.
Conflict-of-interest disclosure: T. Tanaka received honoraria from Celgene. S.F. received honoraria from Kyowa-Kirin. H.N. received honoraria from Otsuka, Celgene, Kyowa-Kirin, Bristol-Myers Squibb, and Pfizer and research funding from Bristol-Myers Squibb, Otsuka, Pfizer, and Kyowa-Kirin. T. Takahashi received honoraria from Kyowa-Kirin, Chugai, Bristol-Myers Squibb, and Celgene. K.K. received honoraria from Takeda, MSD, Kyowa-Kirin, Janssen, Celgene, Ono, Mundi, Bristol-Myers Squibb, and Dainippon-Sumitomo and research funding from Chugai, Takeda, Kyowa Kirin, AbbVie, Novartis, Eisai, Janssen, Celgene, Daiichi Sankyo, and Ono and has a consulting or advisory role for AbbVie, AstraZeneca, Chugai, Novartis, Eisai, Janssen, Daiichi Sankyo, and Celgene. R.S. received honoraria from Chugai, Kyowa-Kirin, Ono, Eisai, Takeda, Celgene, Mundipharma, Nippon Shinyaku, and Janssen and research funding from Chugai, Kyowa-Kirin, Ono, and Eisai. K.U. received honoraria from Takeda and Daiichi Sankyo. Y.I. received honoraria from Kyowa-Kirin. A.U. received honoraria from Kyowa-Kirin and Celgene. T.F. received honoraria from Kyowa-Kirin. The remaining authors declare no competing financial interests.
Correspondence: Takahiro Fukuda, Department of Hematopoietic Stem Cell Transplantation, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: tafukuda@ncc.go.jp.
References
Author notes
The authors agree to share publication-related data. Please contact the corresponding author via e-mail: tafukuda@ncc.go.jp.
The full-text version of this article contains a data supplement.