Key Points
CWP291, the active product from CWP232291, is a first-in-class drug that inhibits the Wnt pathway to cause apoptosis in cancer cells.
In a phase 1 study, CWP232291 was safe and demonstrated single-agent activity in AML patients, with plans for future combination studies.
Abstract
CWP232291 (CWP291) is a small-molecule inhibitor of Wnt signaling that causes degradation of β-catenin via apoptosis induction through endoplasmic reticulum stress activation. This first-in-human, open-label, dose-escalation study to evaluate the safety, maximum tolerated dose (MTD), and preliminary efficacy of CWP291 enrolled 69 patients with hematologic malignancies (acute myeloid leukemia [AML], n = 64; myelodysplastic syndrome, n = 5) in 15 dose-escalation cohorts of 4 to 334 mg/m2 using a modified 3+3 design and 1 dose-expansion cohort. CWP291 was administered IV daily for 7 days every 21 days. The most common treatment-emergent adverse events (TEAEs) were nausea (n = 44, 64%), vomiting (n = 32, 46%), diarrhea (n = 25, 36%), and infusion-related reactions (n = 20, 29%). Grade ≥3 TEAEs in >3 patients (5%) were pneumonia (n = 8, 12%); hypophosphatemia (n = 6, 8%); leukocytosis, nausea, cellulitis, sepsis, and hypokalemia (n = 5 each, 7% each); and hypertension (n = 4, 6%). Dose-limiting toxicities included nausea (n = 3) and abdominal pain, anaphylactic reaction, myalgia, and rash (n = 1, each); the MTD was defined at 257 mg/m2. CWP232204, the active metabolite of CWP291, showed pharmacokinetic linearity on both days 1 and 7, and a terminal half-life of ∼12 hours. Among 54 response-evaluable AML patients, there was one complete response at a dose of 153 mg/m2 and one partial response at 198 mg/m2; bone marrow blast percentage reduced from a median of 58.3% to 3.5% and 15.0% to 4.2%, respectively. Future studies will explore CWP291, with a mechanism of action aimed at eradication of earlier progenitors via Wnt pathway blockade, as combination therapy. This trial was registered at www.clinicaltrials.gov as #NCT01398462.
Introduction
The Wnt signaling pathway plays a critical role in determining the activity and cell fate of hematopoietic stem cells (HSCs) by influencing the balance between homeostasis and regeneration, self-renewal and pluripotency, and differentiation and proliferation.1 The nuclear localization of nonphosphorylated and active β-catenin molecules is a measure of Wnt pathway activation.2 In patients with acute myeloid leukemia (AML),3 aberrant levels of β-catenin are reported in >50% of instances,4,5 a scenario that is associated with poor prognosis.5 Modeling predictions and measurements of β-catenin concentrations suggest that a small fold-change may be sufficient to bring about transcriptional changes.2 Altering Wnt signaling with a target drug presents a potential therapeutic intervention for AML and other clonal HSC disorders.6-8 Furthermore, preclinical studies have shown that blocking Wnt signaling can sensitize leukemia cells to chemotherapy and improve overall survival.9
CWP232291 (CWP291) is a small-molecule inhibitor of the Wnt pathway. In serum, CWP291 is converted to its active form, CWP232204. Molecular studies have shown CWP232204 to induce endoplasmic reticulum (ER) stress, leading to the activation of caspases that reduce the concentration of β-catenin. Ensuing dampening of expression of Wnt target genes culminated in selective cancer cell apoptosis.10 Others have described the unfolded protein response (UPR), an ER stress–induced signaling cascade, as a critical network in the selection, adaptation, and survival of cancer cells.11 The UPR is a conserved adaptive signaling pathway that restores protein homeostasis, primarily in the ER. Recent studies suggest an important function of the UPR in acute leukemias.12
In preclinical studies, CWP291 demonstrated significant antineoplastic activity in cell cultures and animal models, including bone marrow engraftment models with AML and other cell lines.13,14 Pharmacokinetics (PK) data indicated a rapid conversion to CWP232204, which was metabolized and excreted as a glucuronide conjugate form. No serious adverse events (AEs) were detected in animal studies. The no-observed-adverse-effect level (NOAEL) was 2.5 mg/kg (50 mg/m2) in dogs and 10 mg/kg (60 mg/m2) in rats. Protein binding and stability were similar in BALB/c mouse, CS-1 mouse, Sprague Dawley rat, cynomolgus monkey, beagle dog, and human serum samples. Based on preclinical safety, toxicology, and efficacy data, we undertook this first-in-human study of CWP291 to assess the maximum tolerated dose (MTD) in patients with AML and myelodysplastic syndrome (MDS).
