Abstract
Patients with myeloproliferative neoplasms (MPNs), polycythemia vera, essential thrombocythemia, and primary myelofibrosis, have an increased risk of thrombosis. Risk of recurrent thrombosis can be reduced with antithrombotic therapy and/or cytoreduction, but the optimal long-term management in patients with MPN with a history of venous thromboembolism (VTE) is unknown, and clinical practice is heterogeneous. We performed a systematic review and meta-analysis of randomized trials and observational studies evaluating anticoagulant and/or antiplatelet therapy, with or without cytoreduction, in MPN patients with a history of VTE. A total of 5675 unique citations were screened for eligibility. No randomized trials were identified. Ten observational studies involving 1295 patients with MPN were included in the analysis. Overall, 23% had an arterial or recurrent venous thrombotic event on follow-up. The recurrence risk was lowest for patients on oral anticoagulation plus cytoreduction (16%); 55 of 313 (18%) with vitamin K antagonists (VKA) and 5 of 63 (8%) with direct oral anticoagulants (DOACs). In 746 analyzed patients, the risk of recurrent VTE ranged up to 33% (median 13%) and was low in 63 DOAC plus cytoreduction-treated patients (3.2%). All types of antithrombotic treatments were associated with a lower risk of recurrent VTE when combined with cytoreduction. Most studies had a high risk of bias, whereas clinical and statistical heterogeneity led to inconsistent and imprecise findings. In summary, evidence on the optimal antithrombotic treatment of VTE in patients with MPN is based on observational studies only with low certainty for all strategies. Our data suggest that a combination of anticoagulation and cytoreduction may provide the lowest recurrence risk.
Introduction
Polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) belong to the BCR-ABL1–negative myeloproliferative neoplasms (MPNs).1 MPNs are characterized by clonal proliferation of terminal myeloid cells with variable predominance of cell lines in the peripheral blood. PV or ET can also transform to myelofibrosis. All MPNs can progress into acute myeloid leukemia.
Arterial and venous thrombosis are common events in patients with MPN and have a high incidence across all MPN subtypes.2 The incidence of thrombotic events is highest in patients with PV (5.5 per 100 patient-years) and similar in patients with ET (1-3 per 100 patient-years) and PMF (2 per 100 patient-years).3-5 Thrombosis can occur in patients with clinically overt MPN but can also be the first presenting symptom preceding diagnosis of MPN in up to 40% of patients with splanchnic vein thrombosis.6 Its pathophysiology is generally multifactorial. Traditional risk factors of MPN-associated thrombosis include advanced age (>60 years), sex, and a history of thrombosis. Leukocytosis and the presence of the JAK2V617F mutation have been identified as specific risk factors for thrombosis in patients with MPN, whereas the risk of thrombosis appears to be lower in those with CALR mutation or triple-negative MPN.7-9 Although arterial thrombosis is more prevalent, a recent population-based study of 9429 patients with MPN and 35 820 matched controls found the risk of venous thromboembolism (VTE) up to 3 times higher in patients with MPN than in controls at all time points during 5-year follow-up.2 Although VTE manifests most often as deep vein thrombosis of the leg or pulmonary embolism, VTE also occurs relatively frequently at uncommon sites such as splanchnic or cerebral veins in patients with MPN.6,10,11 Patients with MPN have a higher risk of recurrence compared with individuals without MPN but the risk of recurrence may differ per site of VTE.12
One of the main therapeutic goals in MPN treatment is to prevent thrombosis and recurrence of thrombosis, but the optimal antithrombotic strategy and treatment duration are uncertain.12-14 Antiplatelet therapy has proven to be effective for primary prevention of thrombotic complications in PV, but evidence for long-term prevention of recurrent thrombosis in patients with MPN is limited.15-17 A recent systematic review on antithrombotic therapy in essential thrombocythemia showed that evidence on the risk-benefit ratio of antiplatelet therapy was highly uncertain but did not report on the use of oral anticoagulants.17 In the general population, direct oral anticoagulants (DOACs) have been shown to be at least as effective as vitamin K antagonists (VKA) for prevention of recurrent VTE, but with a lower rate of major bleeding complications. DOACs are currently the first-choice treatment of acute VTE in the general population. Based on recent studies, DOACs can also be given for treatment of cancer-associated acute VTE, but data on DOACs in patients with MPN are scarce.18-20 Cytoreductive treatment has demonstrated to play an important role in the prevention of thrombosis in patients with MPN and risk reduction of recurrent arterial thrombosis, but its efficacy in patients with a history of VTE is uncertain.12,13
Because MPNs are chronic diseases, the risk of recurrent thrombosis remains increased, and therefore, an argument can be made for indefinite treatment. We performed a systematic review with the aim to assess the efficacy (ie, recurrent thrombosis) and safety (ie, bleeding) of antithrombotic treatments with or without cytoreductive therapy in patients with MPN with a history of VTE.
