Key Points
VSTs are effective in treating adenoviral infections, a common posttransplant complication in pediatrics that lacks effective antivirals.
Antiviral response driven by VSTs is primarily through CD4+ cells and is restricted through class II HLA alleles.
Abstract
Infection with adenoviruses is a common and significant complication in pediatric patients after allogeneic hematopoietic stem cell transplantation. Treatment options with traditional antivirals are limited by poor efficacy and significant toxicities. T-cell reconstitution is critical for the management of adenoviral infections, but it generally takes place months after transplantation. Ex vivo–generated virus-specific T cells (VSTs) are an alternative approach for viral control and can be rapidly generated from either a stem cell donor or a healthy third-party donor. In the context of a single-center phase 1/2 clinical trial, we treated 30 patients with a total of 43 infusions of VSTs for adenoviremia and/or adenoviral disease. Seven patients received donor-derived VSTs, 21 patients received third-party VSTs, and 2 received VSTs from both donor sources. Clinical responses were observed in 81% of patients, with a complete response in 58%. Epitope prediction and potential epitope identification for common HLA molecules helped elucidate HLA restriction in a subset of patients receiving third-party products. Intracellular interferon-γ expression in T cells in response to single peptides and response to cell lines stably transfected with a single HLA molecule demonstrated HLA-restricted CD4+ T-cell response, and these results correlated with clinical outcomes. Taken together, these data suggest that VSTs are a highly safe and effective therapy for the management of adenoviral infection in immunocompromised hosts. The trials were registered at www.clinicaltrials.gov as #NCT02048332 and #NCT02532452.
Introduction
Viral infections, whether they are new or a reactivation of latent infections, are a significant contributor to morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT).1 In particular, infection with double-stranded DNA viruses is an independent risk factor for nonrelapse mortality, and routine prospective monitoring for asymptomatic viremia is frequently performed at many transplant centers.2-4
Adenoviruses are a ubiquitous group of double-stranded DNA viruses. Exposure to an adenovirus in children is considered to be almost universal, and in immunocompetent hosts, it causes self-limiting and generally mild respiratory, conjunctival, and/or gastrointestinal illness.5 In immunocompromised individuals, infection with adenovirus can result in a broad range of symptoms from asymptomatic viremia to invasive disease such as diarrheal enteritis, respiratory failure, and hepatitis.5,6 Adenoviremia is much more common in pediatric HSCT recipients than in adult patients and is an independent risk factor for nonrelapse mortality.7-10
Treatment options for adenoviral infection are limited. Cidofovir is the most commonly used agent, but it is associated with a significant risk for nephrotoxicity, and response rates are poor in patients awaiting immune reconstitution, particularly of the T-cell compartment.6,11 Brincidofovir, a lipid conjugate prodrug of cidofovir, has improved oral bioavailability and clinical outcomes, but it is no longer available in most countries.12 In recent years, allogeneic virus-specific T-cell (VST) therapy has been shown to be safe and effective in the treatment of double-stranded DNA viruses in immunocompromised patients, particularly after HSCT and solid organ transplantation, with minimal risk of de novo graft-vs-host disease (GVHD).13,14 In this strategy, donor peripheral blood mononuclear cells (PBMCs) are expanded after exposure to viral peptide libraries in vitro. Innovations in manufacturing VSTs have decreased production time to between 10 days and 3 weeks, and the generation of banks of partially HLA-matched third-party products has increased the applicability and accessibility of this therapy.15 Here we report on our experience using VSTs that are either partially HLA matched from third-party donors or VSTs derived from a patient’s stem cell donor (donor-derived VSTs) for treating adenoviremia and invasive adenoviral disease. In addition, we report in vitro experiments that were performed to better understand the epitope specificity and HLA restriction of VST products.
Materials and methods
Patient enrollment and clinical trial information
Two phase 2 studies occurred concurrently that examined the use of donor-derived (#NCT02048332) and third-party (#NCT02532452) VSTs, both cleared by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC) and the US Food and Drug Administration. Patients treated at CCHMC and those who received a transplant and were treated at other institutions were eligible for enrollment. Patients who met the eligibility criteria and completed consent forms were able to receive VST products at the discretion of the treatment team. Eligibility for VST infusion required plasma adenovirus polymerase chain reaction (PCR) results of ≥1000 copies per mL or documentation of invasive adenoviral infection by tissue diagnosis or PCR from an additional site (such as nares, bronchoalveolar fluid, or stool). Failure of previous adenoviral-focused therapy was not required for eligibility. Patients were required to be at least 28 days from their stem cell infusion, receiving ≤0.5 mg/kg per day of prednisone-equivalent steroids, and without active GVHD. Patients who received alemtuzumab or antithymocyte globulin within 2 weeks of receiving VSTs were excluded, although previous exposure to those agents was allowed. The primary study end point was the manufacturing and safe infusion of adenoviral-specific VSTs into recipients of HSCTs and solid organ transplantations. Secondary end points included the efficacy of VSTs in treating adenoviremia and invasive adenoviral disease.
VST manufacturing process
Quadrivalent VSTs specific for adenovirus, BK virus, cytomegalovirus, and Epstein-Barr virus were generated as previously described.16 Briefly, PBMCs were collected from either a patient’s individual stem cell donor (donor-derived) or a healthy adult volunteer who had consented and was designated as an eligible third-party donor. After separation from whole blood by density centrifugation (Ficoll-Paque Premium, GE Healthcare), PBMCs were then stimulated with pools of viral peptides (Pepmix) encompassing antigen epitopes. The adenovirus Pepmix included Hexon-AdV3 and Penton-AdV5. Cells were cultured in G-Rex 10-M culture devices for 11 to 12 days in the presence of interleukin-4 (IL-4) and IL-7, with replenishment of cytokines on day 3 or 4. Products were infused only if they met all release criteria for safety, sterility, and alloreactivity.
In silico epitope binding prediction
Class I HLA epitope binding prediction was performed using HLA restrictor 1.2 (cbs.dtu.dk/services/HLArestrictor/; Technical University of Denmark, Lyngby, Denmark) and class II HLA epitope prediction was performed using the Immune Epitope Database and Analysis Resource (IEDB.org).17,18 Prediction was made by using peptide sequences of hexon and penton adenovirus proteins. Predicted 9- to 15-mer peptides were synthesized by GenScript (Piscataway, NJ).