The study was conducted in compliance with the ethical principles in the Declaration of Helsinki, the International Conference on Harmonization, and applicable requirements and approvals at participating institutions. Written informed consent was obtained from all participants prior to beginning treatment. This trial was registered at www.clinicaltrials.gov as #NCT01398462.
Methods
Study design
This was a multicenter, phase 1, open-label, single-arm, dose-escalation study to assess the safety and PK profile of CWP291 in patients with AML or MDS. The study was conducted in compliance with ethical principles in the Declaration of Helsinki, the International Conference on Harmonization, and applicable requirements and approvals at participating institutions. Written informed consent was obtained from all participants prior to beginning treatment. This trial was registered at www.clinicaltrials.gov as #NCT01398462. All authors had access to primary clinical trial data, which were analyzed by JW Pharmaceutical.
The primary objective was to determine the MTD of CWP291 under specified dosing schedule in patients with AML or MDS. Secondary objectives were to characterize the safety profile of CWP291, determine the plasma and urine PK profile of CWP291 and CWP232204, assess the preliminary anticancer efficacy of CWP291 in patients with AML or MDS, and explore biomarkers of CWP291 activity.
Patients
Patients were included if they had a pathologically confirmed diagnosis of AML that was relapsed or refractory or if they were not eligible to receive therapy of recognized curative potential. Patients were also included if they had MDS that failed ≥3 cycles of hypomethylating therapy. Other inclusion criteria were age ≥18 years, Eastern Cooperative Oncology Group performance status 0 to 2, ≥2-week interval following any prior cytotoxic agents or ≥5 half-lives for noncytotoxic agents, ≥24-hour interval following any hydroxyurea treatment, recovery of any nonhematological toxicities from prior therapy to grade ≤1, and adequate renal and hepatic function. Exclusion criteria included heart disease, central nervous system disease, uncontrolled intercurrent illness, eligibility for hematopoietic cell transplantation at the time of enrollment, other hematological malignancy treatments, or therapy with anticoagulant or antithrombotic agents within 7 days of enrollment.
Study treatment
Patients were enrolled in a 3+3 dose-escalation scheme using a modified Fibonacci scheme.15 The starting dose of CWP291 was 4 mg/m2 per day, which was half of the starting dose level extrapolated from animal studies. Each treatment cycle was 21 days, with daily IV infusion for 7 consecutive days followed by 2 weeks without study drug. This represents a less dose-dense schedule than that used in preclinical 5-cycle animal toxicity studies, where dosing was 7 days on followed by 7 days off for each cycle. Starting at 198 mg/m2 per day and in subsequent higher-dose cohorts, the duration of IV infusion for all patients was a minimum of 30 minutes; furthermore, all patients received prophylaxis with antihistamines and hydrocortisone from day 1, per institutional practice, to avoid potentially severe allergic reactions. Prophylaxes and doses used in this study were hydrocortisone 10 to 500 mg (mode 25-100 mg), promethazine 12.5 to 25 mg (mode 12.5 mg), chlorpheniramine 2 to 8 mg (mode 4 mg), azelastine 1 mg (n = 3), hydroxyzine 1 to 25 mg (n = 3), levocetirizine 5 mg (n = 2), loratadine 10 mg (n = 2), and bepotastine 10 mg (n = 1).
The MTD was the highest dose level at which <2 of 6 patients developed a dose-limiting toxicity (DLT). Once the MTD was identified, 12 patients were to be enrolled at this dose level to further define safety, with consideration of a dose fallback by 2 mg/m2 per day to determine a new MTD if necessary. DLTs were assessed during cycle 1 and included any clinically significant AE considered unrelated to underlying disease, comorbidities, or concomitant medications. DLTs excluded grade 3 nausea, vomiting, or diarrhea, which did not last >2 days and did not require hospitalization, total parenteral nutrition, or tube feeding. Any AE during cycle 1 requiring dose modification was considered a DLT. Patients with grade 2-3 non-hematologic AEs had their study drug withheld and dosing resumed at the next lower dose level when their AEs resolved to grade 1 or baseline. Study treatment was continued until disease progression or unacceptable AE’s, investigator decision, or patient withdrawal from study.
