TO THE EDITOR:
Hereditary dehydrated stomatocytoses or xerocytoses (HX) are described as dominant chronic hemolyses caused by cation channel defects at the red cell membrane.1,2 Although mutations in SLC4A1 encoding Band3 and KCNN4 encoding the Gardos channel are associated with HX, and gain-of-function mutations in PIEZO1 are found in about 90% of cases.3-7 Piezo1 is a nonselective cation channel activated by mechanic pressure. Activated Piezo1 drives Ca2+ entry in red blood cells (RBC), activating the calmodulin-binding Gardos channel, leading to K+ leak out of the cell. Gain-of-function mutations in Piezo1-HX result in increased cation flux and altered RBC ion homeostasis. Specifically, increased Ca2+ intracellular concentrations have several consequences, including increased Gardos channel activation, K+ efflux, and RBC dehydration. The clinical consequence is chronic hemolysis that may be associated with iron overload, perinatal edema, or postsplenectomy thromboembolism. Surprisingly, two-thirds of the Piezo1-HX cases present with a compensated hemolysis characterized by high reticulocytes associated with normal hemoglobin (Hb) level.6 Furthermore, some Piezo1-HX patients were addressed for undiagnosed polycythemia, an unusual feature in hemolytic disorders.6-8 A role of activated Piezo1 in erythropoiesis, including delayed erythroid differentiation and reticulocyte maturation, has been recently demonstrated.9,10 Here, using a liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS), we studied the metabolome of HX-RBC and showed how Piezo1 HX affects red cell glycolysis and leads to increased Hb oxygen affinity.
We studied 20 patients (14 families) diagnosed with HX; a metabolome study was performed in 10 subjects and 10 other HX subjects were tested for p50 or 2,3-bisphosphoglycerate (2,3-BPG) only as were 14 subjects with hereditary spherocytosis (HS) (supplemental Tables 1 and 2). HX subjects were included in a cohort already described.6 Data were collected through the French Cohort of Stomatocytosis approved by the National Commission on Informatics and Liberty. All subjects gave their informed consent for gene analysis and research according to the Helsinki protocol and this study followed the French legislation in terms of noninterventional studies. Untargeted metabolomic experiments by LC-HRMS was performed as previously described (supplemental Methods). The 2,3-BPG level was measured using a commercial enzymatic assay (Roche Diagnostics GmbH, Mannheim, Germany). Hb oxygen binding from fresh RBC suspensions was monitored using an Hemox Analyzer spectrophotometer (TCS, New Hope, PA). Alternately, p50 was recorded using an automated venous blood gas analyzer (Radiometer, Copenhagen, Denmark). Statistical analyses were performed using 2-tailed P value and parametric tests. Statistical significance was defined by P ≤ .05. We used a Student t test for quantitative variables. All numeric values are expressed as mean values ± standard error of the mean.
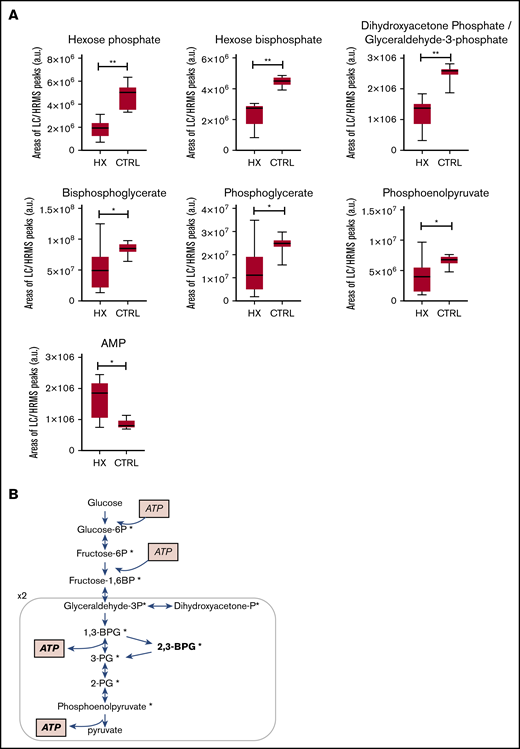
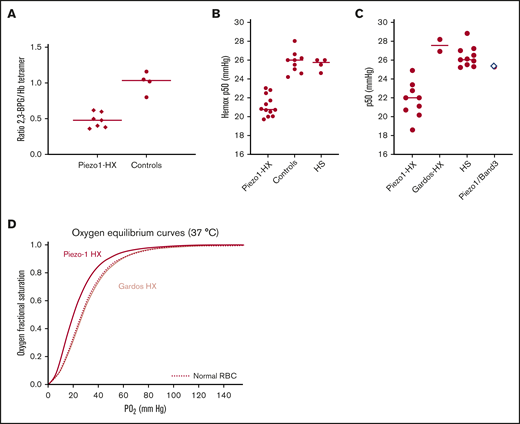
