Key Points
Early death after hospital admission remains one of the major determinants of outcome in APL in the modern era.
Delays in administration of ATRA and hypofibrinogenemia are major predictors of early death.
Abstract
Despite therapeutic advances, early death (ED) remains a major factor curtailing survival of acute promyelocytic leukemia (APL). Studies examining factors that cause early death (ED; within 30 days of admission) and the correlation of survival with the timing of administration of all-trans retinoic acid (ATRA) and hemostatic parameters are scarce. We performed a cohort analysis of nonselect patients with newly diagnosed APL who presented to the health care system in Hong Kong, where oral arsenic trioxide was used. From 1 January 2007 to 30 April 2020, 358 patients (median age, 47 [1-97] years) with newly diagnosed APL were identified. ED occurred in 56 patients (16%): 11 (3%) died in the first 2 days after admission (intracranial hemorrhage [ICH], n = 6; APL-differentiation syndrome [APL-DS], n = 4; infection, n = 1); 22 (6%) died within 3 to 7 days (ICH, n = 12; APL-DS, n = 8; infections, n = 2), and 23 (6%) died within 8 to 30 days (ICH, n = 7; APL-DS, n = 11; infection, n = 5). Factors significantly associated with ED by multivariate analysis included male sex (P = .01); presenting leukocyte count ≥10 × 109/L (P = .03); fibrinogen <1.5 g/L (P = .02); and ATRA administration >24 hours after hospital admission (P < .001). After a median follow-up of 47 (0-166) months, the 5- and 10-year overall survival (OS) was 68.6% and 61.2%, respectively. Excluding EDs, the 5- and 10-year post–30-day OS improved to 81.3% and 72.5%. Early administration of ATRA (<24 hours) and vigorous correction of hemostatic abnormalities, including hypofibrinogenemia, are key to reducing ED.
Introduction
Acute promyelocytic leukemia (APL) arises from t(15;17)(q24;21) and fusion of the PML and RARA genes. Patients have life-threatening bleeding related to thrombocytopenia and characteristic coagulopathy.1 However, clinical trials have shown that, with optimal supportive care and the use of all-trans retinoic acid (ATRA) and chemotherapy, first complete remission (CR1) rates of >90% and long-term survivals of >85% can be achieved.2-5 Current induction protocols also incorporate IV arsenic trioxide (As2O3).6-10 Regimens that include As2O3, ATRA, and chemotherapy result in CR rates of 90% to 100% and long-term survival of 86% to 97%, so that a cure may be expected in most patients receiving treatment.
Early death (ED) caused by bleeding therefore constitutes the main challenge in APL. In multicenter clinical trials of patients treated with ATRA, As2O3, and anthracyclines, ED rates of only 3% to 10% were reported.7,8,11-13 However, population-based and single-institution studies in nonselect patients reported ED rates ranging from 9.6% to 61.5%, depending on the population studied.14-27 Efforts to develop international recommendations have led to a gradual improvement in ED rates over the past 3 decades, from 28% in the 1990s to ∼15% over the past 2 decades.27-29 Major risk factors for ED include advanced age, high-risk disease, poor performance status, and coexisting infection.30 Hemorrhage is the leading cause of ED.31-36 High white blood cell (WBC) count, high lactate dehydrogenase levels, low fibrinogen, prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT), and the presence of differentiation syndrome are associated with fatal bleeding.31-34 Intracranial hemorrhage (ICH) in the presence of hypofibrinogenemia often portends a fatal outcome.35 In addition, large data sets have shown that a delay in administering ATRA is a major factor contributing to ED.37
We have formulated an oral preparation of As2O3 (oral As2O3)38 and have shown that it is efficacious for APL in the first relapse, inducing CR2 in more than 90% of patients.39,40 Further, in an effort to prevent relapses, we have moved oral As2O3 forward to the induction and maintenance of CR1.41,42 This strategy results in favorable overall survival (OS) and leukemia-free-survival. Similar to regimens using IV As2O3, strategies to reduce ED are needed to improve treatment results with oral As2O3.
In this study, we described the incidence, characteristics, and prognostic indicators of deaths that occur early in hospital admission in a retrospective, nonselect cohort of patients with newly diagnosed APL treated with oral As2O3.
Patients and methods
Patients
In Hong Kong, there are 18 public hospitals with 24-hour accident and emergency service, covering a population of 7.5 million people. Patients with newly diagnosed APL presenting to these hospitals from 1 January 2007 to 30 April 2020 were identified by using the Clinical Data Analysis and Reporting System of the Hong Kong Hospital Authority. The data retrieved included the diagnosis according to International Classification of Diseases coding, ninth revision (ICD-9), date and time of first admission, sex, age, time of administration of first dose of ATRA, date and time of death, and cause of death, according to coding. The Laboratory Information System within the Clinical Data Analysis and Reporting System was used to retrieve basic presentation parameters, comprising complete blood count, PT, activated partial thromboplastin time (APTT), and levels of fibrinogen and D-dimer; and information on APL, comprising the morphologic categories, karyotype, and reverse transcription polymerase chain reaction for PML-RARA. APL was diagnosed according to standard morphologic, cytogenetic, and molecular criteria. The study was approved by the Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster and formed part of the Acute Promyelocytic Leukaemia Asian Consortium Project (registered at www.clinicaltrials.gov as #NCT04251754). The study was conducted in accordance with the Declaration of Helsinki.