Single-antigen cell lines and in vitro estimation of reactivity
Immortalized mouse fibroblastoid DAP.3 cells stably transfected with a single class II HLA molecule were previously generated.19 Cells were thawed in a water bath and then cultured in either RPMI with 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10% fetal bovine serum (FBS), 100 mM sodium pyruvate, 1% nonessential amino acids, 1% glutamax with 200 μg/mL G418 or Dulbecco’s modified Eagle medium (DMEM) with 10% FBS, 4 mM L-glutamine, 100 mM sodium pyruvate, and 200 μg/mL G418, depending on the cell line. Adherent cells were dissociated with 0.05% trypsin/EDTA, seeded into a 96-well plate at a density of 0.5 × 105 cells per well, and incubated at 37° overnight. The next morning, cells were washed with phosphate-buffered saline (PBS), and fresh media was added containing 0.5 μg/mL of either adenoviral Pepmix or individual peptides derived from hexons or pentons. After a 20-hour incubation, the cells were washed 3 times with PBS, and then between 2 × 105 and 3.5 × 105 thawed VSTs were added to fibroblasts in the VST complete media. Intracellular flow cytometry was performed after a 6-hour incubation with peptides.
Intracellular flow cytometry
VST products were rapidly thawed in a water bath and then transferred into complete media with RPMI-1640 media with 10% FBS and 2 mM glutamine. Viable cells were pelleted, counted, and then seeded into 96-well plates at a density of 2 × 105 to 3.5 × 105 cells per well and stimulated with either Pepmix or individual peptides. Cells were incubated at 37° for 6 hours and then harvested, washed with PBS, and stained with CD3-phycoerythrin (PE), CD4- allophycocyanin (APC)-eFluor 780, CD8- fluorescein isothiocyanate (FITC), and Zombie Violet viability dye. Cells were fixed and permeabilized and then stained with an antibody against intracellular interferon-γ (IFN-γ)-APC. Flow cytometry analysis was performed using the MACSQuant Analyzer (Miltenyi Biotec).
ELISpot assay
The presence of VSTs in the peripheral blood of VST recipients was measured using the IFN-γ enzyme-linked immune absorbent (ELISpot) assay. PBMCs were collected from the VST recipient immediately before infusion and then once per week for the first 4 weeks after infusion. PBMCs were stimulated with adenoviral Pepmix at a concentration of 0.5 μg/mL for 14 to 16 hours. Spot-forming cells (SFCs) were developed using the Human IFN-γ ELISpotPLUS kit (Mabtech, Nacka Strand, Sweden) and quantitated using the Immunospot S6 Analyzer (Cellular Technology Limited, Cleveland, OH). Background SFC counts in the media were subtracted from experimental conditions to determine the reported SFC count.
VST product selection and response criteria
When they were available, donor-derived VSTs were preferred over third-party VSTs because of the higher degree of HLA match. Product selection for recipients of third-party products was based on maximizing the degree of HLA match between the product and recipient. Attempts were made to match at least 1 class I and 1 class II allele. If there were multiple products with equivalent match, IFN-γ production measured by intracellular flow cytometry was considered. Both donor-derived and third-party products could be infused on a once-per month basis if there had been no safety concerns such as the development of GVHD. In the third-party protocol, subsequent infusions could be from the same initial donor or a different donor. A different donor could be chosen if the original product was no longer available or if there was a less-than-desired clinical response. Cells were infused at a fixed dose of 5 × 107 VSTs per m2.
Response was evaluated 4 weeks after the VST infusion and was re-evaluated after each VST infusion for patients who received more than 1 infusion. Clinical response was assessed by the principal investigator and designees. A complete response (CR) was defined as resolution of viremia and/or symptomatic disease in the 4-week period after infusion. A partial response (PR) was defined as greater than 50% reduction in viremia and/or improvement in symptomatic disease in the 4-week period after infusion.15,20 No response was defined as no change or an increase in adenoviremia or adenoviral symptomatic disease in the 4-week period after infusion.
Results
VSTs are an effective therapy for the treatment of adenoviremia and adenovirus-related disease
Thirty patients received a total of 43 infusions of VSTs for the treatment of adenoviremia and/or invasive adenoviral disease in the study period (2016 to 2019). Patient and disease characteristics are provided in Table 1. Seven of 30 patients received VSTs derived from their stem cell donor after allo-HSCT, 21 received partially HLA-matched, off-the-shelf, third-party VSTs, and 2 received both donor-derived and third-party products (because both patients received third-party products first, they were included in the third-party cohorts for subsequent analyses). Twenty-seven patients were previous recipients of allo-HSCT, 1 patient received an orthotopic heart transplant, and 2 patients had inherited primary immunodeficiency syndromes (ataxia-telangiectasia in both cases). HSCT was performed at 15 separate transplantation centers.