The RBC metabolome of 10 HX subjects (8 families) and 10 age-matched controls was investigated using a LC-HRMS protocol previously established to study the RBC metabolome in sickle cell disease and in overhydrated stomatocytosis.11,12 Eight patients carried 1 or 2 distinct PIEZO1 mutations.6 One patient (HX7) carried a Piezo1 p.Ser338Tyr polymorphism and a new Band3 p.Glu681Gln mutation, he was diagnosed with HX based on the clinical and biological phenotype and a typical ektacytometric profile. The last tested subject (HX3) carried the recurrent p.Arg352His Gardos channel mutation. Multivariate data analyses using unsupervised principal component analysis indicated distinct metabolic profiles for the clustered controls compared with HX patients, with the exception of the subject carrying a Band3 substitution whose RBC clustered with the controls (supplemental Figure 1). Among the 127 metabolites annotated, 19 discriminated HX from controls, 3 were significantly increased, and 16 significantly decreased (supplemental Table 3). These metabolites are involved in pathways related to redox metabolism (decreased ergothioneine, NAD phosphate, glutamic acid), lipid synthesis (mevalonolactone, hydroxycaproic acid, malonic acid, and caproic acid), and glycolysis. We focused on glycolysis because the level of the 6 glycolytic intermediates was significantly lower in HX-RBC (Figure 1). No significant difference was found in hexose, pyruvate, or lactate concentration. These results are consistent with a rapid turnover of glycolytic intermediates and an enhanced glycolysis rate. Decreased levels of glycolytic intermediates were associated with an increase in adenosine 5′-monophosphate (AMP) level and no significant variation in adenosine 5′-diphosphate or adenosine triphosphate (ATP), predicting a decreased ATP/AMP ratio and an altered RBC energy level. Glycolysis is the only source of ATP in RBC, so enhanced glycolysis will provide the ATP used by membrane pumps, including the plasma membrane calcium pump and the Na+K+ATPase, whose function is increased to compensate for the constitutive ion leak.13 Interestingly, decreased glycolytic metabolites and increased AMP levels were also observed in the overhydrated stomatocytosis RBC.11,14 Our results were also in agreement with a study of hereditary over- and dehydrated stomatocytoses diagnosed before gene analysis was available; this work showed a normal ATP level, an increase in adenosine 5′-diphosphate, and a decrease in 2,3-BPG.13 Accordingly, our data showed a decreased BPG level, but metabolome analysis could not discriminate between 1,3 and 2,3-BPG (Figure 1B), so we used a specific enzymatic assay to measure the 2,3-BPG/Hb ratio in HX-RBC. This ratio was decreased in all tested Piezo1-HX RBC compared with that of controls (0.48 ± 0.09, n = 7 vs 1.01 ± 0.1, n = 4, P < .001) (Figure 2A). The 2,3-BPG/Hb ratio was normal in 1 HS case and 1 Gardos-HX (1.1 and 0.95, respectively). Because 2,3-BPG is a potent allosteric inhibitor of Hb oxygen affinity, a decrease in 2,3-BPG is predicted to increase Hb oxygen affinity, so we measured p50 using an Hemox Analyzer or an automated blood gas analyzer. The p50 value was significantly lower in Piezo1-HX cases compared with controls using both continuous recording (Piezo1-HX: n = 11, 21.1 ± 0.9 mm Hg; controls: n = 10, 25.9 ± 0.7 mm Hg) and the automated test (Piezo1-HX: n = 9, 22.2 ± 1.1 mm Hg; reference range: 25-29 mm Hg) (Figure 2B-C). These results demonstrated a higher Hb oxygen affinity in Piezo1-HX with different mutations. The p50 decrease was variable and most often moderate compared with Hb variants with increased oxygen affinity.15,16 In Gardos-HX, Piezo1-Band3, or HS cases, p50 was not significantly different from controls (Gardos-HX: n = 1, 27.5 mm Hg; HS: n = 4, 25.5 ± 0.5 mm Hg; controls: n = 10, 25.9 ± 0.7 mm Hg using Hemox analyzer; Gardos-Hx: n = 2, 28.2 and 26.9 mm Hg, respectively; Piezo1-Band3: n = 1, 25.3 mm Hg; HS: n = 11, 26.4 ± 0.8 mm Hg; reference range 25-29 mm Hg using blood gas test) (Figure 2B-C).