Supportive care and treatment regimens
Owing to the retrospective and nontrial nature of this study, there were no explicitly stated guidelines for supportive care and management of patients with APL. In patients diagnosed at regional hospitals, however, platelet counts of <50 × 109/L and fibrinogen concentrations of <1 g/L triggered the transfusion of platelet concentrates and cryoprecipitate/fresh frozen plasma, respectively. In patients diagnosed at or referred to Queen Mary Hospital (QMH), the only academic center with expertise in APL in Hong Kong, protocol-driven, vigorous investigations and supportive care were instituted that included keeping fibrinogen levels >1.5 g/L and platelet counts >50 × 109/L during the first 21 days of presentation. Prophylactic corticosteroids were not administered to prevent APL differentiation syndrome (APL-DS). Dexamethasone (10 mg IV every 12 hours) was given to patients with 1 or more features compatible with differentiation (unexplained fever, weight gain >5 kg, hypotension, dyspnea, radiographic pulmonary infiltrates, pleural or pericardial effusion, and acute renal failure).43,44 Treatment strategies for patients aged ≥18 years were divided into 2 periods according to the induction regimens. From 2007 through 2012, standard induction comprised ATRA (45 mg/m2 per day for 42 days) with or without daunorubicin (supplemental File 1). In patients in CR1, the choices of maintenance, including maintenance with ATRA, oral As2O3, and ascorbic acid (AAA),45,46 were determined by the attending physicians. The number of patients referred to QMH for AAA maintenance during this period was 130. Patients given AAA maintenance were referred to QMH, the only hospital where oral As2O3 was available, for treatment and follow-up. From 2013 onward, induction with ATRA and oral As2O3 were tested in clinical trials (supplemental File 1).41 Eligible patients were referred to and treated at Queen Mary Hospital. The number of patients recruited to frontline oral As2O3 and ATRA-based clinical trials during this period was 104. In patients aged <18 years, the International Consortium for Childhood Acute Promyelocytic Leukemia Study 01 protocol was used throughout the study period (supplemental File 1). ATRA was included in induction, consolidation, and maintenance therapy.
Definitions of end points
ED was defined as death within the first 30 days of presentation. Thirty-day survival was defined as the number of days from diagnosis to death within 30 days (event). OS was defined as time from diagnosis to death (event) or last follow-up (censor). Post–30-day OS was defined as time from day 31 of presentation to death (event) or last follow-up (censor). Data were censored at 31 December 2020. The primary end points were ED and 30-day survival, and the secondary end points were OS and post–30-day OS.
Statistical analyses
Categorical variables were analyzed by χ2 test and continuous variables by a nonparametric test. Time of survival was analyzed by the Kaplan-Meier method, and the difference between the groups was determined with the log-rank test and Cox proportional hazards model. A receiver operating characteristic analysis was performed, and cutoffs of the prognostic parameters were determined based on optimal sensitivities and specificities. Prognostic impacts on survival were evaluated for the following parameters: year of diagnosis (before vs after 2013), sex, age (< vs ≥50 years), presentation hemoglobin (< vs ≥8 g/dL), leukocyte count (< vs ≥10 × 109/L), platelet count (< vs ≥40 × 109/L), PT (< vs ≥16 seconds), APTT (< vs ≥40 seconds), fibrinogen (< vs ≥1.5 g/L), and timing of ATRA administration (≤ vs >24 hours). Prognostic factors with P < .10 on univariate analysis were further examined by multivariate analyses. Two-tailed P < .05 was regarded as significant. All statistical analyses were performed with the SPSS version 26.0 (Chicago, IL).
Results
Patient characteristics
During the study period, 358 patients were diagnosed with APL (Table 1). Twenty-nine patients (8.1%) were aged <18 years on presentation. At diagnosis, 306 patients (85.5%) presented to regional hospitals, and 52 patients (14.5%) presented to QMH, the only academic and quaternary referral center for APL in Hong Kong. On presentation, 84 (23%), 145 (41%), and 129 (36%) patients were in the low-, intermediate-, and high-risk categories, respectively, according to the Sanz score.12 Most patients had coagulopathy at presentation. Fibrinogen <1.5 g, PT >16, and APTT >40 seconds occurred in 50.8%, 28%, and 3.4% of the patients, respectively. Notably, a presentation coagulation profile was not available in 5 patients, and fibrinogen concentration not available in 37.