Patient and disease characteristics of HSCT recipients
| . | Donor-derived VST . | Third-party VST . | Total no. of patients . |
|---|---|---|---|
| Sex | |||
| Male | 4 | 13 | 17 |
| Female | 3 | 7 | 10 |
| Median age at transplantation, y (range) | 12.79 (0.85-23.33) | 11.51 (1.08-63.32) | |
| Diagnosis | |||
| Malignancy | 2 | 12 | 14 |
| Immune deficiency | 4 | 5 | 9 |
| Marrow failure | 1 | 3 | 4 |
| Stem cell donor type | |||
| Related (MRD, MMRD, haploidentical) | 4 | 3 | 7 |
| Unrelated (MUD, MMUD) | 3 | 16 | 19 |
| Not available | 0 | 1 | 1 |
| Degree of HLA match | |||
| Full | 4 | 8 | 12 |
| Partial | 3 | 8 | 11 |
| Not available | 0 | 4 | 4 |
| Stem cell source | |||
| Bone marrow | 4 | 4 | 8 |
| PBSCs | 3 | 9 | 12 |
| Cord blood | 0 | 3 | 3 |
| Not available | 0 | 4 | 4 |
| Conditioning regimen | |||
| Myeloablative | 5 | 10 | 15 |
| Reduced intensity | 2 | 7 | 9 |
| Not available | 0 | 3 | 3 |
| GVHD prophylaxis (allo-HSCT only) | |||
| Calcineurin (cyclosporine A, tacrolimus) | 4 | 12 | 16 |
| Other (sirolimus + other) | 0 | 3 | 3 |
| Ex vivo T-cell depletion (CD34 selection) | 2 | 1 | 3 |
| Antithymocyte globulin | 0 | 1 | 1 |
| None | 1 | 3 | 4 |
| . | Donor-derived VST . | Third-party VST . | Total no. of patients . |
|---|---|---|---|
| Sex | |||
| Male | 4 | 13 | 17 |
| Female | 3 | 7 | 10 |
| Median age at transplantation, y (range) | 12.79 (0.85-23.33) | 11.51 (1.08-63.32) | |
| Diagnosis | |||
| Malignancy | 2 | 12 | 14 |
| Immune deficiency | 4 | 5 | 9 |
| Marrow failure | 1 | 3 | 4 |
| Stem cell donor type | |||
| Related (MRD, MMRD, haploidentical) | 4 | 3 | 7 |
| Unrelated (MUD, MMUD) | 3 | 16 | 19 |
| Not available | 0 | 1 | 1 |
| Degree of HLA match | |||
| Full | 4 | 8 | 12 |
| Partial | 3 | 8 | 11 |
| Not available | 0 | 4 | 4 |
| Stem cell source | |||
| Bone marrow | 4 | 4 | 8 |
| PBSCs | 3 | 9 | 12 |
| Cord blood | 0 | 3 | 3 |
| Not available | 0 | 4 | 4 |
| Conditioning regimen | |||
| Myeloablative | 5 | 10 | 15 |
| Reduced intensity | 2 | 7 | 9 |
| Not available | 0 | 3 | 3 |
| GVHD prophylaxis (allo-HSCT only) | |||
| Calcineurin (cyclosporine A, tacrolimus) | 4 | 12 | 16 |
| Other (sirolimus + other) | 0 | 3 | 3 |
| Ex vivo T-cell depletion (CD34 selection) | 2 | 1 | 3 |
| Antithymocyte globulin | 0 | 1 | 1 |
| None | 1 | 3 | 4 |
MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor.
The median time from transplantation to first activation of adenovirus was transplant day +28 (range, day –7 to day +242). Twenty-five of the patients had received either cidofovir or brincidofovir for the management of adenoviral infection at some point before their first VST infusion. Median adenovirus viral load at the closest check to VST infusion was 43 323 copies per mL (range, 0-450 000 000 copies per mL). Six infusions were given primarily for invasive adenoviral disease rather than viremia (5 patients with adenoviral diarrhea, 1 with diarrhea and respiratory disease); disregarding those patients, the median adenovirus viral load closest to VST infusion was 46 593 copies per mL (range, 500-450 000 000 copies per mL). A total of 18 different VST products were used across the study population, and each product was used for between 1 and 5 infusions (median, 1 infusion). Baseline absolute lymphocyte count was available for 36 infusions, with a median of 295 cells per µL (range, 0-2500 cells per µL). The median HLA match (high resolution, at the allelic level) between the third-party VST product and the recipient was 5/10 (range, 3/10-7/10) with a median class II match (HLA-DR and HLA-DQ) of 2/4 (range, 0/4-4/4) whereas the median HLA match in donor-derived infusions was 9.5/10 (range, 5/10-10/10).
Twenty-six of the recipients were deemed evaluable for response. Three patients died within 10 days of their sole adenovirus-directed infusion, and 1 patient transitioned to hospice after 11 days; all of these events occurred before the expected timeframe for clinical efficacy. These patients were considered not evaluable for response. Twenty-one (81%) of 26 evaluable patients achieved a clinical response after at least 1 infusion. A CR occurred in 15 (58%) of 26 patients and a PR in 6 (23%) of 26 patients. All PRs were clinically significant and, in all cases, the patients approached but did not quite achieve a CR; specifics are provided in Table 2. Eight (89%) of 9 patients who received at least 1 donor-derived VST product achieved at least a PR. Two patients who received a donor-derived product did not respond to their initial infusions but had response (1 CR, 1 PR) on repeat infusion. Of patients with a clinical response, 17 (80.9%) of 21 had previous and/or concurrent exposure to cidofovir and/or brincidofovir whereas 5 (100%) of 5 patients who never achieved a response had previous and/or concurrent exposure to the same medications. Of the 5 patients who never achieved a response, 4 received third-party products and 1 received a third-party product followed by a donor-derived product. Of the 5 nonresponders, 3 of 5 received >1 infusions. No response was seen with any subsequent infusion, even when a different VST product was chosen, even though in each case the subsequent product had a higher degree of class II HLA match, including 1 patient who received a donor-derived product for the second infusion. Specific sites of HLA match for all nonresponders are detailed in supplemental Table 2. The median HLA match between third-party recipients who responded was 4.5/10 (range, 3-6), and it was 3/10 (range, 3-6) in nonresponders, although the median number of HLA class II matches in both groups was 2/4. No infusion reactions were seen. GVHD occurred within 30 days in only 1 evaluable infusion (2.6%). This patient developed grade 2 skin GVHD and rapidly responded to treatment with systemic steroids. However, this patient had no clinical response to VSTs. Full details on clinical responses in the recipients of VSTs after HSCT are provided in Table 3.