RBC metabolome analysis showed decreased levels of 6 glycolysis intermediates in 10 HX subjects compared with 10 controls. (A) Individual levels of 6 glycolysis intermediates (*P < .05, **P < .01). (B) The decreased metabolites (marked with an asterisk) are shown on as simplified scheme of glycolysis, hexose, and pyruvate levels were not affected. a.u., arbitrary units; CTRL, control.
RBC metabolome analysis showed decreased levels of 6 glycolysis intermediates in 10 HX subjects compared with 10 controls. (A) Individual levels of 6 glycolysis intermediates (*P < .05, **P < .01). (B) The decreased metabolites (marked with an asterisk) are shown on as simplified scheme of glycolysis, hexose, and pyruvate levels were not affected. a.u., arbitrary units; CTRL, control.
Decreased 2,3 BPG level accounts for increased Hb oxygen affinity in Piezo1 HX. The 2,3-BPG level, expressed as 2,3-BPG/Hb ratio, was measured from fresh blood kept on ice and deproteinized using perchloric acid. (A) The 2,3-BPG/Hb ratio was significantly decreased in Piezo1 HX patients compared with controls (0.48 ± 0.09, n = 7 vs 1.01 ± 0.1, n = 4; P < .001). (B) The p50 values measured using an Hemox Analyzer showed that hemoglobin-oxygen affinity was higher in Piezo1-HX RBC compared with HS or controls RBC (Piezo1-HX: n = 11, p50 = 21.1 ± 0.9 mm Hg; HS: n = 4, p50 = 25.5 ± 0.5 mm Hg; controls: n = 10, p50 = 25.9 ± 0.7 mm Hg). The difference between Piezo1-HX and other conditions was statistically significant (P < .001). (C) p50 values measured using a venous blood gas analyzer confirmed the increased hemoglobin-oxygen affinity in Piezo1-HX RBC compared with Gardos-HX or HS-RBC (Piezo1-HX: n = 9, p50 = 22.2 ± 1.1 mm Hg; HS: n = 11, p50 = 26.4 ± 0.8 mm Hg; Gardos-HX: n = 2, p50 = 28.2 and 26.9 mm Hg, respectively; reference range, 25-29 mm Hg). One patient with Piezo1/Band3 double heterozygosity has a normal p50 (p50 = 25.4 mm Hg). The differences between Piezo1-HX and HS was statistically significant (P < .001). (D) Hemox Analyzer oxygen equilibrium curve showed a typical increase in Hb oxygen affinity for a Piezo1-HX RBC suspension (red line) compared with a normal control (dashed line) and Gardos-HX RBC (light red line).
Decreased 2,3 BPG level accounts for increased Hb oxygen affinity in Piezo1 HX. The 2,3-BPG level, expressed as 2,3-BPG/Hb ratio, was measured from fresh blood kept on ice and deproteinized using perchloric acid. (A) The 2,3-BPG/Hb ratio was significantly decreased in Piezo1 HX patients compared with controls (0.48 ± 0.09, n = 7 vs 1.01 ± 0.1, n = 4; P < .001). (B) The p50 values measured using an Hemox Analyzer showed that hemoglobin-oxygen affinity was higher in Piezo1-HX RBC compared with HS or controls RBC (Piezo1-HX: n = 11, p50 = 21.1 ± 0.9 mm Hg; HS: n = 4, p50 = 25.5 ± 0.5 mm Hg; controls: n = 10, p50 = 25.9 ± 0.7 mm Hg). The difference between Piezo1-HX and other conditions was statistically significant (P < .001). (C) p50 values measured using a venous blood gas analyzer confirmed the increased hemoglobin-oxygen affinity in Piezo1-HX RBC compared with Gardos-HX or HS-RBC (Piezo1-HX: n = 9, p50 = 22.2 ± 1.1 mm Hg; HS: n = 11, p50 = 26.4 ± 0.8 mm Hg; Gardos-HX: n = 2, p50 = 28.2 and 26.9 mm Hg, respectively; reference range, 25-29 mm Hg). One patient with Piezo1/Band3 double heterozygosity has a normal p50 (p50 = 25.4 mm Hg). The differences between Piezo1-HX and HS was statistically significant (P < .001). (D) Hemox Analyzer oxygen equilibrium curve showed a typical increase in Hb oxygen affinity for a Piezo1-HX RBC suspension (red line) compared with a normal control (dashed line) and Gardos-HX RBC (light red line).