Clinicopathologic features of 358 patients with newly diagnosed APL
| Clinicopathologic parameters . | Value . |
|---|---|
| Patients, n | 358 |
| Period of diagnosis | |
| 2007-2012 | 158 (44.1) |
| 2013-2020 | 200 (55.9) |
| Sex | |
| Male | 173 (48.3) |
| Female | 185 (51.7) |
| Median age (range), y | 48 (1-97) |
| Age ≥50 y | 171 (47.8) |
| Age groups, y | |
| ≤17 | 29 (8.1) |
| 18-29 | 39 (10.9) |
| 30-39 | 44 (12.3) |
| 40-49 | 75 (20.9) |
| 50-59 | 77 (21.5) |
| 60-69 | 52 (14.5) |
| ≥70 | 42 (11.7) |
| Institution at initial presentation | |
| Regional hospitals | 306 (85.5) |
| QMH | 52 (14.5) |
| Hematologic features at first presentation | |
| Hemoglobin, median (range), g/dL | 8.4 (2.4-15.6) |
| Hemoglobin <8 g/dL | 155 (43.3) |
| Leucocyte count, median (range), ×109/L | 5.1 (0.1-337.6) |
| Leucocyte count ≥10 × 109/L | 129 (36) |
| Platelet count, median (range), ×109/L | 26 (2-481) |
| Platelet count, <40 × 109/L, | 239 (66.8) |
| PT, median (range), s* | 14.4 (9.9-120) |
| PT ≥16 s* | 99 (28.0) |
| Activated partial thromboplastin time, median (range), s* | 29.6 (19.7-180) |
| Activated partial thromboplastin time ≥40 s* | 12 (3.4) |
| Fibrinogen, median (range), g/L* | 1.5 (0-8.1) |
| Fibrinogen <1.5 g/L* | 163 (50.8) |
| Risk according to Sanz score | |
| Low | 84 (23) |
| Intermediate-high | 145 (41) |
| High | 129 (36) |
| Timing of ATRA administration | |
| 0-24 h of presentation | 287 (80.2) |
| >24 h after presentation | 71 (19.8) |
| Deaths within 30 d of presentation | |
| Total | 56 (15.6) |
| ICH | 25 (44.6) |
| Complications from APL-DS | 23 (41.1) |
| Infections | 8 (14.3) |
| Total deaths | 119 (33.2) |
| Median duration of follow-up (range), mo | 47 (0-166) |
| Clinicopathologic parameters . | Value . |
|---|---|
| Patients, n | 358 |
| Period of diagnosis | |
| 2007-2012 | 158 (44.1) |
| 2013-2020 | 200 (55.9) |
| Sex | |
| Male | 173 (48.3) |
| Female | 185 (51.7) |
| Median age (range), y | 48 (1-97) |
| Age ≥50 y | 171 (47.8) |
| Age groups, y | |
| ≤17 | 29 (8.1) |
| 18-29 | 39 (10.9) |
| 30-39 | 44 (12.3) |
| 40-49 | 75 (20.9) |
| 50-59 | 77 (21.5) |
| 60-69 | 52 (14.5) |
| ≥70 | 42 (11.7) |
| Institution at initial presentation | |
| Regional hospitals | 306 (85.5) |
| QMH | 52 (14.5) |
| Hematologic features at first presentation | |
| Hemoglobin, median (range), g/dL | 8.4 (2.4-15.6) |
| Hemoglobin <8 g/dL | 155 (43.3) |
| Leucocyte count, median (range), ×109/L | 5.1 (0.1-337.6) |
| Leucocyte count ≥10 × 109/L | 129 (36) |
| Platelet count, median (range), ×109/L | 26 (2-481) |
| Platelet count, <40 × 109/L, | 239 (66.8) |
| PT, median (range), s* | 14.4 (9.9-120) |
| PT ≥16 s* | 99 (28.0) |
| Activated partial thromboplastin time, median (range), s* | 29.6 (19.7-180) |
| Activated partial thromboplastin time ≥40 s* | 12 (3.4) |
| Fibrinogen, median (range), g/L* | 1.5 (0-8.1) |
| Fibrinogen <1.5 g/L* | 163 (50.8) |
| Risk according to Sanz score | |
| Low | 84 (23) |
| Intermediate-high | 145 (41) |
| High | 129 (36) |
| Timing of ATRA administration | |
| 0-24 h of presentation | 287 (80.2) |
| >24 h after presentation | 71 (19.8) |
| Deaths within 30 d of presentation | |
| Total | 56 (15.6) |
| ICH | 25 (44.6) |
| Complications from APL-DS | 23 (41.1) |
| Infections | 8 (14.3) |
| Total deaths | 119 (33.2) |
| Median duration of follow-up (range), mo | 47 (0-166) |
Data are number of patients (percentage of total group), unless otherwise indicated. Hematologic features at first presentation are shown as median levels in the patients tested with the number of patients tested on the following line.
Only 353 and 321 patients had clotting profile and fibrinogen level tested at presentation, respectively.
