Key Points
Intermittent-bolus VWF concentrate dosing may have suboptimal efficacy in MGUS-associated acquired VWD.
Continuous-infusion VWF concentrate is effective in maintaining target ristocetin cofactor levels in acquired VWD.
Abstract
Acquired von Willebrand disease (aVWD) is a rare disorder associated with a reduction in von Willebrand factor (VWF) activity, leading to increased bleeding risk. Monoclonal gammopathy of undetermined significance (MGUS) is the most common cause of lymphoproliferative disorder-associated aVWD and is caused by accelerated clearance of circulating VWF. Standard VWF replacement protocols for congenital VWD based on intermittent bolus dosing are typically less effective for aVWD because of antibody-mediated clearance. Intermittent bolus dosing of VWF concentrates often leads to inadequate peak response and profoundly shortened VWF half-life in aVWD. Intravenous immune globulin (IVIG) has demonstrated efficacy in aVWD; however, treatment effect is delayed up to 4 days, limiting its efficacy in acutely bleeding patients. We report the successful use of continuous-infusion VWF concentrate (with or without concomitant IVIG) in 3 patients with MGUS-associated aVWD who had demonstrated an inadequate response to bolus dosing. VWF concentrate doses required in this cohort were higher than typical doses for bleeding treatment in congenital VWD. This report illustrates that continuous-infusion VWF concentrate administration with or without intravenous immunoglobulin rapidly achieves target ristocetin cofactor activity and provides adequate hemostasis in aVWD associated with immunoglobulin G MGUS.
Introduction
Acquired von Willebrand disease (aVWD) is a rare disorder associated with increased bleeding risk resulting from a qualitative or quantitative reduction in von Willebrand factor (VWF).1 Acquired VWD has been associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular conditions.2
Monoclonal gammopathy of undetermined significance (MGUS) is the most common cause of lymphoproliferative disorder-associated aVWF.3 MGUS-associated aVWD results from accelerated VWF clearance by monoclonal immunoglobulins (Ig).1,3 Consequently, standard treatment protocols for congenital VWD based on intermittent bolus dosing of VWF concentrates are often suboptimal because of inadequate peak response and profoundly reduced VWF half-life.3 Intravenous Ig (IVIG) has been used to treat aVWD, with increased efficacy in IgG MGUS compared with IgM, and an overall success rate of 85%.3,4 However, maximal effect of IVIG is seen ∼4 days after administration, highlighting the need for faster acting therapies for actively bleeding patients.4
We report successful management of three patients with aVWD with continuous-infusion (CI) plasma-derived VWF concentrate (Humate-P) with or without concomitant IVIG.
Case descriptions
Patient 1
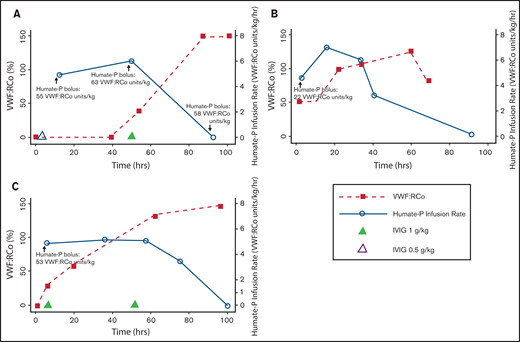