Taken together, our results are consistent with a high ATP need in Piezo1 HX-RBC that stimulates the main glycolytic pathway at the expense of the 2,3-BPG shunt or Rapoport-Luebering shunt (Figure 1B). The metabolome results suggest that same mechanism might affect the pentose phosphate pathway as well because a decreased concentration of NAD phosphate was observed. They mainly showed that the energetic state of RBCs is altered in Piezo1-HX and, notably, the increased AMP/ATP ratio is predicted to stimulate the AMP-activated protein kinase.17,18 AMP-activated protein kinase is critically involved in cell adaptation to energy depletion by regulating several pathways such as glucose uptake, glycolysis, and oxidative response, and therefore is likely involved in the cell response. The decrease in 2,3-BPG is therefore the RBC response to adapt to the energetic stress. These data suggest that this response is specific to Piezo1-HX because 2,3-BPG level was normal in the tested Band3 or Gardos-HX, although this should be confirmed on a higher number of non-Piezo1-HX cases. Significant differences in Piezo1 and Gardos HX physiopathology have been already reported.19 Indeed, increased Ca2+ influx occurs in Piezo1 and not in Gardos-HX, and Ca2+ has several effects on RBC physiology.20,21 In vitro data have shown that RBC Ca2+ is involved in regulating phosphofructokinase activity. Phosphofructokinase, a rate-limiting enzyme of glycolysis, is a calmodulin-binding protein.22-24 Therefore, increased Ca2+ concentration might stimulate glycolysis enzymes. In addition, this Ca2+ influx will add to the electrolytic dysequilibrium and account for a higher ATP need to fuel the plasma membrane calcium pump.20,21 Interestingly, another cause of high ATP need in Piezo1-HX is a specific effect of Piezo1 activation on ATP extracellular release described by several teams.25,26 It has been known for a long time that elongation triggers ATP release from RBC and Piezo1 has been demonstrated to regulate this process by controlling shear-induced Ca2+ influx. This further links Piezo1 with RBC energy level and might affect ATP release to neighboring cells and tissues as well. In most hereditary hemolytic anemia such as thalassemia, G6PD deficiency, sickle cell disease, and pyruvate kinase deficiency, 2,3-BPG levels are increased.12,27-29 One exception is the rare bisphosphoglyceratemutase deficiency characterized by decreased 2,3-BPG levels and erythrocytosis.30,31 Similarly, in Piezo1-HX, the high Hb oxygen affinity is predicted to decrease oxygen release to the tissues, mimicking a relative hypoxia and providing a basis for the reticulocytosis and compensated hemolysis observed in most Piezo1-HX subjects. Nonetheless, this describes one of several factors regulating Hb level, a number of other parameters such as iron status, hemolysis degree, delayed reticulocyte maturation, and enhanced erythropoiesis are involved as well. Notably, no clinical signs of hypoxia were observed in these patients, possibly because most cells rely on an aerobic energetic metabolism. Decreased oxygen delivery might be too weak to translate into clinical signs, although we cannot exclude that some frequent symptoms such as tiredness or cramps are related to decreased tissue oxygenation. Finally, measuring p50 using venous blood gas is a routine test that might prove useful in diagnosing Piezo1-HX, characterized by a heterogeneous clinical phenotype, the lack of screening tests in routine laboratories, and many mutations and polymorphisms in PIEZO1. We have therefore shown how Piezo1-HX affects RBC energy metabolism and glycolysis, providing a basis for the unexpected increased Hb levels in this disorder and suggesting a role of Piezo1 in erythrocytosis.6-8 These results therefore have an impact on the diagnosis of erythrocytosis and suggest that some cases are related to PIEZO1 mutations. Finally, our metabolomic analysis in HX RBC indicate an effect on redox pathways and lipid metabolism that deserve to be further investigated.
For original data, e-mail the corresponding author, Veronique Picard (veronique.picard@aphp.fr).
Acknowledgments:
The authors thank all the families, patients, and the French Cohort of Hereditary Stomatocytosis; all technicians and biologists of the Hematology Laboratory of Hôpital Bicêtre for their contribution, and Hélène Ponsin for excellent data management; and all investigators and contributors, especially Madeleine Fénéant-Thibault and Gérard Tertian.
This work was supported by the Association Recherche et Formation en Hématopathologie.
Contribution: V. Picard, C.G., L.G., and P.-H.R. designed the study and wrote the paper; V. Picard, L.B., and V. Proulle performed xerocytoses diagnosis and genetic testing; L.K. performed hemoglobin affinity and 2,3-BPG assays; K.G. performed sodium dodecyl sulfate polyacrylamide gel electrophoresis membrane protein analysis and automated p50 measurements; L.O., F.F., and C.J. were in charge of metabolome analysis and analyzed and interpreted the data; C.G., F.G., and L.G. followed patients and actively participated in recording data; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Veronique Picard, Service d’Hématologie Biologique, CHU Bicêtre, AP-HP, 94275 Le Kremlin-Bicêtre, France; e-mail: veronique.picard@aphp.fr.
References
Author notes
The full-text version of this article contains a data supplement.