Materials and methods
Search strategy
We systematically searched Medline, Embase, The Cochrane Central Register of Controlled Trials, Web of Science conference proceedings, clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform, with the last paper identified on February 5, 2020. Studies identified by the electronic search as eligible for inclusion in the review were used as “seeds” for the “related article” feature in PubMed and “find similar” feature in Embase to identify more potentially relevant articles. A citation search was performed for these studies in Web of Science to identify articles that have cited these. Additionally, reference lists of the included studies and of previous reviews on the subject were hand searched for potentially relevant studies. We did not apply any language or date restrictions. Relevant terms in the search strategy are specified in the supplemental Data; the full search strategy is available upon request. This systematic review was registered at the Prospective Register of Systematic Reviews (PROSPERO) International Prospective Register of Systematic Reviews as #CRD42019112344.
Selection of studies
Two authors (E.N.H. and M.N.L.) screened all titles and abstracts identified by the search strategy independently for potentially eligible studies, using Rayyan.21 Studies were eligible if they met all of the following criteria: (1) were a randomized controlled trial or observational study with at least 10 patients; (2) included adults (age ≥18 years) with an MPN (PV, ET, PMF, or pre-PMF) diagnosed according to the contemporary diagnostic World Health Organization criteria at the time of study conduct; (3) included patients with a history of VTE; (4) evaluated anticoagulant therapy and/or antiplatelet therapy with or without cytoreductive treatment, compared with another, no treatment, or placebo; and (5) reported data on recurrent thrombotic or bleeding events. Conference abstracts were only considered if sufficient data were available for data extraction. Eligible publications were reviewed in full text to determine whether they met the inclusion criteria by 2 authors independently (E.N.H. and M.N.L.). Any disagreements were resolved through discussion between authors, and if necessary, a third author (S.M.) was involved for the final vote. Agreement between reviewers was measured with the Cohen κ coefficient at screening and full-text review.
Data extraction and synthesis
For every eligible study, data were extracted by 2 authors (E.N.H. and M.N.L.) independently using a standardized data extraction form. In case of uncertainties, we contacted the authors of the study and requested additional information. Disagreements were resolved as previously described. Outcomes of interest includes recurrent thrombotic events, bleeding, all-cause mortality, fatal thrombosis, and leukemic transformation. If eligible studies included a mixed population with regard to history of thrombosis, we only extracted data of the patients with a history of VTE for our primary outcome analysis. Recurrent symptomatic VTE was defined as recurrent deep vein thrombosis (of the leg or arm), pulmonary embolism, cerebral (sino)venous thrombosis, and splanchnic vein thrombosis (including portal vein thrombosis, hepatic vein thrombosis [Budd-Chiari syndrome], or mesenteric vein thrombosis). Bleeding events, including major, clinically relevant nonmajor, or minor bleeding episodes, were defined by the International Society on Thrombosis and Haemostasis (ISTH) criteria, the study’s local protocol, or according to the treating physician. Other extracted data included year of publication, number of participants, type and dose of antithrombotic treatment, risk factors, and sites of thrombosis.
Treatment strategies
We aimed to evaluate the efficacy, by means of the recurrent thrombosis rate, and safety, expressed as occurrence of bleeding events for the following treatment strategies: (1) antiplatelet therapy with or without cytoreduction; (2) oral anticoagulation with or without cytoreduction; (3) oral anticoagulation plus antiplatelet therapy with or with cytoreduction; and (4) cytoreduction only or no antithrombotic or cytoreductive treatment. Long-term nonoral anticoagulant treatment (eg, low-molecular-weight heparin or synthetic pentasaccharide) was not assessed separately.
Study quality assessment
Two authors (E.N.H. and M.N.L.) independently assessed risk of bias for each study using the Cochrane ROBINS-I tool for nonrandomized observational studies of interventions.22 Any discrepancies were resolved by discussion or by involving a third assessor (S.M.). Our treatment recommendations are based on the quality of available evidence as outlined in the Grading of Recommendations Assessment Development and Evaluation tool.23
Statistical analysis
Primary and secondary outcomes were reported per antithrombotic treatment strategy with and without concomitant cytoreductive therapy. Using a random effects model, 95% confidence intervals (CIs) were calculated for proportions and relative risks (RRs). Heterogeneity of the pooled estimates was assessed using I2 statistics. Significant heterogeneity was defined as I2 >50%, and considerable heterogeneity was defined as I2 >30%. All statistical analyses were carried out using StatsDirect version 3.3.3 (StatsDirect Ltd, Chesire, United Kingdom).
Results
Study identification and selection
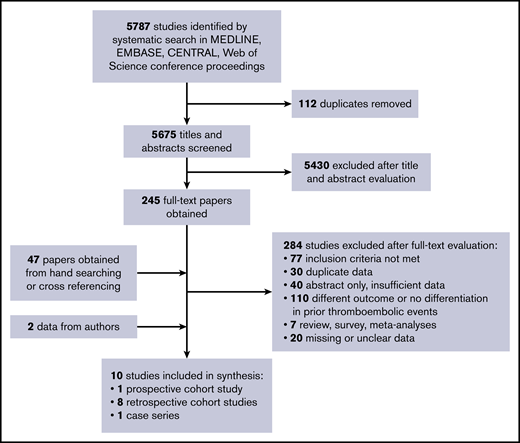
We identified 5787 studies using our search strategy. After removal of 112 duplicates, we excluded 5430 papers based on title and abstract screening. Screening agreement was excellent (κ = 0.89). We obtained 245 full-text papers for further evaluation and to identify possibly overlapping study populations. By handsearching and cross-referencing retrieved papers, we identified an additional 47 papers. A total number of 282 papers was subsequently excluded after full-text evaluation: 77 did not meet the inclusion criteria; 30 had overlapping study populations or data; 108 evaluated a different outcome or did not report on patients with prior thrombotic events; 7 were reviews, surveys, or meta-analyses; 40 were abstract only with insufficient data for proper assessment; and 20 papers had missing or unclear data. Agreement at full-text stage was also excellent (κ = 0.91).