Outcome
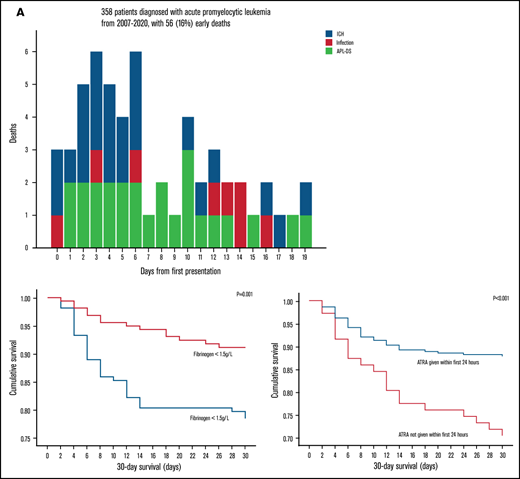
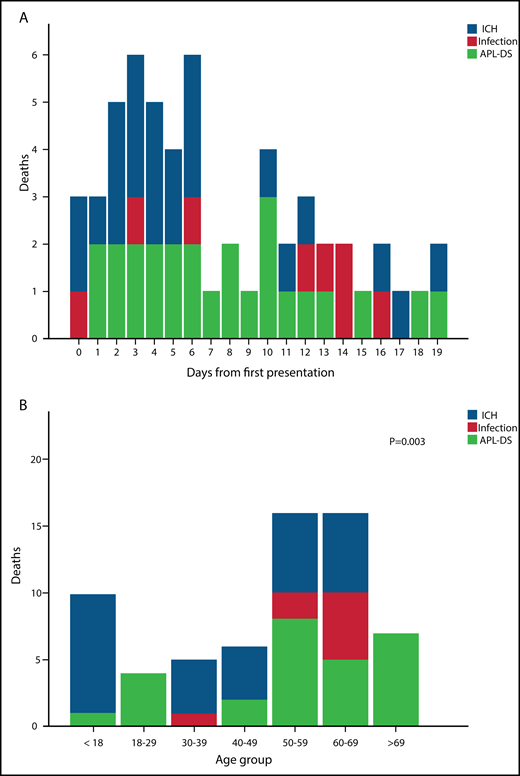
Five patients died before any treatment could be given. ATRA was administered within the first 24 hours in 287 patients (80.2%). ED occurred in 56 patients (15.6%) (Table 2), with 4 deaths at QMH (7.1%) and 52 deaths at other regional hospitals (92.9%). Of note, the median time from admission to the first dose of ATRA in those patients was within the first 24 (6-192) hours. There was no statistically significant difference in the time from admission to the first dose of ATRA in patients diagnosed in regional hospitals and QMH (P = .38). The major causes of deaths were ICH (25 patients, 44.6%), complications of APL-DS (noncardiogenic pulmonary edema, pulmonary hemorrhage, and acute renal failure; 23 patients; 41.1%), and infections (8 patients; 14.3%; Table 2; Figure 1A). Eleven patients (3.1%) died in the first 2 days (ICH, n = 6; APL-DS, n = 4; infection, n = 1), 22 patients (6.2%) died on days 3 to 7 (ICH, n = 12; APL-DS, n = 8; infections, n = 2), and 23 (6.4%) died on days 8 to 30 (ICH, n = 7; APL-DS, n = 11; infection, n = 5; Table 1). Most deaths occurred in patients aged 50 to 59 years (n = 16; 28.6%) followed by those aged 60 to 69 years (n = 13; 23.2%). The major causes of death in patients differed with age: ICH in patients aged <50 years and APL-DS–related complications in those aged ≥50 years (P = .003; Figure 1B). Of interest was the impact of clinical trials on ED. During the study period, 104 patients were recruited into induction trials with oral As2O3, all in QMH. ED did not occur in any of these patients.
Cause-of-death statistics. (A) Daily incidence and causes of death in 56 patients with newly diagnosed APL. (B) Causes of death in different age groups in 56 patients with newly diagnosed APL.
Cause-of-death statistics. (A) Daily incidence and causes of death in 56 patients with newly diagnosed APL. (B) Causes of death in different age groups in 56 patients with newly diagnosed APL.
Clinicopathologic features of 56 EDs in patients with newly diagnosed APL
| Clinicopathologic parameter . | Value . |
|---|---|
| Period of diagnosis | |
| 2007-2012 | 30 (53.6) |
| 2013-2020 | 26 (46.4) |
| Sex | |
| Male | 35 (62.5) |
| Female | 21 (37.5) |
| Median age (range), y | 54.5 (1-97) |
| Age ≥50 years | 36 (64.3) |
| Age groups, y | |
| ≤17 | 5 (8.9) |
| 18-29 | 4 (7.1) |
| 30-39 | 5 (8.9) |
| 40-49 | 6 (10.7) |
| 50-59 | 16 (28.6) |
| 60-69 | 13 (23.2) |
| ≥70 | 7 (12.5) |
| Institution at initial presentation | |
| Regional hospitals | 52 (92.9) |
| QMH | 4 (7.1) |
| Hematologic features at presentation | |
| Hemoglobin, median (range), g/dL | 8 (3.9-14.8) |
| Hemoglobin <8 g/dL | 28 (50) |
| Leucocyte count, median (range), ×109/L | 15.8 (0.9-337.6) |
| Leucocyte count, ≥10 × 109/L | 33 (58.9) |
| Platelet count, median (range), ×109/L | 22 (4-168) |
| Platelet count <40 × 109/L | 41 (73.2) |
| PT, median (range), s* | 16.2 (11.5-65.5) |
| PT ≥16 s* | 30 (53.6) |
| Activated partial thromboplastin time, median (range), s* | 30.3 (21.4-47.2) |
| Activated partial thromboplastin time ≥40 s* | 5 (8.9) |
| Fibrinogen, median (range), g/L* | 1.1 (0.3-6.0) |
| Fibrinogen <1.5 g/L* | 35 (62.5) |
| Time from admission to first dose of ATRA, median (range), h | 24 (6-192) |
| Timing of ATRA administration | |
| 0-24 h of presentation | 35 (62.5) |
| >24 h after presentation | 21 (37.5) |
| Deaths within 30 d of presentation | |
| 0-2 d | 11 (19.6) |
| 3-7 d | 22 (39.3) |
| 8-30 d | 23 (41.2) |
| Cause of death within 30 d of presentation | |
| ICH | 25 (44.6) |
| Complications from APL-DS | 23 (41.1) |
| Infections | 8 (14.3) |
| Clinicopathologic parameter . | Value . |
|---|---|
| Period of diagnosis | |
| 2007-2012 | 30 (53.6) |
| 2013-2020 | 26 (46.4) |
| Sex | |
| Male | 35 (62.5) |
| Female | 21 (37.5) |
| Median age (range), y | 54.5 (1-97) |