Clinical details of patients with PR to VSTs
| Patient with PR . | Third-party or donor-derived VST . | No. of infusions . | Details of PR . |
|---|---|---|---|
| 1 | Third-party | 1 | Viremia improved from 4400 to <100 copies per mL |
| 2 | Third-party | 2 | Viremia improved from 1 million to 7135 copies per mL after first infusion; no further decrease with second infusion |
| 3 | Third-party followed by donor-derived | 2 | Viremia improved from 500 292 to 54 891 copies per mL after first infusion, then to 863 copies per mL after second infusion |
| 4 | Third-party | 1 | Viremia decreased from 3140 to <1000 copies per mL with improvement in adenoviral diarrhea |
| 5 | Third-party | 1 | No viremia; reduction but not resolution of adenoviral diarrhea |
| 6 | Donor-derived | 2 | Viremia worsened from 4694 to 8103 copies per mL after first infusion but decreased to 810 copies per mL after second infusion |
| Patient with PR . | Third-party or donor-derived VST . | No. of infusions . | Details of PR . |
|---|---|---|---|
| 1 | Third-party | 1 | Viremia improved from 4400 to <100 copies per mL |
| 2 | Third-party | 2 | Viremia improved from 1 million to 7135 copies per mL after first infusion; no further decrease with second infusion |
| 3 | Third-party followed by donor-derived | 2 | Viremia improved from 500 292 to 54 891 copies per mL after first infusion, then to 863 copies per mL after second infusion |
| 4 | Third-party | 1 | Viremia decreased from 3140 to <1000 copies per mL with improvement in adenoviral diarrhea |
| 5 | Third-party | 1 | No viremia; reduction but not resolution of adenoviral diarrhea |
| 6 | Donor-derived | 2 | Viremia worsened from 4694 to 8103 copies per mL after first infusion but decreased to 810 copies per mL after second infusion |
Adenoviral disease presentation and responses to each VST infusion
| . | Total . | Donor-derived VST . | Third-party VST . |
|---|---|---|---|
| No. of patients with an indication for VST | 30 | 7 | 23 |
| Viremia | 9 | 3 | 6 |
| Invasive disease | 6 | 0 | 6 |
| Viremia + invasive disease | 15 | 4 | 11 |
| ADV infection (viremia) presentation before first VST infusion | |||
| Median no. of adenovirus copies per mL before first VST infusion (range) | 40 977 (0-450 000 000) | 5184 (0-191 965) | 116 599 (0-450 000 000) |
| Site of invasive disease | |||
| Diarrhea/enteritis | 18 | 3 | 15 |
| Respiratory tract | 5 | 2 | 3 |
| Antiviral therapy before first VST infusion | |||
| Cidofovir | 12 | 1 | 11 |
| Brincidofovir | 8 | 3 | 5 |
| Cidofovir and brincidofovir | 5 | 0 | 5 |
| None | 5 | 2 | 3 |
| Antiviral therapy at the time of first VST infusion | |||
| Cidofovir | 7 | 1 | 6 |
| Brincidofovir | 4 | 0 | 4 |
| Acyclovir/valacyclovir | 9 | 3 | 6 |
| Ganciclovir | 3 | 1 | 2 |
| None | 9 | 2 | 7 |
| Adenovirus best response to VSTs in evaluable patients (%) | |||
| CR | 54 | 86 | 42 |
| PR | 27 | 14 | 32 |
| CR+PR | 81 | 100 | 74 |
| NR | 19 | 0 | 26 |
| Median No. of evaluable VST infusions (range) | 1 (1-2) | 1 (1-3) | |
| Infusion reaction | 0 | 0 | 0 |
| aGVHD within 30 days of VST infusion | 1 | 0 | 1 |
| . | Total . | Donor-derived VST . | Third-party VST . |
|---|---|---|---|
| No. of patients with an indication for VST | 30 | 7 | 23 |
| Viremia | 9 | 3 | 6 |
| Invasive disease | 6 | 0 | 6 |
| Viremia + invasive disease | 15 | 4 | 11 |
| ADV infection (viremia) presentation before first VST infusion | |||
| Median no. of adenovirus copies per mL before first VST infusion (range) | 40 977 (0-450 000 000) | 5184 (0-191 965) | 116 599 (0-450 000 000) |
| Site of invasive disease | |||
| Diarrhea/enteritis | 18 | 3 | 15 |
| Respiratory tract | 5 | 2 | 3 |
| Antiviral therapy before first VST infusion | |||
| Cidofovir | 12 | 1 | 11 |
| Brincidofovir | 8 | 3 | 5 |
| Cidofovir and brincidofovir | 5 | 0 | 5 |
| None | 5 | 2 | 3 |
| Antiviral therapy at the time of first VST infusion | |||
| Cidofovir | 7 | 1 | 6 |
| Brincidofovir | 4 | 0 | 4 |
| Acyclovir/valacyclovir | 9 | 3 | 6 |
| Ganciclovir | 3 | 1 | 2 |
| None | 9 | 2 | 7 |
| Adenovirus best response to VSTs in evaluable patients (%) | |||
| CR | 54 | 86 | 42 |
| PR | 27 | 14 | 32 |
| CR+PR | 81 | 100 | 74 |
| NR | 19 | 0 | 26 |
| Median No. of evaluable VST infusions (range) | 1 (1-2) | 1 (1-3) | |
| Infusion reaction | 0 | 0 | 0 |
| aGVHD within 30 days of VST infusion | 1 | 0 | 1 |
aGVHD, acute graft-versus-host disease; NR, no response.
Adenovirus-specific T cells show robust expansion in peripheral blood of responding patients after infusion
PBMCs were collected at the time of infusion and then once per week for the first month after each infusion to detect the presence of adenovirus (ADV)-specific T cells by IFN-γ ELISpot. There were adequate numbers of lymphocytes for performing ELISpot assays on 21 of 30 patients, including 6 patients who received donor-derived VSTs, 14 patients who received third-party VST infusions, and 1 patient who received both third-party and donor-derived VSTs. ELISpot was performed on PBMCs from 18 patients who achieved a clinical response.
An increase in ADV-specific T cells was seen in the peripheral blood after the first infusion in which a response was detected in 14 (77.8%) of 18 patients. Two of 4 patients with a clinical response but no increase in peripheral blood T cells were infused with VSTs for adenoviral diarrhea without viremia. In the 14 patients with increased ADV-specific T-cell numbers after infusion, the frequency of ADV-specific T cells increased from a median baseline of 11 SFCs in 4 × 105 PBMCs (range, 0-273 PBMCs) to a median peak of 126 SFCs in 4 × 105 PBMCs (range, 11-1050 PBMCs) (Figure 1A). Representative examples are shown for patients for whom only 1 infusion was required vs when the infusion provided no clinical benefit (Figure 1B-C). ELISpot showed no or a very low peak T-cell frequency (range, 0-45 SFCs in 4 × 105 T cells) in the 3 patients who did not achieve any response from VST therapy.