An 85-year-old female with a 6-year history of IgG κ MGUS associated aVWD presented for evaluation before epidural corticosteroid injection for spinal stenosis. Baseline laboratory evaluation revealed factor VIII (FVIII) activity of 18%, ristocetin cofactor (VWF:RCo) <20%, and VWF antigen of 10% (Table 1). She received IVIG 0.5 mg/kg without improvement in VWF:RCo levels. A VWF concentrate bolus dose of 64 VWF:RCo units/kg was administered to assess pharmacokinetic response in preparation for epidural injection. Preinfusion VWF:RCo was below the limit of detection and FVIII activity was 14%. Thirty minutes after the infusion, the VWF:RCo rose to 45% and FVIII activity to 76%, which declined to 26% and 54%, respectively, after 45 minutes. Because of inadequate response to either IVIG or VWF concentrate alone, both IVIG (0.5 g/kg per day for 2 days) followed by CI VWF were administered. Preinfusion VWF:RCo and FVIII activity were 22% and 87%, respectively. VWF concentrate was administered as a bolus of 53 VWF:RCo units/kg immediately followed by CI at 4 VWF:RCo units/kg per hour. One hour into the infusion, her VWF:RCo and FVIII activity were 104% and 141%, respectively. VWF concentrate CI was continued and she underwent epidural injection with adequate hemostasis during and after the procedure. Seven months later, she received similar periprocedural management for lumbar radiofrequency ablation. Her response to IVIG and CI VWF during this encounter is shown in Figure 1A. She received 0.5 g/kg IVIG followed by VWF 55 VWF:RCo units/kg bolus and initiation of CI thereafter at a rate of 5 VWF:RCo units/kg per hour. Her response (VWF:RCo <20%, FVIII activity 49%) was considered inadequate for her impending procedure, so she received an additional VWF concentrate bolus of 63 VWF:RCo units/kg, an increase in the CI VWF rate to 6 VWF:RCo units/kg per hour, and IVIG 1 g/kg. An additional VWF concentrate bolus of 58 VWF:RCo units/kg was administered the following day to optimize levels preoperatively, after which her VWF:RCo activity increased to >150% and her FVIII activity measured 204% (Figure 1A). CI VWF concentrate was continued through the procedure, which was completed successfully without hemostatic complications.
VWF concentrate (Humate-P) dosing and ristocetin cofactor activity response in 3 patients with aVWD. (A) Patient 1’s treatment course during second admission; (B) patient 2’s treatment course; and (C) patient 3’s treatment course.
VWF concentrate (Humate-P) dosing and ristocetin cofactor activity response in 3 patients with aVWD. (A) Patient 1’s treatment course during second admission; (B) patient 2’s treatment course; and (C) patient 3’s treatment course.
Summary of studies reporting continuous-infusion VWF in aVWD
| Publication, n . | aVWD etiology . | Baseline laboratory values . | Continuous-infusion indication . | Continuous-infusion dose . | VWF:RCo activity . | Adjunctive therapies . |
|---|---|---|---|---|---|---|
| Patient 1 | IgG κ MGUS | M spike 0.33 g/dL FVIII 18% VWF Ag 10% VWF:RCo <20% VWF propeptide 92% | Epidural steroid injection | 4 VWF:RCo units/kg per hour | 104% | IVIG |
| Lumbar radiofrequency ablation | 6 VWF:RCo units/kg per hour | >150% | IVIG | |||
| Patient 2 | IgG κ MGUS | M spike 0.29 g/dL FVIII 13% VWF Ag <25% VWF:RCo <20% | Gluteal hematoma | 7 VWF:RCo units/kg per hour | 99% | None, IVIG given 3 d later |
| Patient 3 | IgG κ MGUS | M spike 0.7 g/dL FVIII 25% VWF Ag <5% VWF:RCo <20% VWF propeptide 149% | Ankle fracture and hemarthrosis | 5 VWF:RCo units/kg per hour | 135% | IVIG |