Twenty studies with unclear data (ie, no clear specification of thrombotic events during follow-up or per treatment strategy) were contacted for additional data; 12 responded, but only 2 provided the requested data. Hence, the other 10 studies were excluded because of missing data. Finally, we included 10 nonrandomized observational studies in this systematic review with a total number of 1295 participants (Figure 1).24-33
Study characteristics
Characteristics of the included studies are presented in Table 1. No randomized controlled trials were identified. The included studies were predominantly retrospective, single center cohort studies, conducted in European centers. Study populations size ranged from 14 to 494 patients, and the median duration of follow-up was between 2.1 and 10 years. The median age of study participants ranged from 47 to 75 years and 57% of the overall study population was female. ET was the most prevalent MPN (54%), followed by PV (37%). Of 1295 patients in the included studies, 87% (N = 1121) had a history of any thrombosis and 13% did not have a history of thrombosis (N = 174). The majority of patients with previous thrombosis had a history of VTE (738 of 1121; 66%). These patients were included in our analysis as main study population. JAK2V617F was the most common reported mutation in patients in whom MPN mutational status was assessed (Table 2).
Characteristics of the 10 included studies
| Study year . | Study characteristics . | Study population . | MPN . | Follow-up, y . | Antithrombotic treatment . | Reference . |
|---|---|---|---|---|---|---|
| 1991 | Retrospective cohort | N = 103 | ET | Mean 4.8 | Antiplatelet ± cytoreduction (N = 74) | 24 |
| Single center | Age: 9-88 y | Cytoreduction only (N = 15) | ||||
| Italy | ♀/♂: 59/44 | No treatment (N = 14) | ||||
| 2002 | Retrospective cohort | N = 118 | ET | Median 10 | Antiplatelet ± cytoreduction (N = 118) | 25 |
| Single center | Age: mean 62 y | |||||
| Italy | ♀/♂: 69/49 | |||||
| 2008 | Retrospective cohort | N = 494 | ET, PV | Median 5.3 | Antiplatelet ± cytoreduction (N = 351) | 26 |
| Multicenter | Age: median 62 y | VKA ± cytoreduction (N = 79) | ||||
| Italy | ♀/♂: 255/239 | VKA + antiplatelet ± cytoreduction (N = 11) | ||||
| Cytoreduction only (N = 40) | ||||||
| No treatment (N = 13) | ||||||
| 2011 | Retrospective cohort | N = 44 | ET, PV, MF, Unclassified | Median 5.8 | Antiplatelet only (N = 6) | 27 |
| Single center | Age: median 48 y | VKA only (N = 9) | ||||
| Netherlands | ♀/♂: 31/13 | VKA + antiplatelet (N = 6) | ||||
| No treatment (N = 23) | ||||||
| 2016 | Retrospective cohort | N = 181 | ET, PV, PMF | Median 3.2 | Antiplatelet + cytoreduction (N = 6) | 28 |
| Multicenter | Age: median 48 y | Anticoagulation ± cytoreduction (N = 156) | ||||
| Multinational | ♀/♂: 118/63 | Anticoagulation + antiplatelet ± cytoreduction (N = 10) | ||||
| Cytoreduction only (N = 8) | ||||||
| No treatment (N = 1) | ||||||
| 2016 | Retrospective cohort | N = 206 | ET, PV, PMF | Median 2.6 | Antiplatelet ± cytoreduction (N = 11) | 29 |
| Multicenter | Age: median 72 y | Anticoagulation ± cytoreduction (N = 163) | ||||
| Multinational | ♀/♂: 114/92 | Anticoagulation + antiplatelet ± cytoreduction (N = 19) | ||||
| Cytoreduction only (N = 9) | ||||||
| No treatment (N = 5) | ||||||
| 2017 | Prospective cohort | N = 25 | ET, PV | Median 2.1 | DOAC ± cytoreduction (N = 25) | 30 |
| Single center | Age: median 75 y | |||||
| France | ♀/♂: 12/13 | |||||