Expansion of adenovirus-specific T cells in peripheral blood of recipients of VSTs by ELISpot. (A) Quantitation of the number of adenoviral T cells before VSTs compared with the peak number in the first 4 weeks after infusion presented as SFCs. Graph shows data from patients with clinical response and an increase in T-cell number after infusion. For patients with multiple infusions, data reflect T-cell increase after the first infusion that resulted in a response. (B-C) Inverse relationship between frequency of circulating VSTs and adenoviral burden. (B) Representative examples of 3 VST recipients who had CRs after infusion with a corresponding increase in T-cell number by ELISpot. (C) ELISpot in 1 patient with no clinical response; note small scale of the y-axis demonstrating minimal T-cell numbers. Open symbols denote number of SFCs per 400 000 PBMCs. Solid symbols denote adenoviral burden in peripheral blood. UPN, unique patient number.
Expansion of adenovirus-specific T cells in peripheral blood of recipients of VSTs by ELISpot. (A) Quantitation of the number of adenoviral T cells before VSTs compared with the peak number in the first 4 weeks after infusion presented as SFCs. Graph shows data from patients with clinical response and an increase in T-cell number after infusion. For patients with multiple infusions, data reflect T-cell increase after the first infusion that resulted in a response. (B-C) Inverse relationship between frequency of circulating VSTs and adenoviral burden. (B) Representative examples of 3 VST recipients who had CRs after infusion with a corresponding increase in T-cell number by ELISpot. (C) ELISpot in 1 patient with no clinical response; note small scale of the y-axis demonstrating minimal T-cell numbers. Open symbols denote number of SFCs per 400 000 PBMCs. Solid symbols denote adenoviral burden in peripheral blood. UPN, unique patient number.
Anti-adenoviral response and adenovirus epitopes can be predicted in silico and are primarily driven by CD4+ T cells
We generated 206 VST products with in vitro activity against adenovirus that met previously defined safety release criteria through 2019.16 Intracellular flow cytometry was performed as part of the preclinical testing process on all products to determine the percentage of cells that produce IFN-γ when stimulated with adenoviral Pepmix. The median IFN-γ was 1.94% (range, 0.0% to 22.09%). Only 3 products had 0% IFN-γ, consistent with the known high prevalence of previous adenoviral exposure in the general population.
We next sought to better understand the adenovirus-specific HLA restriction of VST products. Epitope prediction for the 2 proteins in the adenoviral Pepmix (hexon and penton) was performed for common HLA alleles using HLA restrictor 1.2 for class I alleles and IEDB.org for class II alleles and compared with previously validated and published epitopes. Twenty 9mer to 15mer peptides representing predicted epitopes predicted to bind HLA alleles were synthesized (supplemental Table 1).
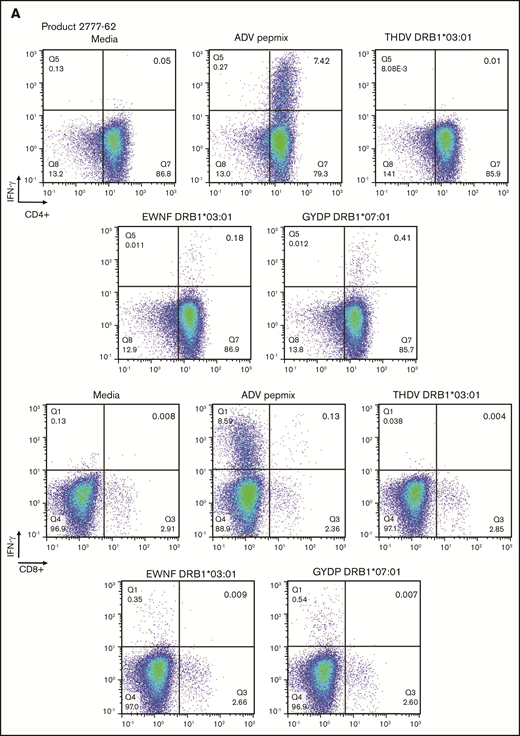
Intracellular flow cytometry for IFN-γ was performed on 12 VST products after stimulation with adenovirus Pepmix in addition to 2 to 4 individual peptides (median, 4 individual peptides) predicted to bind HLA alleles present on the product. At least some IFN-γ positivity was seen in 11 to 12 products, with response to more than 1 peptide being seen in 6 of the products. The predominant response was in CD4+ cells more so than CD8+ cells, with a representative example shown in Figure 2A.
In silico predicted adenoviral epitopes elicit a VST response by intracellular flow cytometry. (A) Representative example of VST product was stimulated with both the entire adenoviral Pepmix and single peptides synthesized on the basis of epitope prediction. Each single peptide is predicted to be presented by the noted HLA molecule present within the cellular product. Differential degree of IFN-γ positivity seen by intracellular flow cytometry, indicating that multiple epitopes are involved in provoking a T-cell response in this product. CD4-predominant response is seen, but little CD8 response is observed.
In silico predicted adenoviral epitopes elicit a VST response by intracellular flow cytometry. (A) Representative example of VST product was stimulated with both the entire adenoviral Pepmix and single peptides synthesized on the basis of epitope prediction. Each single peptide is predicted to be presented by the noted HLA molecule present within the cellular product. Differential degree of IFN-γ positivity seen by intracellular flow cytometry, indicating that multiple epitopes are involved in provoking a T-cell response in this product. CD4-predominant response is seen, but little CD8 response is observed.
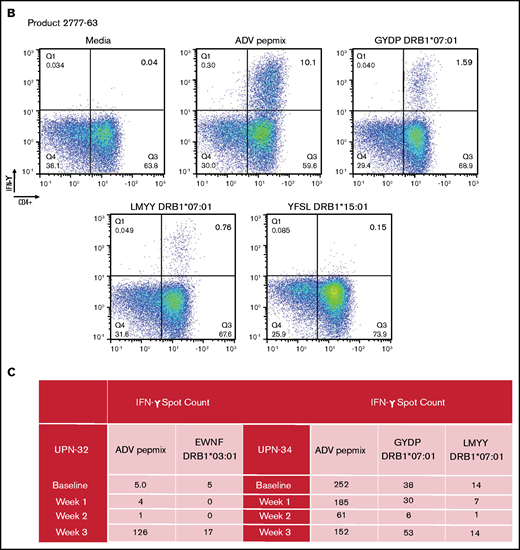
In silico predicted adenoviral epitopes elicit a VST response by intracellular flow cytometry. (B) Representative example of a product that had been given to a patient with a complete clinical response. This product has a response to peptides predicted to be presented by DRB1*07:01 but not to the peptide predicted to be presented by DRB1*15:01. (C) Quantitation of the number of adenoviral T cells by ELISpot in 2 recipients of third-party VSTs. PBMCs were stimulated with either adenoviral Pepmix or single peptides with the HLA predicted to present the epitope indicated in the table. At all timepoints, the background media spot counts have been subtracted.