| Age ≥50 years | 36 (64.3) |
| Age groups, y | |
| ≤17 | 5 (8.9) |
| 18-29 | 4 (7.1) |
| 30-39 | 5 (8.9) |
| 40-49 | 6 (10.7) |
| 50-59 | 16 (28.6) |
| 60-69 | 13 (23.2) |
| ≥70 | 7 (12.5) |
| Institution at initial presentation | |
| Regional hospitals | 52 (92.9) |
| QMH | 4 (7.1) |
| Hematologic features at presentation | |
| Hemoglobin, median (range), g/dL | 8 (3.9-14.8) |
| Hemoglobin <8 g/dL | 28 (50) |
| Leucocyte count, median (range), ×109/L | 15.8 (0.9-337.6) |
| Leucocyte count, ≥10 × 109/L | 33 (58.9) |
| Platelet count, median (range), ×109/L | 22 (4-168) |
| Platelet count <40 × 109/L | 41 (73.2) |
| PT, median (range), s* | 16.2 (11.5-65.5) |
| PT ≥16 s* | 30 (53.6) |
| Activated partial thromboplastin time, median (range), s* | 30.3 (21.4-47.2) |
| Activated partial thromboplastin time ≥40 s* | 5 (8.9) |
| Fibrinogen, median (range), g/L* | 1.1 (0.3-6.0) |
| Fibrinogen <1.5 g/L* | 35 (62.5) |
| Time from admission to first dose of ATRA, median (range), h | 24 (6-192) |
| Timing of ATRA administration | |
| 0-24 h of presentation | 35 (62.5) |
| >24 h after presentation | 21 (37.5) |
| Deaths within 30 d of presentation | |
| 0-2 d | 11 (19.6) |
| 3-7 d | 22 (39.3) |
| 8-30 d | 23 (41.2) |
| Cause of death within 30 d of presentation | |
| ICH | 25 (44.6) |
| Complications from APL-DS | 23 (41.1) |
| Infections | 8 (14.3) |
55 patients and 49 patients had clotting profile and fibrinogen level tested at presentation, respectively.
Prognostic indicators for 30-day survival
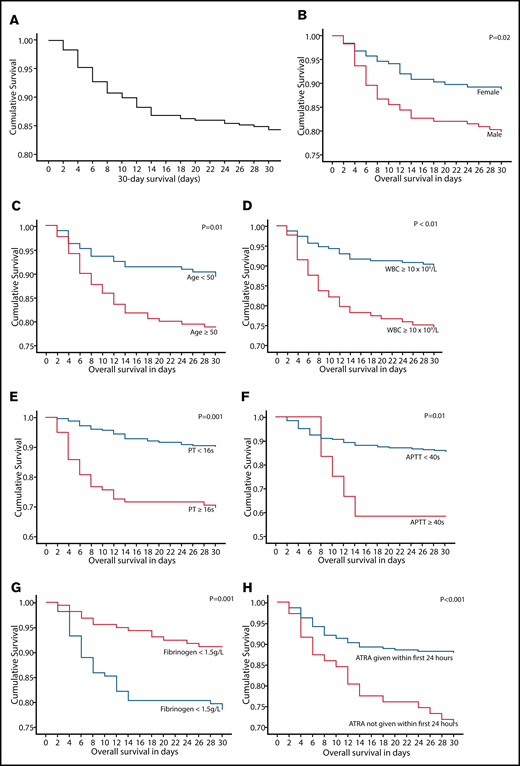
OS at 2, 7, and 30 days was 96.9%, 90.8%, and 84.4%, respectively (Figure 2). On univariate analysis, inferior 30-day survival was significantly associated with male sex (P = .02), age ≥50 years (P = .01), leukocyte count ≥10 × 109/L (P < .001), PT ≥16 seconds (P < .001), APTT ≥40 seconds (P = .02), fibrinogen <1.5 g/L (P = .002), and ATRA administration >24 hours (P = .001; Table 3; Figure 2). On multivariate analysis, male sex (P = .01), leukocyte count ≥10 × 109/L (P = .03), fibrinogen <1.5 g/L (P = .02), and ATRA administration >24 hours (P < .001) remained significant adverse prognostic factors (Table 3). On receiver operating characteristics analysis, the WBC count had an area under the curve of 0.71 (95% confidence interval [CI], 0.63-0.78; P < .001) and fibrinogen had an area under the curve of 0.68 (95% CI, 0.59-0.76; P < .001) in predicting death within 30 days of admission. There was a nonstatistically significant trend toward better 30-day survival in patients diagnosed at QMH compared with those diagnosed at regional hospitals (hazards ratio [HR], 0.48; 95% CI, 0.16-2.21; P = .11). The number of patients with the 3 risk factors male sex, WBC count ≥10 × 109/L, and fibrinogen <1.5 g/L together was 35 (30 patients at regional hospitals and 5 patients at QMH) with 13 deaths (all at regional hospitals). In this group of patients, there was a trend toward better 30-day survival in the group of patients diagnosed at QMH compared with those diagnosed at regional hospitals (P = .09). There was a nonstatistically significant trend toward better 30-day survival in patients diagnosed in the period from 2013 through 2020 compared with those diagnosed from 2007 through 2012 (HR, 0.69; 95% CI, 0.41-1.16; P = .16). A subgroup analysis of the impact of delayed ATRA administration was also performed. ATRA administration at >24 hours after admission was associated with worse 30-day survival in male patients (HR, 2.22; 95% CI, 1.10-4.54; P = .03), female patients (HR, 3.64; 95% CI, 1.54-8.56; P = .03), patients with WBC count >10 × 109/L (HR, 3.79; 95% CI, 1.86-7.72; P < .001), patients with WBC count ≤10 × 109/L (HR, 2.32; 95% CI, 1.01-5.38; P = .048), and patients with fibrinogen <1.5 g/L (HR, 3.20; 95% CI, 1.62-6.29; P = .001). ATRA administration >24 hours did not affect 30-day survival in patients with fibrinogen ≥1.5 g/L (P = .20).