| 2018 | Case-series | N = 14 | ET, PV, Unclassified | Median 7.4 | VKA ± cytoreduction (N = 9) | 31 |
| Single center | Age: mean 47 y | Antiplatelet ± cytoreduction (N = 2) | ||||
| Northern Ireland | ♀/♂: 11/3 | VKA + antiplatelet ± cytoreduction (N = 3) | ||||
| No treatment (N = 2) | ||||||
| 2019 | Retrospective cohort | N = 78 | ET, PV, MF | Median 5.4 | Anticoagulation ± cytoreduction (N = 71) | 32 |
| Single center | Age: median 53 y | No anticoagulation (N = 7) | ||||
| Germany | ♀/♂: 57 /25 | |||||
| 2020 | Retrospective cohort | N = 32 | ET, PV, MF, Unclassified | Median 2.1 | 33 | |
| Single center | Age: median 50 y | DOAC + cytoreduction (N = 28) | ||||
| United Kingdom | ♀/♂: 18/14 | DOAC + antiplatelet (N = 4) |
| Study year . | Study characteristics . | Study population . | MPN . | Follow-up, y . | Antithrombotic treatment . | Reference . |
|---|---|---|---|---|---|---|
| 1991 | Retrospective cohort | N = 103 | ET | Mean 4.8 | Antiplatelet ± cytoreduction (N = 74) | 24 |
| Single center | Age: 9-88 y | Cytoreduction only (N = 15) | ||||
| Italy | ♀/♂: 59/44 | No treatment (N = 14) | ||||
| 2002 | Retrospective cohort | N = 118 | ET | Median 10 | Antiplatelet ± cytoreduction (N = 118) | 25 |
| Single center | Age: mean 62 y | |||||
| Italy | ♀/♂: 69/49 | |||||
| 2008 | Retrospective cohort | N = 494 | ET, PV | Median 5.3 | Antiplatelet ± cytoreduction (N = 351) | 26 |
| Multicenter | Age: median 62 y | VKA ± cytoreduction (N = 79) | ||||
| Italy | ♀/♂: 255/239 | VKA + antiplatelet ± cytoreduction (N = 11) | ||||
| Cytoreduction only (N = 40) | ||||||
| No treatment (N = 13) | ||||||
| 2011 | Retrospective cohort | N = 44 | ET, PV, MF, Unclassified | Median 5.8 | Antiplatelet only (N = 6) | 27 |
| Single center | Age: median 48 y | VKA only (N = 9) | ||||
| Netherlands | ♀/♂: 31/13 | VKA + antiplatelet (N = 6) | ||||
| No treatment (N = 23) | ||||||
| 2016 | Retrospective cohort | N = 181 | ET, PV, PMF | Median 3.2 | Antiplatelet + cytoreduction (N = 6) | 28 |
| Multicenter | Age: median 48 y | Anticoagulation ± cytoreduction (N = 156) | ||||
| Multinational | ♀/♂: 118/63 | Anticoagulation + antiplatelet ± cytoreduction (N = 10) | ||||
| Cytoreduction only (N = 8) | ||||||
| No treatment (N = 1) | ||||||
| 2016 | Retrospective cohort | N = 206 | ET, PV, PMF | Median 2.6 | Antiplatelet ± cytoreduction (N = 11) | 29 |
| Multicenter | Age: median 72 y | Anticoagulation ± cytoreduction (N = 163) | ||||
| Multinational | ♀/♂: 114/92 | Anticoagulation + antiplatelet ± cytoreduction (N = 19) | ||||
| Cytoreduction only (N = 9) | ||||||
| No treatment (N = 5) | ||||||
| 2017 | Prospective cohort | N = 25 | ET, PV | Median 2.1 | DOAC ± cytoreduction (N = 25) | 30 |
| Single center | Age: median 75 y | |||||
| France | ♀/♂: 12/13 | |||||
| 2018 | Case-series | N = 14 | ET, PV, Unclassified | Median 7.4 | VKA ± cytoreduction (N = 9) | 31 |
| Single center | Age: mean 47 y | Antiplatelet ± cytoreduction (N = 2) | ||||
| Northern Ireland | ♀/♂: 11/3 | VKA + antiplatelet ± cytoreduction (N = 3) | ||||
| No treatment (N = 2) | ||||||
| 2019 | Retrospective cohort | N = 78 | ET, PV, MF | Median 5.4 | Anticoagulation ± cytoreduction (N = 71) | 32 |
| Single center | Age: median 53 y | No anticoagulation (N = 7) | ||||
| Germany | ♀/♂: 57 /25 | |||||
| 2020 | Retrospective cohort | N = 32 | ET, PV, MF, Unclassified | Median 2.1 | 33 | |
| Single center | Age: median 50 y | DOAC + cytoreduction (N = 28) | ||||
| United Kingdom | ♀/♂: 18/14 | DOAC + antiplatelet (N = 4) |
DVT, deep vein thrombosis; PV, polycythemia vera.