In silico predicted adenoviral epitopes elicit a VST response by intracellular flow cytometry. (B) Representative example of a product that had been given to a patient with a complete clinical response. This product has a response to peptides predicted to be presented by DRB1*07:01 but not to the peptide predicted to be presented by DRB1*15:01. (C) Quantitation of the number of adenoviral T cells by ELISpot in 2 recipients of third-party VSTs. PBMCs were stimulated with either adenoviral Pepmix or single peptides with the HLA predicted to present the epitope indicated in the table. At all timepoints, the background media spot counts have been subtracted.
On a retrospective analysis, 6 of the 12 products had been used clinically to treat 7 different patients (1 recipient of donor-derived product and 6 recipients of third-party VSTs). Six of 7 patients treated with these products achieved at least a PR. We identified a match between the VST product and the recipient at an HLA site expected to bind 1 of the tested individual peptides in 5 of the 6 patients who responded to infusion. Representative examples are shown in Figure 2B, and full details are summarized in Table 4. Confirmatory ELISpot was performed using samples from 2 third-party recipients of the products shown in Figure 2: 1 with a very good PR (patient 1 in Table 2) and 1 with a CR. PBMCs were exposed to both the entire adenoviral Pepmix and to individual peptides predicted to be presented by the class II allele shared by the recipient and product. In both patients at the peak time of T-cell expansion, the number of T cells responding to each single peptide was detectable although to a lower overall extent than T cells responding to the Pepmix as a whole, again suggesting an HLA restricted but polyclonal response (Figure 2C).
Clinical response is correlated with matching at sites of predicted HLA restriction as determined by single peptide intracellular flow cytometry
| VST recipient . | VST source . | VST product received . | Predicted sites of HLA restriction based on single peptide flow cytometry . | Sites of HLA match between products and recipient . | Clinical response . |
|---|---|---|---|---|---|
| UPN-20 | Third-party | 2777-62 | A*01:01, DRB1*03:01, DRB1*07:01 | A*01:01, B*08:01, C*07:01, DRB1*03:01, DQB1*02:01 | CR |
| UPN-32 | Third-party | 2777-62 | A*01:01, DRB1*03:01, DRB1*07:01 | A*01:01, B*08:01, C*07:01, DRB1*03:01, DQB1*02:01 | PR |
| UPN-34 | Third-party | 2777-63 | DRB1*07:01 | A*02:05, B*50:01, C*06:02, DRB1*07:01, DQB1*02:02 | CR |
| UPN-59 | Third-party | 2777-113 | DRB1*03:01, DRB1*04:01 | A*02:01, DRB1*03:01, DQB1*02:01 | PR |
| UPN-69 | Third-party | 2777-167 | DRB1*03:01. DRB1*04:01 (minimal activity) | A*02:01, C*07:01, DRB1*04:01 | NR |
| UPN-105 | Donor-derived | 2777-105 | B*35:08, DRB1*03:01, DRB1*15:01 | A*03:01, A*31:01, B*35:08, B*50:01 C*04:01, C*06:02, DRB1*03:01, DQB1*06:02 | CR |
| VST recipient . | VST source . | VST product received . | Predicted sites of HLA restriction based on single peptide flow cytometry . | Sites of HLA match between products and recipient . | Clinical response . |
|---|---|---|---|---|---|
| UPN-20 | Third-party | 2777-62 | A*01:01, DRB1*03:01, DRB1*07:01 | A*01:01, B*08:01, C*07:01, DRB1*03:01, DQB1*02:01 | CR |
| UPN-32 | Third-party | 2777-62 | A*01:01, DRB1*03:01, DRB1*07:01 | A*01:01, B*08:01, C*07:01, DRB1*03:01, DQB1*02:01 | PR |
| UPN-34 | Third-party | 2777-63 | DRB1*07:01 | A*02:05, B*50:01, C*06:02, DRB1*07:01, DQB1*02:02 | CR |
| UPN-59 | Third-party | 2777-113 | DRB1*03:01, DRB1*04:01 | A*02:01, DRB1*03:01, DQB1*02:01 | PR |
| UPN-69 | Third-party | 2777-167 | DRB1*03:01. DRB1*04:01 (minimal activity) | A*02:01, C*07:01, DRB1*04:01 | NR |
| UPN-105 | Donor-derived | 2777-105 | B*35:08, DRB1*03:01, DRB1*15:01 | A*03:01, A*31:01, B*35:08, B*50:01 C*04:01, C*06:02, DRB1*03:01, DQB1*06:02 | CR |
Numbers in bold represent alleles that are shared by VST donor and recipient and are predicted to be sites of HLA restriction.
The single patient who did not have a clinical response was infused with a product that matched at DRB1*04:01. Peptides predicted to be presented by this HLA allele were able to elicit an IFN-γ response; however, the response was minimal and lower than the response seen for the DRB1*03:01-presented peptide. Taken together, these data suggest that epitope prediction and confirmatory single-peptide stimulation are additional tools to help determine HLA presentation and indirectly determine HLA restriction for VST products.
Single HLA transfected cell lines are an effective method for directly confirming HLA restriction
Epitope prediction allows for inferences regarding HLA restriction, but because peptides could be presented by more than 1 HLA allele, it is a somewhat indirect method. Our data, along with data from previously published studies, suggest that class II HLA restriction is important for anti-adenoviral activity.21,22 We therefore focused on determining class II restrictions. We used mouse fibroblastoid DAP.3 cells that inherently lack any HLA expression and have been stably transfected with a single HLA allele to more specifically determine the HLA restriction of VST product. Class II single antigen-expressing cell lines (SALs) were evaluated for 7 common HLA alleles (DRB1*01:01, DRB1*03:01, DRB1*04:01, DRB1*07:01, DRB1*11:01, DRB1*13:01, and DRB1*15:01). Class II HLA expression was confirmed by flow cytometry.