Thirty-day survival and the prognostic indicators on univariate analysis in 358 patients with newly diagnosed APL. (A) Thirty-day survival in 358 patients with newly diagnosed APL. Impact of sex (B), age (C), WBC count (D), presenting PT (E), presenting APTT (F), presenting fibrinogen level (G), and timing of ATRA administration (H) on 30-day survival.
Thirty-day survival and the prognostic indicators on univariate analysis in 358 patients with newly diagnosed APL. (A) Thirty-day survival in 358 patients with newly diagnosed APL. Impact of sex (B), age (C), WBC count (D), presenting PT (E), presenting APTT (F), presenting fibrinogen level (G), and timing of ATRA administration (H) on 30-day survival.
Prognostic factors for 30-d survival in patients with newly diagnosed APL
| . | . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| Features . | n . | 30-d survival (%) . | P . | HR . | 95% CI . | P . | HR . | 95% CI . |
| Patients, n | 358 | 84.4 | — | — | — | — | — | — |
| Period of diagnosis | ||||||||
| 2007-2012 | 158 | 81 | .16 | 1.46 | 0.86-2.47 | — | — | — |
| 2013-2020 | 200 | 87 | — | 0.69 | 0.41-1.16 | — | — | — |
| Sex | ||||||||
| Male | 173 | 79.8 | .02 | 1.88 | 1.10-3.23 | .01 | 2.32 | 1.26-4.28 |
| Female | 185 | 88.6 | — | 0.53 | 0.31-0.91 | — | 0.43 | 0.23-0.79 |
| Age at diagnosis, y | ||||||||
| ≥50 | 171 | 82.5 | .01 | 2.10 | 1.21-3.61 | .09 | 1.66 | 0.93-2.98 |
| <50 | 187 | 89.3 | — | 0.48 | 0.28-0.83 | — | 0.60 | 0.34-1.10 |
| Institution at initial presentation | ||||||||
| Regional hospital | 306 | 83.0 | .11 | 2.29 | 0.83-6.33 | — | — | — |
| QMH | 52 | 92.3 | — | 0.44 | 0.16-1.21 | — | — | — |
| Hematologic features at presentation | ||||||||
| Hemoglobin, g/dL | ||||||||
| ≥8 | 203 | 86.2 | .30 | 0.76 | 0.45-1.28 | — | — | — |
| <8 | 155 | 81.9 | — | 1.32 | 0.78-2.23 | — | — | — |
| Leucocyte count, ×109/L | ||||||||
| ≥10 | 129 | 76.0 | <.001 | 2.80 | 1.64-4.76 | .03 | 1.93 | 1.05-3.54 |
| <10 | 229 | 90.0 | — | 0.36 | 0.21-0.61 | — | 0.52 | 0.28-0.95 |
| Platelet count, ×109/L | ||||||||
| ≥40 | 119 | 87.4 | .25 | 0.71 | 0.39-1.27 | — | — | — |
| <40 | 239 | 82.8 | — | 1.42 | 0.78-2.56 | — | — | — |
| PT, s | ||||||||
| ≥16 | 99 | 69.7 | <.001 | 3.64 | 2.14-6.18 | .052 | 1.88 | 1.00-3.53 |
| <16 | 254 | 90.2 | — | 0.28 | 0.16-0.47 | — | .53 | 0.28-1.00 |
| APTT, s | ||||||||
| ≥40 | 12 | 58.3 | .02 | 3.10 | 1.23-7.78 | .20 | 1.85 | 0.72-4.76 |
| <40 | 341 | 85.3 | — | 0.32 | 0.13-0.81 | — | 0.54 | 0.21-1.39 |
| Fibrinogen, g/L | ||||||||
| ≥1.5 | 158 | 91.1 | .002 | 0.38 | 0.20-0.71 | .02 | 0.47 | 0.24-0.90 |
| <1.5 | 163 | 78.5 | — | 2.63 | 1.42-4.90 | — | 2.15 | 1.11-4.14 |
| Timing of ATRA administration | ||||||||
| 0-24 h of presentation | 287 | 87.8 | .001 | 0.38 | 0.22-0.66 | <.001 | 0.32 | 0.18-0.57 |
| >24 h after presentation | 71 | 70.4 | — | 2.61 | 1.52-4.48 | — | 3.16 | 1.75-5.69 |
| . | . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| Features . | n . | 30-d survival (%) . | P . | HR . | 95% CI . | P . | HR . | 95% CI . |
| Patients, n | 358 | 84.4 | — | — | — | — | — | — |
| Period of diagnosis | ||||||||
| 2007-2012 | 158 | 81 | .16 | 1.46 | 0.86-2.47 | — | — | — |
| 2013-2020 | 200 | 87 | — | 0.69 | 0.41-1.16 | — | — | — |
| Sex | ||||||||
| Male | 173 | 79.8 | .02 | 1.88 | 1.10-3.23 | .01 | 2.32 | 1.26-4.28 |
| Female | 185 | 88.6 | — | 0.53 | 0.31-0.91 | — | 0.43 | 0.23-0.79 |
| Age at diagnosis, y | ||||||||