Safety assessments
All patients who received ≥1 dose or any partial dose of CWP291 were evaluable for toxicity from the time of their first dose. Clinical AEs and laboratory abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. As the Wnt signaling pathway and β-catenin may have an important role in regulating the biological function of osteoblasts and osteoclasts,16 measurement of bone turnover was done to assess the effect of Wnt signaling pathway inhibition on this function. This assessment was performed in a central laboratory by measuring bone-specific alkaline phosphatase in serum samples collected on protocol-specified days.
PK assessments
PK studies were performed in all patients in the safety population who received any amount of CWP291. Plasma samples were collected on days 1 and 7 of cycle 1 at time 0 (predose); 0.25, 0.5, 1, 1.5, 2, 4, 8, and 24 hours after dosing; and at 48 and 72 hours after the last dose (days 9 and 10). Urine samples were collected on days 1 and 7 after infusion at 0 to 2, 2 to 8, and 8 to 24 hours and 48 and 72 hours after the last dose (ie, days 9 and 10). CWP291 and CWP232204 concentrations were determined using a validated liquid chromatography tandem mass spectrometry assay (QPS, Newark, DE). PK parameters of CWP291 and CWP232204 were derived by standard noncompartmental PK approaches using Phoenix WinNonlin Version 6.1 (Pharsight Corporation, a Certara Company, Mountain View, CA).
Efficacy assessment
All patients who received at least 5 of the 7 planned doses in the first cycle and had at least 1 evaluation of response were evaluable for response assessment (efficacy population). Response was assessed according to the International Working Groups recommendations for AML17 and MDS.18 Complete blood counts were obtained at baseline and at least weekly. Bone marrow biopsy/aspiration assessments were at baseline, on day 22, and at the investigator’s discretion.
Pharmacodynamics assessment
Pharmacodynamics assessment included all patients who received ≥1 dose of CWP291, had a baseline assessment, and had ≥1 postbaseline assessment for biomarkers. Whole-blood samples were collected at the same time points as plasma samples in cycle 1 and at predose on days 3, 4, 5, 6, 15, and 22. In subsequent cycles, whole-blood samples were collected at predose on days 1, 3, 4, 5, 6, 7, and 15. Bone marrow samples, collected for efficacy assessment, were also assayed for survivin. Survivin was assayed by enzyme-linked immunosorbent assay (QPS) or polymerase chain reaction (AltheaDx, San Diego, CA).
Statistical analyses
This study was planned to enroll 48 to 68 patients. This sample size was dependent on the number of cohorts required until determination of MTD and was not determined by statistical power considerations. Additional patients could be enrolled to replace unevaluable patients in cases of discontinuations unrelated to treatment-emergent AEs (TEAEs). Appropriate descriptive statistics were to be reported. Statistical analyses were performed using SAS version 9.4.
Results
Baseline patient characteristics
Sixty-nine patients were enrolled in the study (Table 1), 5 with MDS and 64 with AML (Table 2). The median age was 64 years (range, 25-81 years). Prior therapies ranged from 1 to 14 for AML patients and from 1 to 2 for MDS patients (Table 2). All MDS patients had received ≥4 cycles of hypomethylating agents.
CWP291 dose cohorts evaluated in this study
| Cohort . | Number of patients . | CWP291 dose, mg/m2 . | Increment, % . |
|---|---|---|---|
| Dose escalation | |||
| 1 | 4 | 4 | — |
| 2 | 3 | 8 | 100 |
| 3 | 3 | 13 | 67 |
| 4 | 3 | 19 | 50 |
| 5 | 3 | 26 | 40 |
| 6 | 5 | 33 | 30 |
| 7 | 3 | 42 | 30 |
| 8 | 6 | 54 | 30 |
| 9 | 3 | 70 | 30 |
| 10 | 4 | 91 | 30 |
| 11 | 4 | 118 | 30 |
| 12 | 6 | 153 | 30 |
| 13 | 6 | 198 | 30 |
| 14 | 6 | 257 | 30 |
| 15 | 4 | 334 | 30 |
| Dose expansion | |||
| 14.1 | 6 | 257 | — |
| Cohort . | Number of patients . | CWP291 dose, mg/m2 . | Increment, % . |
|---|---|---|---|
| Dose escalation | |||
| 1 | 4 | 4 | — |
| 2 | 3 | 8 | 100 |
| 3 | 3 | 13 | 67 |
| 4 | 3 | 19 | 50 |
| 5 | 3 | 26 | 40 |
| 6 | 5 | 33 | 30 |
| 7 | 3 | 42 | 30 |
| 8 | 6 | 54 | 30 |
| 9 | 3 | 70 | 30 |
| 10 | 4 | 91 | 30 |
| 11 | 4 | 118 | 30 |
| 12 | 6 | 153 | 30 |
| 13 | 6 | 198 | 30 |
| 14 | 6 | 257 | 30 |
| 15 | 4 | 334 | 30 |
| Dose expansion | |||
| 14.1 | 6 | 257 | — |
The study drug was infused over a 5-minute duration for cohorts 1 to 13 and over at least a 30-minute duration for cohorts 14 and 15. For dose-expansion cohort 14.1, the study drug was infused over a 2-hour duration. Patients in cohort 13 onward received prophylaxis with antihistamines and hydrocortisone from day 1, per institutional practice, to avoid potentially severe allergic reactions.