SALs were used to retrospectively screen VST products for class II HLA restriction of viral epitopes. An experimental model is shown in Figure 3A. In this model system, the peptides are initially incubated only with the SALs and then washed, so that the only viral antigen present is what has been loaded and presented by the transfected HLA molecule. This method contrasts with the previously described intracellular flow cytometry experiments in which VSTs serve as their own antigen-presenting cells after loading with Pepmix or single epitopes. VSTs were then added into culture with the peptide-loaded SALs followed by intracellular flow cytometry for IFN-γ. The SALs are adherent cells and the VSTs do not adhere, which allows for simple isolation of only the VSTs for flow cytometric analysis. Seven products were tested. Each product was tested using SALs corresponding to the 2 DRB1 alleles present in the product. At least 1 SAL showed IFN-γ positivity above the media control background. In 4 of the patients, the restriction occurred through both shared alleles in each product. Both alleles showed strong degrees of restriction in 2 products, whereas in the other 2 products, 1 allele was much stronger than the other.
SALs for the direct determination of HLA restriction. (A) Schematic demonstrating the model system. Mouse fibroblasts are stably transfected with a single HLA molecule, and Pepmix is added to these cells in culture. After incubation, Pepmix is removed so that the only antigen present is what is bound to and presented by the SAL. SALs are then cocultured with VSTs and intracellular flow cytometry is performed. (B-C) Two retrospective examples of using SALs to determine HLA restriction and correlate with clinical outcomes. In panel B, the coculture of a VST product given to patient UPN-69 shows IFN-γ positivity only with DRB1*03:01 SALs, indicating restriction through that allele. The patient and product did not match at DRB1*03:01 and had no clinical response. By contrast, in Figure 2C, a coculture of the VST product given to patient UPN-32 shows restriction by SALs through both DRB1*03:01 and DRB1*07:01. The patient and product matched at DRB1*07:01 and had a robust clinical response.
SALs for the direct determination of HLA restriction. (A) Schematic demonstrating the model system. Mouse fibroblasts are stably transfected with a single HLA molecule, and Pepmix is added to these cells in culture. After incubation, Pepmix is removed so that the only antigen present is what is bound to and presented by the SAL. SALs are then cocultured with VSTs and intracellular flow cytometry is performed. (B-C) Two retrospective examples of using SALs to determine HLA restriction and correlate with clinical outcomes. In panel B, the coculture of a VST product given to patient UPN-69 shows IFN-γ positivity only with DRB1*03:01 SALs, indicating restriction through that allele. The patient and product did not match at DRB1*03:01 and had no clinical response. By contrast, in Figure 2C, a coculture of the VST product given to patient UPN-32 shows restriction by SALs through both DRB1*03:01 and DRB1*07:01. The patient and product matched at DRB1*07:01 and had a robust clinical response.
Two of the assessed products had previously been given to patients. One product was given to a patient who had no clinical response. This patient’s HLA type matched the VST product at DRB1*04:01 but not at DRB1*03:01. By using SALs, it was determined that this product was restricted through DRB1*03:01. Our SAL data on a different product showed that HLA restriction occurred through both DRB1*03:01 and DRB1*07:01. The product was infused into 2 different recipients, both matched at DRB1*03:01 and both had a clinical response (1 CR, 1 PR) (Figure 3B). In summary, class II SALs are a robust system for directly elucidating DRB1-specific HLA restriction in VST products.
Discussion
Here we present our single-institution experience administering VSTs for the treatment of adenovirus infection and disease in immunocompromised patients. In addition to the large overall cohort size, this is the largest published cohort of patients treated with third-party VSTs for this indication. In addition, in vitro approaches were used to increase our understanding of the epitope specificity and HLA restriction of VST products used in a partially HLA-matched third-party setting.
Adenoviremia and associated invasive adenoviral infections are a major cause of morbidity and mortality after allo-HSCT, especially in the pediatric setting. The incidence of adenoviral infection after HSCT varies across different studies but has been reported to be between 10% and 30% of pediatric patients, with lower frequencies in adults.6,8,23 Prospective screening by PCR is commonly performed for adenovirus in the peripheral blood of children receiving HSCT, and adenovirus is frequently found in the stool of those with diarrhea.4,24 Risk factors for the development of adenoviral infections include ex vivo or in vivo T-cell depletion, use of cord blood grafts, and development of acute GVHD.25-27 Development of adenoviral infection, a high adenoviral load, and the area under the curve of viral copy number have been independently associated with risk for transplant-related mortality.28-30 Notably, both adenoviral infection and mortality from adenovirus are much more common in pediatric recipients of HSCT compared with adult HSCT recipients.23 This could be a result of previous exposure to cross-reactive serotypes, which is likely to be higher in older patients.31 Routine screening is therefore performed much less frequently at centers that perform HSCTs with adult patients.
Treatment options for adenovirus are limited. Recently published results from 2 large surveys of HSCT providers (the Infectious Disease Working Party of the European Society for Blood and Marrow Transplantation and the multinational AdVance study) indicate that a large majority of pediatric providers prefer a preemptive approach to treatment, often when blood PCR values are at or around 1000 copies per mL.23,32 The first-choice therapy historically has been off-label use of cidofovir. However, cidofovir does not reduce the risk of progression to more significant disease.6 In particular, cidofovir is particularly ineffective at controlling adenovirus without T-cell reconstitution in the host33,34 and is also a highly nephrotoxic agent.35 Brincidofovir, an oral lipid-conjugated prodrug of cidofovir that has superior bioavailability compared with cidofovir and high intracellular penetration, is effective in the treatment of adenoviremia without nephrotoxicity or myelosuppression, but unfortunately, it is not commercially available at this time.12,36 It is notable that in our cohort, all nonresponders had previous or concurrent exposure to cidofovir whereas 4 of 21 responding patients had no previous exposure. One explanation for this is that responding patients had infections that were easier to treat, but this conclusion is confounded by our recent institutional trend toward using VSTs as first-line therapy to avoid cidofovir-related toxicities.