| ≥50 | 171 | 82.5 | .01 | 2.10 | 1.21-3.61 | .09 | 1.66 | 0.93-2.98 |
| <50 | 187 | 89.3 | — | 0.48 | 0.28-0.83 | — | 0.60 | 0.34-1.10 |
| Institution at initial presentation | ||||||||
| Regional hospital | 306 | 83.0 | .11 | 2.29 | 0.83-6.33 | — | — | — |
| QMH | 52 | 92.3 | — | 0.44 | 0.16-1.21 | — | — | — |
| Hematologic features at presentation | ||||||||
| Hemoglobin, g/dL | ||||||||
| ≥8 | 203 | 86.2 | .30 | 0.76 | 0.45-1.28 | — | — | — |
| <8 | 155 | 81.9 | — | 1.32 | 0.78-2.23 | — | — | — |
| Leucocyte count, ×109/L | ||||||||
| ≥10 | 129 | 76.0 | <.001 | 2.80 | 1.64-4.76 | .03 | 1.93 | 1.05-3.54 |
| <10 | 229 | 90.0 | — | 0.36 | 0.21-0.61 | — | 0.52 | 0.28-0.95 |
| Platelet count, ×109/L | ||||||||
| ≥40 | 119 | 87.4 | .25 | 0.71 | 0.39-1.27 | — | — | — |
| <40 | 239 | 82.8 | — | 1.42 | 0.78-2.56 | — | — | — |
| PT, s | ||||||||
| ≥16 | 99 | 69.7 | <.001 | 3.64 | 2.14-6.18 | .052 | 1.88 | 1.00-3.53 |
| <16 | 254 | 90.2 | — | 0.28 | 0.16-0.47 | — | .53 | 0.28-1.00 |
| APTT, s | ||||||||
| ≥40 | 12 | 58.3 | .02 | 3.10 | 1.23-7.78 | .20 | 1.85 | 0.72-4.76 |
| <40 | 341 | 85.3 | — | 0.32 | 0.13-0.81 | — | 0.54 | 0.21-1.39 |
| Fibrinogen, g/L | ||||||||
| ≥1.5 | 158 | 91.1 | .002 | 0.38 | 0.20-0.71 | .02 | 0.47 | 0.24-0.90 |
| <1.5 | 163 | 78.5 | — | 2.63 | 1.42-4.90 | — | 2.15 | 1.11-4.14 |
| Timing of ATRA administration | ||||||||
| 0-24 h of presentation | 287 | 87.8 | .001 | 0.38 | 0.22-0.66 | <.001 | 0.32 | 0.18-0.57 |
| >24 h after presentation | 71 | 70.4 | — | 2.61 | 1.52-4.48 | — | 3.16 | 1.75-5.69 |
OS and post–30-day survival
After a median follow-up of 47 (0-166) months, the 5- and 10-year OS was 68.6% and 61.2% (supplemental File 2; supplemental File 3A-E). On univariate analysis, inferior OS was significantly associated with male sex (P = .001), age ≥50 years (P < .001), leukocyte count ≥10 × 109/L (P < .001), and PT ≥16 seconds (P = .001). On multivariate analysis, male sex (P < .001), age ≥50 years (P < .001), and leukocyte count ≥10 × 109/L (P = .004) remained significant adverse prognostic factors. The 5- and 10-year post–30-day survival was 81.3% and 72.5%, respectively. On univariate analysis, inferior post–30-day survival was significantly associated with male sex (P = .01), age ≥50 years (P = .001), leukocyte count ≥10 × 109/L (P = .002), and fibrinogen ≥1.5 g/L (P = .02; supplemental File 3F-H). On multivariate analysis, age ≥50 years (P = .001), leukocyte count ≥10 × 109/L (P = .002), and fibrinogen ≥1.5 g/L (P = .02) remained significant adverse prognostic factors.
Discussion
In this population-based study of patients with newly diagnosed APL, we evaluated the clinicopathologic features of and risk factors for ED. Our observation of a 16% rate of ED was somewhat lower than the ∼20% rate reported in other population-based registries.30,47 Severe thrombotic events that were described in up to 12% of patients in other studies were not observed by us.48 Other causes of ED (ICH, APL-DS, and infections) in this study agreed with those found in previous reports.30 Of note, 12 patients died of APL-DS in the first 7 days, accounting for 33% of EDs within the first 7 days. Forty-one percent of EDs within 30 days were caused by APL-DS in our cohort compared with ∼11% reported in large cohorts treated with ATRA and chemotherapy induction.43 A higher median age of patients compared with that reported may partly explain the observation, as most deaths from APL-DS occurred in patients >50 years of age.