| Frank et al, 20025 (n = 1) | IgG MGUS | FVIII 48% VWF Ag ND VWF:RCo <20% | Total hip replacement | 6 VWF:RCo units/kg per hour | 35%-70% | Desmopressin, IVIG, |
| Lipkind et al, 20056 (n = 1) | SLE | FVIII 2% VWF:RCo <5% | Labor and delivery | 7 FVIII units/kg per hour (∼3 VWF:RCo units/kg per hour) | 200% | Dexamethasone, IVIG |
| Patel et al, 20147 (n = 1; 2 episodes) | CLL | FVIII 9% VWF Ag 16% VWF:RCo <10% | Right hip total arthroplasty and postoperative wound hematoma | 15 VWF:RCo units/kg per hour | 22%-76% | Antifibrinolytics |
| Hand and forearm hematoma | 30 VWF:RCo units/kg per hour | 23%-104% | None | |||
| Hematuria | 30 VWF:RCo units/kg per hour | 37%-80% | None |
| Publication, n . | aVWD etiology . | Baseline laboratory values . | Continuous-infusion indication . | Continuous-infusion dose . | VWF:RCo activity . | Adjunctive therapies . |
|---|---|---|---|---|---|---|
| Patient 1 | IgG κ MGUS | M spike 0.33 g/dL FVIII 18% VWF Ag 10% VWF:RCo <20% VWF propeptide 92% | Epidural steroid injection | 4 VWF:RCo units/kg per hour | 104% | IVIG |
| Lumbar radiofrequency ablation | 6 VWF:RCo units/kg per hour | >150% | IVIG | |||
| Patient 2 | IgG κ MGUS | M spike 0.29 g/dL FVIII 13% VWF Ag <25% VWF:RCo <20% | Gluteal hematoma | 7 VWF:RCo units/kg per hour | 99% | None, IVIG given 3 d later |
| Patient 3 | IgG κ MGUS | M spike 0.7 g/dL FVIII 25% VWF Ag <5% VWF:RCo <20% VWF propeptide 149% | Ankle fracture and hemarthrosis | 5 VWF:RCo units/kg per hour | 135% | IVIG |
| Frank et al, 20025 (n = 1) | IgG MGUS | FVIII 48% VWF Ag ND VWF:RCo <20% | Total hip replacement | 6 VWF:RCo units/kg per hour | 35%-70% | Desmopressin, IVIG, |
| Lipkind et al, 20056 (n = 1) | SLE | FVIII 2% VWF:RCo <5% | Labor and delivery | 7 FVIII units/kg per hour (∼3 VWF:RCo units/kg per hour) | 200% | Dexamethasone, IVIG |
| Patel et al, 20147 (n = 1; 2 episodes) | CLL | FVIII 9% VWF Ag 16% VWF:RCo <10% | Right hip total arthroplasty and postoperative wound hematoma | 15 VWF:RCo units/kg per hour | 22%-76% | Antifibrinolytics |
| Hand and forearm hematoma | 30 VWF:RCo units/kg per hour | 23%-104% | None | |||
| Hematuria | 30 VWF:RCo units/kg per hour | 37%-80% | None |
Ag, antigen; CLL, chronic lymphocytic leukemia; ND, not detectable; SLE, systemic lupus erythematosus.
Patient 2
An 88-year-old male with a 9-year history of IgG κ MGUS-associated aVWD presented with a gluteal hematoma after a fall. As he had had no prior bleeding and had not previously received treatment of asymptomatic aVWD. He received VWF concentrate 34 VWF:RCo units/kg before transfer to our facility. Laboratory evaluation on arrival revealed VWF:RCo activity 50% and factor VIII activity 107%. Because he was actively bleeding and the onset of IVIG effect is delayed, he received a bolus dose of 23 VWF:RCo units/kg, followed by initiation of CI VWF concentrate at 4.5 VWF:RCo units/kg per hour. The CI rate was escalated to 7 VWF:RCo units/kg per hour because of inadequate response (VWF:RCo 49%). After 19 hours at this rate, the infusion rate was tapered over the subsequent 2.5 days because of elevated VWF:RCo activity and improvement of his gluteal hematoma. Thereafter, intermittent doses of IVIG 0.5 g/kg were administered to maintain normal VWF:RCo levels during the remainder of his hospitalization (Figure 1B).