VSTs have become increasingly important as an effective treatment for adenoviral infection. Faster manufacturing protocols and third-party banks have made them more accessible, although they are still not available at most transplantation centers. The studies that have been published in recent years of both donor-derived and third-party VSTs using rapid Pepmix technology and IFN-γ capture technology are mostly small ones.15,20,37-39 Response rates in these studies range from 70% to 91%, in line with our results.40,41 Importantly, the 21 patients in our cohort who received third-party products represent the largest cohort of such patients to date and support the use of this therapy for centers that do not have the ability to make individualized donor-derived products for each HSCT recipient. It is notable that our CR and overall response rates were higher in patients treated with donor-derived VSTs compared with third-party recipients (86% vs 42% CR; 74% vs 100% overall response). This could be partially explained by the high degree of overall and class II HLA match for donor-derived recipients, although there was not a large difference in the degree of match between responders and nonresponders in the third-party cohort. This suggests that patients who responded were more likely to be matched at the restricting alleles and that this is the critical determinant of response more than the overall degree of match per se. There are likely determinants of response other than HLA restriction because nonresponse was seen in patients with a high degree of HLA match, including matching at all class II sites (supplemental Table 2). Another consideration is that this difference in response is in part a result of patient selection. Patients who received donor-derived VSTs all received their HSCT at our institution, whereas the majority of third-party recipients were referrals from other institutions. Our own institutional practice has moved toward early delivery of VSTs and, overall, the donor-derived recipients had less and shorter exposure to adenoviral-directed antiviral medications compared with third-party recipients.
Third-party VST banks that provide VSTs to HLA-mismatched recipients face the challenge of selecting the optimal VST product for each recipient. A key component of product selection in the third-party setting is to ensure match at the HLA alleles through which the antiviral response is restricted.42-44 In contrast to previous studies in which restriction was determined largely through loading autologous phytohemagglutinin blasts with epitopes of interest, we used SALs to increase understanding of the HLA restriction of certain VST products and to ideally allow optimization of product selection. We found that class II HLA restriction seems to be critical for the anti-adenoviral activity. The degree of IFN-γ positivity was lower in each case for single peptides compared with the Pepmix. One possible reason for this is that there are no professional antigen-presenting cells in this system, and the ability to process and present 14mers of the single peptides may be diminished compared with the 9mers in the Pepmix. Another possibility is that the adenoviral response is predominantly polyclonal. This is supported by the single epitope flow cytometry data in which there is a differential degree of response elicited by peptides presented by different HLA alleles, although further mechanistic study is needed for confirmation. The predominance of a CD4+ T-cell–driven response is in keeping with previous data showing that in healthy donors the majority of cells that secrete IFN-γ that respond to adenovirus are CD4+ T cells.45 Moreover, in vitro models demonstrate that CD4+ T-cell clones that recognize antigen from the hexon protein (restricted through class II HLA molecules) can lyse virally infected target cells.46 Accordingly, in pediatric HSCT recipients, delayed CD4 reconstitution is associated with increased risk for adenoviral infection, and a large majority of patients who did not develop adenoviremia after HSCT demonstrated CD4-mediated anti-adenoviral responses by 3 months after transplantation.47,48 Taken together, these data all suggest that HLA class II–mediated restriction is critical and that optimizing class II HLA match when choosing third-party VST products may be a helpful strategy.
We present one of the largest groups of patients with adenovirus infection and disease treated with VSTs reported to date. We have also used laboratory strategies to optimize third-party product selection and have used our experience to validate our predictions. However, determination of HLA restriction using SALs was performed only retrospectively. In addition, our third-party bank is large with more than 100 products, which makes rapid prospective screening a logistical hurdle. Two recent studies have shown that mini-banks of products enriched in high-frequency HLA haplotypes allow for large-scale product availability from only a small number of donors.49,50 We are currently generating our own mini-bank of targeted products and will use SAL products to prospectively characterize their HLA restriction, which will allow for improved product selection and ideally higher clinical response rates.
Acknowledgments
The authors thank the staff of the Regenerative Medicine and Cellular Therapies Division at Hoxworth Blood Center for their critical aid in managing and manufacturing VSTs and infusions; the CCHMC Bone Marrow Tissue Repository and the Cell Processing Core laboratories for ongoing technical assistance; Brian Clark for discussions on the design and methods for determining HLA restriction of viral responses; and the patients and their families for taking part in this study.
This work was supported by divisional funds from the Division of Bone Marrow Transplantation and Immune Deficiency.
Authorship
Contribution: J.D.R., A.S.N., S.M.D., M.S.G., and C.L designed the study, performed research, and wrote the manuscript; X.Z., G.P., L.R., and S.E. performed functional studies and analysis; T.L. manufactured VSTs; O.G., D.C., and T.C. performed data collection and analysis; S.J., J.A.C., S.T., C.M.B., P.J.H., M.D.K., C.S.L.A., and A.S. provided vital conceptual insights for study design, assisted with study subject accrual and data collection, and prepared scientific support; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: C.M.B. is on the advisory board for Cellectis, is on the scientific advisory boards for Catamaran Bio and Mana Therapeutics with stock/or ownership, is on the board of directors for Caballeta Bio with stock options, and has stock in Neximmune and Torque Therapeutics. P.J.H. is a cofounder of and on the board of directors for Mana Therapeutics, on the scientific advisory board of Cellevolve, and has intellectual property related to virus-specific T cells. S.M.D. has a US patent application under review, received research support from Alexion Pharmaceuticals, and served as a consultant for Novartis (unrelated to this work). S.J. has a US patent application under review and received travel support and consultancy fees from Omeros (unrelated to this work). The remaining authors declare no competing financial interests.
Correspondence: Jeremy D. Rubinstein, Division of Oncology, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, MLC 7018, 3333 Burnet Ave, Cincinnati OH 45229; e-mail: jeremy.rubinstein@cchmc.org.
References
Author notes
For original data, please contact Jeremy D. Rubinstein via e-mail at jeremy.rubinstein@cchmc.org.
The full-text version of this article contains a data supplement.