Baseline characteristics of studied patient population (n = 1295)
| Characteristic . | n (%) . |
|---|---|
| Sex, n (%) | ♀ 744 (57) |
| ♂ 555 (43) | |
| Age, y | Range: 47-75 |
| MPN, n (%) | |
| Essential thrombocythemia | 700 (54) |
| Polycythemia vera | 473 (37) |
| Primary myelofibrosis | 103 (8) |
| Unclassified | 19 (1) |
| Mutational status,*n/N (%) | |
| JAK2+ | 485/550 (88) |
| CALR | 24/190 (13) |
| MPL | 7/196 (4) |
| JAK2ex12 | 1/31 (3) |
| Triple− | 9/131 (7) |
| Prior thrombosis, n (%) | |
| Arterial thrombosis | 383 (30) |
| Venous thrombosis | 738 (57) |
| DVT/PE | 278 (38) |
| Splanchnic vein thrombosis | 286 (39) |
| Cerebral (sino)venous thrombosis | 8 (1) |
| Not specified | 162 (22) |
| Both arterial and venous thrombosis | 8 (0.6) |
| Characteristic . | n (%) . |
|---|---|
| Sex, n (%) | ♀ 744 (57) |
| ♂ 555 (43) | |
| Age, y | Range: 47-75 |
| MPN, n (%) | |
| Essential thrombocythemia | 700 (54) |
| Polycythemia vera | 473 (37) |
| Primary myelofibrosis | 103 (8) |
| Unclassified | 19 (1) |
| Mutational status,*n/N (%) | |
| JAK2+ | 485/550 (88) |
| CALR | 24/190 (13) |
| MPL | 7/196 (4) |
| JAK2ex12 | 1/31 (3) |
| Triple− | 9/131 (7) |
| Prior thrombosis, n (%) | |
| Arterial thrombosis | 383 (30) |
| Venous thrombosis | 738 (57) |
| DVT/PE | 278 (38) |
| Splanchnic vein thrombosis | 286 (39) |
| Cerebral (sino)venous thrombosis | 8 (1) |
| Not specified | 162 (22) |
| Both arterial and venous thrombosis | 8 (0.6) |
Proportion of patients in whom MPN mutational status was assessed.
CALR, calreticulin gene mutation; DVT, deep vein thrombosis; JAK2, janus kinase 2 (V617F) mutation; JAKex12, janus kinase 2 exon 12 mutation; MPL, myeloproliferative leukemia virus oncogene mutation.
Various antithrombotic treatment strategies were evaluated. In total, 623 patients received antiplatelet therapy, in most cases low-dose aspirin (80 mg). VKA was the most common oral anticoagulant treatment (467 patients), whereas more recent studies included 77 patients treated with DOACs.28-30,32,33 Few studies specified the indication of antithrombotic treatment. A change of treatment during study follow-up was common, but reasons for changing or a rationale behind a different type of antithrombotic therapy were frequently not reported. Treatment with cytoreductive agents was common but rarely given without concomitant antithrombotic therapy; 69% vs 8%, respectively.
Study quality
We addressed study quality using the ROBINS-I tool. Full details of the risk of bias assessment for each study can be found in the supplemental Data. Two studies had serious risk of bias, and the remaining 9 studies were judged to have moderate risk of bias, leading to an overall rating of moderate risk of bias. The main sources of bias were confounding and the selection of patients. Deviations from the intended interventions with patients crossing over to another treatment differed largely per study and was in almost half of the studies not assessable.
Outcomes
Recurrent thrombotic events.
Our main study population consisted of the patients with a history of VTE in the included studies (N = 738). Overall, 22.6% of evaluated patients had a recurrent thrombotic event during follow-up, either arterial or venous thrombosis (293 of 1295 patients; 10 studies).24-33 The observed recurrence risk was highest in patients with single antithrombotic treatment modalities or patients receiving neither antithrombotic treatment nor cytoreduction. The overall evidence was rated as very uncertain for all interventions because of the observational nature of the data, high risk of bias in the included studies, inconsistency in the results and imprecision in the estimates.
Six studies (555 patients) described patient cohorts in whom all patients had a VTE at study baseline; 254 patients with splanchnic vein thrombosis, 298 with deep vein thrombosis and/or pulmonary embolism, 2 with cerebral venous sinus thrombosis, and 1 with superficial thrombophlebitis.27-29,31-33 Results of an exploratory comparative analysis of studies in which all patients had either splanchnic vein thrombosis or deep vein thrombosis/pulmonary embolism as baseline event can be found in supplemental Table 1.27-29,31
Few studies specified whether recurrent thrombotic events concerned recurrent arterial or venous thrombosis. In patients with established recurrent VTE, the risk of recurrence ranged from 0.0% to 33.3% (median, 13.0%) and was highest when only anticoagulant treatment was given in 21 of 72 patients (29.2%) on VKA only and in 3 of 14 patients (21.4%) on DOAC only.
Antiplatelet therapy with or without cytoreduction
Five studies reported recurrent arterial or venous thrombotic events in 118 patients on antiplatelet therapy only and found a risk of recurrence of 30.5% (95% CI, 22.4-39.6).24,29,31,32 Seven studies reported recurrence in 452 patients on antiplatelet plus cytoreductive therapy, with recurrent events in 18.6% of patients (95% CI, 15.1-22.5).24-29,31 Of 120 recurrent thrombotic events, nearly all (118 of 120) were arterial. Recurrent VTE occurred in only 1 patient on single antiplatelet therapy and 1 patient on antiplatelet therapy and cytoreduction.
Oral anticoagulation with or without cytoreduction
In 6 studies reporting on 120 patients treated with oral anticoagulation only (either VKA or DOAC), 42 had a recurrent arterial or venous thrombotic event (35.0%; 95% CI, 26.5-44.2).26,28-32
When oral anticoagulation was combined with cytoreductive treatment, a pooled analysis of 8 studies involving 376 patients showed a recurrence risk of 16.0% (95% CI, 12.4%-20.1%), associated with an RR of 0.42 (95% CI, 0.19-0.19; I2 = 62.7%) compared with patients on oral anticoagulation without concomitant cytoreduction.26-33 A sensitivity analysis per type of anticoagulation showed that the risk of recurrence was high for both VKA only and DOAC only treated patients: 39 of 106 (36.8%) and 3 of 14 (21.4%). This risk was significantly lower for both VKA and DOAC when they were combined with cytoreduction (17.6% and 7.9%, respectively; Table 3).