Patient 3
A 53-year-old female with IgG κ MGUS-associated aVWD was transferred to our institution from an outside facility with a closed right ankle fracture and hemarthrosis. Before transfer, she received a VWF concentrate bolus without response (VWF:RCo activity <20% and FVIII activity 25% on arrival). She then received IVIG 1 g/kg followed by a 53 VWF:RCo units/kg VWF bolus. Peak VWF:RCo activity measured 35 minutes after bolus dose administration was lower than expected at 30% (Figure 1C). Thus, CI VWF was initiated at a rate of 5 VWF:RCo units/kg per hour. A second dose of IVIG 1 g/kg was administered 24 hours after the first. VWF CI was continued for 36 hours at 5 VWF:RCo units/kg per hour, at which time her VWF:RCo activity rose to 135%, and the CI rate was reduced by 50%. Twelve hours after dose reduction, the CI was discontinued because of an elevated VWF:RCo activity >150% and FVIII activity of 229%. Her hemarthrosis improved, and 8 days after her initial presentation, she underwent open reduction and internal fixation of her ankle without additional VWF concentrate or IVIG. VWF:RCo activity declined to 63% on postoperative day 4, and an additional IVIG 0.5 g/kg dose was administered with the goal of maintaining VWF:RCo activity 50% to 80% through postoperative day 21 to ensure adequate hemostasis and allow for venous thromboembolism prophylaxis with enoxaparin 30 mg twice daily.
Methods
Bolus followed by CI VWF concentrate was administered to 3 consecutive patients with aVWD for periprocedural optimization (patient 1) or to treat bleeding episodes (patients 2 and 3). We measured FVIII activity using a 1-stage clotting test. Von Willebrand factor activity was measured using Siemens ristocetin cofactor assay (Marburg, Germany).
Results and discussion
CI VWF concentrate was effective in maintaining target VWF:RCo activity to treat bleeding or allowing safe procedures in 3 patients with aVWD. Prior reports suggest similar success with administration of high-dose CI VWF (3-30 VWF:RCo units/kg per hour) in 5 episodes (in 3 patients) (Table 1).5-7 Consistent with these reports, patients with aVWD in our cohort demonstrated poor response to intermittent bolus VWF administration and required higher doses of CI VWF (range 5-7 VWF:RCo units/kg per hour) compared with congenital VWD (3-5 VWF:RCo units/kg per hour) to achieve target VWF:RCo activity. Two of 3 patients in our cohort received concomitant IVIG with CI VWF; therefore, it is difficult to precisely determine the contribution of CI VWF vs IVIG administration to their outcomes. However, patient 1 did not respond to IVIG or intermittent-bolus VWF concentrate alone and achieved target VWF:RCo activity only when CI VWF was added to IVIG administration. Patient 2 only received IVIG during the final hours of CI VWF. Finally, all patients demonstrated increased VWF:RCo and FVIII activities within hours of CI VWF concentrate initiation, a finding unlikely to be explained by IVIG alone. The mechanism by which CI VWF concentrate provides increased VWF:RCo activity in patients with IgG MGUS-associated aVWD without response to intermittent bolus administration has not been fully elucidated. We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy.
Studies in congenital VWD also show that CI vs intermittent bolus dosing results in lower total dose and lower FVIII peaks.8 These results suggest CI VWF concentrate, with or without IVIG, should be considered for treatment of aVWD associated with IgG MGUS. Close monitoring of VWF:RCo and FVIII activity is necessary to prevent overexposure, especially if administered with IVIG.
Acknowledgment
This work was supported by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (K99HL150594) (S.C).
Authorship
Contribution: K.E.D collected data, developed treatment protocols, wrote, edited, and reviewed the manuscript; J.P.L. developed treatment protocols and critically reviewed the manuscript; T.K. oversaw all laboratory assays and critically reviewed the manuscript; M.B.S., A.M., J.Y., R.N., and S.C. edited and reviewed the manuscript; and all authors read and approved the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shruti Chaturvedi, Division of Hematology, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Building, Room 1025, Baltimore, MD 21205; e-mail: schatur3@jhmi.edu.
References
Author notes
For data sharing, e-mail the corresponding author: schatur3@jhmi.edu.