Factors that may contribute to ED include delays in diagnosis, medical attendance, and initiation of ATRA treatment; inadequate correction of hemostatic abnormalities; and problems in the recognition and management of APL-DS. Our record review findings that in the first 24 hours of diagnosis, >10% of patients did not have fibrinogen level tested and 20% of patients (5 of whom died) did not receive ATRA indicated a lack of awareness of the initial risks of APL. For patients recruited into oral As2O3 induction trials, which accounted for 30% of patients in this cohort, there were no EDs. Referral bias may partly explain this favorable outcome. Another major reason was protocol-driven, vigorous investigations and supportive care, which included maintaining fibrinogen levels >1.5 g/L and platelets >50 × 109/L during the first 21 days; a low threshold for dexamethasone in APL-DS; and meticulous control of the fluid balance. The impact of oral As2O3–based induction on ED remains to be evaluated prospectively. Because of the retrospective nature and the long duration of follow-up, data on the quantity and timing of blood product administered for each patient were not available to correlate with the risk of EDs. Detailed information on the administration of corticosteroid for APL-DS was not available for the same reason. In this study, we decided not to examine the impact of various regimens on 30-day survival, as the results may have presented a misleading conclusion caused by potential referral bias and impact of clinical trials.
In this study, several factors were associated with inferior 30-day survival on multivariate analysis. The biologic basis for male sex predisposing to EDs is unclear. However, the healthcare-seeking behavior of men may partly explain delays in receiving medical care. Leucocytosis is a major risk factor for bleeding and APL-DS.30,49,50 Hypofibrinogenemia is a factor in bleeding episodes.48-50 Increased fibrinolysis as part of disseminated intravascular coagulopathy only partly explains the reduced fibrinogen levels. Recent evidence indicates that primary hyperfibrinolysis, mediated by annexin A2 and S100A10, is the predominant mechanism for hypofibrinogenemia.48,50 The observation that hypofibrinogenemia and not prolonged PT and APTT was prognostically important lends support to the thought that primary hyperfibrinolysis is a key contributor to coagulopathy. Prompt administration of ATRA is one of the most important measures in reversing the hemostatic abnormalities in APL, and a delay in its administration contributes to ED caused by bleeding. It is also important to consider the causes that may have led to delays in administering ATRA. An important reason that we postulate is related to delays in making the diagnosis of APL. Delays in making a proper diagnosis of APL could be due to lack of suspicion by the attending clinician, delays in blood draws and testing, and a delay in communication with the hematopathologist for blood film review. Because this was a retrospective cohort analysis, we were not able to perform a root cause analysis of the reasons for delayed ATRA administration.
For OS, ED was a major factor that affected outcome, as shown by 5- and 10-year OS at 68.6% and 61.2% and 5- and 10-year post–30-day OS improving to 81.3% and 72.5%. Several factors were associated with inferior post–30-day OS. The adverse impact of male sex and age may be related to comorbidities typically found in men with advanced age. Leucocytosis is a well-known adverse prognostic indicator, especially in the ATRA era, that may be related to increased risk of relapse. Fibrinogen ≥1.5 g/L was associated with worse post–30-day OS. After adjustment for ED, most patients with fibrinogen <1.5g/L were excluded. High fibrinogen, an acute-phase reactant, correlated positively with leucocytosis, a prognostic indicator of worse post–30-day OS and may explain the observation.
It is also important to emphasize that, when we took into account EDs in nonselect patients in the real-world setting, 5- and 10-year OS was significantly worse than reported in the clinical trial setting. Both 30-day survival and OS should be considered important indicators of overall outcome of APL worldwide. Observations in this study allow for several strategies to be formulated to improve early mortality and thus the overall outcome of APL. Education of generalists, emergency physicians, and hematologists in community hospitals in the early diagnosis and detection of hemostatic abnormalities in APL is needed. Initiating ATRA therapy at the first suspicion of the diagnosis with aggressive correction of hemostatic irregularities is essential. Early and concurrent use of cytoreductive therapy in patients with leucocytosis may help to reduce the risk of APL-DS. In addition, prophylactic or early use of dexamethasone, together with meticulous fluid management, can further reduce risks of APL-DS. Better prevention and management of APL-DS may also prevent unwanted delays or interruptions in administration of ATRA. Recent evidence has shown that a comanagement strategy between academic institutions and community practice may lower EDs to <10%.51 Because APL is rare, centralized expertise, with experience in providing supporting care and managing complications, is essential.
Acknowledgment
The study received no funding support.
Authorship
Contribution: H.G. conceived the study, treated the patients, analyzed the data, and wrote and approved the manuscript; Y.Y., H.-T.C., P.-K.Y., and E.L., analyzed the data and approved the manuscript; W.-Y.A., D.C., and S.-Y.H. treated the patients and approved the manuscript; R.Y., P.L., R.Y.Y.L., and E.S.K.M., performed the laboratory investigations, and approved the manuscript; and C.R.K. and Y.-L.K. invented oral arsenic trioxide, treated the patients, and wrote and approved the manuscript.
Conflict-of-interest disclosure: The University of Hong Kong holds 2 US patents (7c521c071 B2 and 8c906c422 B2), 1 Japanese patent (4786341), and 1 European patent (EP 1562616 B1) for the use of oral arsenic trioxide in the treatment of leukemias and lymphomas. H.G., R.Y., and Y.-L.K. are employees of the University of Hong Kong. The remaining authors declare no competing financial interests.
Correspondence: Harinder Gill, Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong Kong, China; e-mail: gillhsh@hku.hk.
References
Author notes
The full text version of this article contains a data supplement.
For the original data, contact the corresponding author at gillhsh@hku.hk.