Recurrent thrombotic events (arterial or venous) per treatment strategy
| Antithrombotic treatment . | Recurrent events . | Relative risk (95% CI); I2 . | |
|---|---|---|---|
| n/N . | % . | ||
| Antiplatelet therapy only | 36/118 | 30.5 | |
| Antiplatelet + cytoreduction | 84/452 | 18.6 | 0.27 (0.07-1.04); 80.9% |
| Oral anticoagulation (any) | 42/120 | 35.0 | |
| Oral anticoagulation (any) + cytoreduction | 60/376 | 16.0 | 0.42 (0.19-0.92); 62.7% |
| Oral anticoagulation + antiplatelet therapy | 4/16 | 25.0 | |
| Oral anticoagulation + antiplatelet + cytoreduction | 9/37 | 24.3 | 0.60 (0.18-2.01); 36.3% |
| No antithrombotic treatment or cytoreduction | 12/33 | 36.4 | |
| Cytoreduction only | 31/101 | 30.7 | 0.50 (0.37-0.67); 0.0% |
| Recurrent thrombosis per type of oral anticoagulant | |||
| VKA only | 39/106 | 36.8 | |
| VKA + cytoreduction | 55/313 | 17.6 | 0.51 (0.23-1.14); 62.2% |
| DOAC only | 3/14 | 21.4 | |
| DOAC + cytoreduction | 5/63 | 7.9 | 0.21 (0.08-0.60); 16.9% |
| VKA + antiplatelet therapy | 4/11 | 36.4 | |
| VKA + antiplatelet + cytoreduction | 9/37 | 24.3 | 0.43 (0.16-1.15); 0% |
| DOAC + antiplatelet therapy | 0/5 | 0% | — |
| Antithrombotic treatment . | Recurrent events . | Relative risk (95% CI); I2 . | |
|---|---|---|---|
| n/N . | % . | ||
| Antiplatelet therapy only | 36/118 | 30.5 | |
| Antiplatelet + cytoreduction | 84/452 | 18.6 | 0.27 (0.07-1.04); 80.9% |
| Oral anticoagulation (any) | 42/120 | 35.0 | |
| Oral anticoagulation (any) + cytoreduction | 60/376 | 16.0 | 0.42 (0.19-0.92); 62.7% |
| Oral anticoagulation + antiplatelet therapy | 4/16 | 25.0 | |
| Oral anticoagulation + antiplatelet + cytoreduction | 9/37 | 24.3 | 0.60 (0.18-2.01); 36.3% |
| No antithrombotic treatment or cytoreduction | 12/33 | 36.4 | |
| Cytoreduction only | 31/101 | 30.7 | 0.50 (0.37-0.67); 0.0% |
| Recurrent thrombosis per type of oral anticoagulant | |||
| VKA only | 39/106 | 36.8 | |
| VKA + cytoreduction | 55/313 | 17.6 | 0.51 (0.23-1.14); 62.2% |
| DOAC only | 3/14 | 21.4 | |
| DOAC + cytoreduction | 5/63 | 7.9 | 0.21 (0.08-0.60); 16.9% |
| VKA + antiplatelet therapy | 4/11 | 36.4 | |
| VKA + antiplatelet + cytoreduction | 9/37 | 24.3 | 0.43 (0.16-1.15); 0% |
| DOAC + antiplatelet therapy | 0/5 | 0% | — |
The majority of the recurrent events were VTE (61 of 102 thrombotic events); 24 of 42 thrombotic events in patients on oral anticoagulation only, either VKA or DOAC, whereas this was 37 of 60 recurrent events when oral anticoagulation was combined with cytoreduction. Recurrent VTE was diagnosed in 2 of 63 (3.2%; 95% CI, 0.4-11.0) patients treated with DOAC plus cytoreduction and in 35 of 268 (13.1%; 95% CI 9.3-17.7) patients on VKA plus cytoreduction.
Two large cohort studies (387 patients overall) reported incidence rates of recurrent arterial or venous thrombosis on and off VKA therapy. In 181 patients with MPN with splanchnic vein thrombosis the incidence rate of recurrent thrombosis on VKA therapy was 3.9 per 100-patient years compared with 7.2 per 100-patient years off VKA therapy.28 A cohort of 206 patients with MPN with deep vein thrombosis and/or pulmonary embolism reported an incidence rate of recurrent thrombosis of 4.7 per 100-patient years on VKA therapy, as it was 8.9 per 100-patient years off VKA therapy.29
Antiplatelet therapy plus oral anticoagulation with or without cytoreduction
Five studies reported on 53 patients receiving the combination of antiplatelet therapy and oral anticoagulation, with or without cytoreduction.26-29,31 In 9 of 37 (24.3%; 95% CI, 11.8-41.2) patients receiving antiplatelet therapy plus oral anticoagulation and cytoreduction, a recurrent arterial or venous thrombotic even was diagnosed, similar to 4 of 16 patients without additional cytoreduction (25.0%; 95% CI, 7.3-52.4). Approximately half (6 of 13) of these recurrent events were of venous origin.
Cytoreduction only or no antithrombotic/cytoreductive treatment
Six studies included 134 patients treated with cytoreduction only or patients with neither antithrombotic treatment nor cytoreductive treatment. No difference in recurrence risk was observed in patients receiving cytoreduction only or no treatment at all and the risk was high in both groups: 30.7% and 36.4%, respectively (RR, 0.50; 95% CI, 0.37-0.67; I2 = 0%). Of 43 recurrent thrombotic events overall, only 9 were venous events.24,26-29,32
Bleeding events
In total, 94 bleeding events were reported in the 10 included studies.24-33 Most included studies did not discern major, clinically relevant nonmajor, or minor bleeding events or specification per treatment strategy. The majority of bleeding events occurred on antiplatelet therapy only or in combination with oral anticoagulation (60%), primarily of gastrointestinal origin. In the 77 DOAC-treated patients, 3 major bleeding episodes (all related to interventions), 3 clinically relevant nonmajor episodes, and 2 minor bleeding episodes were observed.28-30,32,33
Overall survival
Mortality rates were reported in 8 of 10 studies (950 patients).24-31 The overall survival rate was high (93%). In 15 of 77 patients (19%), cause of death was directly related to the thrombotic event. Leukemic transformation was reported in 31 patients (3%) and was considered the cause of death in 8 patients (10%).
Discussion
Based on data from 10 observational studies, our systematic review shows a high risk of recurrent thrombotic events in patients with MPN with a history of VTE, even with long-term oral anticoagulant treatment. Treatment regimens involving the combination of oral anticoagulation and cytoreduction report the lowest risk of recurrent VTE, with low risks of recurrent VTE in limited numbers of patients in studies with DOACs. As direct comparisons of different treatment strategies are lacking because of the absence of randomized controlled trials, it is not possible to make suggestions on an optimal antithrombotic regimen.
This systematic review summarizes the currently available evidence on antithrombotic treatment in patients with an MPN and a history of VTE. The available evidence, albeit with very low certainty and moderate risk of bias, suggests that the combination of oral anticoagulation and cytoreduction is associated with the lowest risk of recurrent thrombosis in patients with MPN with a prior VTE, in line with current clinical practice. Although the use of VKA is most common in this population, DOACs may be a reasonable alternative. The limited number of studies on DOAC-treated patients identified in our review showed relatively low risks of recurrent thrombotic and bleeding events, but more data are definitely needed. In addition, prospective studies evaluating DOACs for treatment of unusual site VTE are anticipated and will be important for patients with MPN, as these diseases are a known major risk factor for splanchnic vein thrombosis, whereas DOAC prescription is still off-label in these patients.34-36 Recent guidance from the ISTH Scientific and Standardization Committee already suggests using DOACs for treatment of splanchnic vein thrombosis in noncirrhotic patients, including patients with underlying MPN, if not contraindicated by severe liver dysfunction.37 A combination of oral anticoagulation and antiplatelet therapy has been suggested as an effective long-term treatment option in patients with MPN and splanchnic vein thrombosis,26,27 but based on the available data of this review, there seems insufficient evidence to support this combination as first-line treatment.
The majority of our study population received cytoreductive therapy combined with antithrombotic therapy. Details of cytoreductive treatment, such as the type and indication for cytoreduction were frequently not specified, whereas concomitant phlebotomies were only reported in 3 of 10 studies and not in relation to study outcomes. Hence, the net clinical benefit of cytoreductive therapy remains uncertain, as well as the optimal type of cytoreductive treatment (eg, hydroxyurea, [peg]interferon, or ruxolitinib).
This review has clear limitations. Both the amount and the quality of the available data form serious limitations in this review. Initially only 8 studies met our inclusion criteria. An additional 20 studies were potentially eligible but did not specify thrombotic events during follow-up or per treatment strategy. We requested additional data from several research groups, which enabled us to include 2 additional studies and obtain more data. Several landmark randomized trials on antithrombotic treatment in patients with MPN have been performed and were identified by our search but were judged to be ineligible for inclusion in this review based on our criteria because of missing data on either recurrent thrombosis or treatment strategy. Almost all studies included in this review were highly affected by confounding by indication and selection bias because of their retrospective design, which is also reflected in the overall moderate to severe risk of bias of the studies. Although the use of risk of bias tools is disputed in observational studies, we chose to address the study quality using the ROBINS-I tool.
Study outcomes were not consistently reported per treatment and whether with or without cytoreductive therapy, and individual patient data were hardly available. Moreover, we did not intend to perform an individual patient data meta-analysis but on an analysis on study level. This hampered any possibilities of performing a multivariable analysis to adjust for confounding factors in our primary outcome analysis. Planned subgroup analyses based on mutation status or site of VTE were also not possible as sufficient data for meaningful analyses could not be retrieved from the original studies. Therefore, we cannot make any conclusive statements regarding differences in outcomes or strategies for patients with a history of splanchnic vein thrombosis or other types of VTE. We report the proportion of patients with a recurrent thrombotic event, specifically recurrent VTE, but the collected data does not allow exploring the association between recurrence and disease duration, timing of recurrent event, or its relation to treatment duration. Predefined subgroup analyses evaluating outcomes in patients with VTE diagnosed before MPN diagnosis or at or after MPN diagnosis were not possible because of lack of individual patient data. Ideally, for comparative purposes, we would have presented incidence rates per antithrombotic treatment strategy. However, we were unable to do so given the available data. Additionally, with the exception of 1 included case series,31 we were unable to evaluate changes in antithrombotic treatment during study follow-up. This is of importance, as not only the risk of recurrent thrombosis but also bleeding complications may require a change in antithrombotic therapy. Certainty of evidence was further affected by imprecision, inconsistency in results and significant heterogeneity. The comprehensive search strategy, with well-defined inclusion and exclusion criteria and accurate methods, however, are the strengths of this study.
The fact that we only identified 10 studies matching our inclusion criteria implicates a lack of evidence and high-quality studies investigating this important clinical issue. The great variety of treatment strategies and heterogeneity in the studied patients also reflect the clinical variety of patients with MPN and exhibit possible difficulties that can be encountered when conducting research in this population. Prospective studies evaluating antithrombotic treatment of secondary prevention of MPN-related VTE are much needed. A direct comparison between different antithrombotic strategies in this population is lacking and ideally should be evaluated in an adequately powered randomized clinical trial in a clearly defined study population. Such a trial could provide urgently needed data on the safety and efficacy of the different anticoagulants, for instance VKA vs DOAC, in MPN patients. Additionally, it could contribute to clinical decision making regarding the net clinical benefit of adding antiplatelet or cytoreductive therapy to anticoagulation for prevention of recurrent thrombosis.
In summary, evidence on the optimal long-term antithrombotic treatment of VTE in patients with MPN is based on observational studies only, at high risk of bias and with imprecise findings. This systematic review reports a high risk of recurrent thrombosis in patients with MPN with a history of VTE despite anticoagulant treatment. Our data suggest that antithrombotic treatment using a combination of oral anticoagulation and cytoreduction may provide the lowest risk of recurrent thrombosis albeit with very little precision because of limited data and an analysis on study level only. The role of cytoreduction in the different subtypes of patients with VTE in MPN remains to be determined. Adequately powered randomized clinical trials are needed for proper assessment of the optimal antithrombotic treatment strategy in patients with an MPN and a history of VTE.
For data, please contact the corresponding author at e.n.hamulyak@amsterdamumc.nl.
Acknowledgments
The authors thank the following investigators for providing additional data or clarifications: J. Hoekstra (Department of Gastroenterology and Hepatology, Erasmus University Medical Center Rotterdam, Rotterdam, The Netherlands), V. De Stefano (Institute of Hematology, Catholic University, Rome, Italy), P.E. Westerweel (Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands), K. Wille (University Clinic for Haematology, Oncology, Hemostaseology and Palliative Care, Johannes Wesling Medical Center Minden, University of Bochum, Minden, Germany), R. Landolfi (Fondazione Policlinico Universitario A. Gemelli IRCCS, U.O.C. Clinica Medica e Malattie Vascolari, Rome, Italy), A. Vannucchi and G. Coltro (Haematology Unit and Centro di Ricerca e Innovazione per le Malattie Mieloproliferative (CRIMM), Azienda Ospedaliero-Universitaria Careggi, Florence, Italy), J. Hernandez-Boluda (Department of Hematology, Hospital Clínico Universitario-INCLIVA, University of Valencia, Valencia, Spain), S. Koschmieder (Department of Hematology, Oncology, Hemostaseology, and Stem Cell Transplantation, Faculty of Medicine, RWTH Aachen University, Aachen, Germany; Germany Study Group MPN (GSG-MPN), Lüneburg, Germany), S. Parasuraman (Incyte Corporation, Wilmington, DE) and C. Harrison (Department of Hematology, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom).
Authorship
Contribution: E.N.H., J.G.D., F.W.G.L., P.A.W.t.B., S.M., and M.N.L. developed the methods for the systematic review and analyses; J.G.D. conducted the systematic search; E.N.H. and M.N.L. participated in the review and selection of included studies and performed independent data extraction and quality assessment and did the analyses; S.M. was third reviewer for quality assessment; E.N.H. and M.N.L. drafted the manuscript; and all authors reviewed drafts and approved the final draft of the manuscript.
Conflict-of-interest disclosure: F.W.G.L. received research support from CSL Behring, Shire/Takeda, uniQure, and Sobi, not related to this study; is a consultant for uniQure, Novo Nordisk, and Shire/Takeda, of which the fees go to the institution; has received a travel grant from Sobi; and is also a Data Safety Monitoring Board member for a study by Roche. B.J.B. received research grants from Sanquin, Novartis, and GBT and joined advisory boards of Novartis, Celgene, Amgen, GBT, and CSL Behring. P.A.W.t.B. joined advisory boards of Novartis and Celgene. S.M. reports grants and fees paid to her institution from GSK, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. M.N.L. reports grants and fees paid to her institution from GSK, BMS/Pfizer, Bayer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Eva N. Hamulyák, Department of Vascular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam Cardiovascular Sciences, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: e.n.hamulyak@amsterdamumc.nl.
References
Author notes
The full-text version of this article contains a data supplement.