Baseline characteristics
| Safety population . | Value (N = 69) . |
|---|---|
| Age, median (range), y | 64 (25-81) |
| Female sex, n (%) | 32 (46) |
| Race, n (%) | |
| American Indian or Alaska Native | 2 (3) |
| Asian | 29 (42) |
| Black or African American | 3 (4) |
| White | 35 (51) |
| Height, median (range), cm | 165 (142-183) |
| Weight, median (range), kg | 67 (44-109) |
| Body surface area, median (range), m2 | 1.76 (1.4-2.3) |
| Current indication, n (%) | |
| AML | 64 (93) |
| Hypomethylating agents relapse/refractory | 19 (30) |
| Intensive chemotherapy relapsed/refractory | 45 (70) |
| MDS | 5 (7) |
| ECOG performance status, n (%) | |
| 0 | 2 (3) |
| 1 | 54 (78) |
| 2 | 13 (19) |
| Prior treatments, median (range) | |
| AML | 4 (1-14) |
| MDS | 1 (1-2) |
| Safety population . | Value (N = 69) . |
|---|---|
| Age, median (range), y | 64 (25-81) |
| Female sex, n (%) | 32 (46) |
| Race, n (%) | |
| American Indian or Alaska Native | 2 (3) |
| Asian | 29 (42) |
| Black or African American | 3 (4) |
| White | 35 (51) |
| Height, median (range), cm | 165 (142-183) |
| Weight, median (range), kg | 67 (44-109) |
| Body surface area, median (range), m2 | 1.76 (1.4-2.3) |
| Current indication, n (%) | |
| AML | 64 (93) |
| Hypomethylating agents relapse/refractory | 19 (30) |
| Intensive chemotherapy relapsed/refractory | 45 (70) |
| MDS | 5 (7) |
| ECOG performance status, n (%) | |
| 0 | 2 (3) |
| 1 | 54 (78) |
| 2 | 13 (19) |
| Prior treatments, median (range) | |
| AML | 4 (1-14) |
| MDS | 1 (1-2) |
ECOG, Eastern Cooperative Oncology Group.
Safety
All 69 enrolled patients received ≥1 dose of CWP291 and constituted the safety population. Fifty-five (80%) patients had AEs that were considered by the investigator to be related to the study drug. The most commonly reported TEAEs were nausea, vomiting, diarrhea, and infusion-related reaction in 44 (64%), 32 (46%), 25 (36%), and 20 (29%) patients, respectively (Table 3). Grade ≥3 TEAEs in >3 patients (5%) included pneumonia (n = 8, 12%); hypophosphatemia (n = 6, 8%); leukocytosis, nausea, cellulitis, sepsis, and hypokalemia (n = 5, 7% each); and hypertension (n = 4, 6%). Serious AEs, regardless of relationship to study drug, were pneumonia (n = 7, 10%); pyrexia and cellulitis (n = 5, 7% each); sepsis (n = 4, 6%); respiratory failure (n = 3, 4%); and febrile neutropenia, nausea, multiorgan failure, and urinary tract infection (n = 2, 3% each).
TEAEs for conditions with more than 15 events in the total population
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 14.1 . | 15 . | All . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients reporting ≥1 TEAE | 4 (100) [20] | 3 (100) [42] | 3 (100) [41] | 3 (100) [32] | 3 (100) [32] | 5 (100) [91] | 3 (100) [52] | 6 (100) [85] | 3 (100) [26] | 4 (100) [53] | 4 (100) [22] | 6 (100) [109] | 6 (100) [183] | 6 (100) [153] | 6 (100) [158] | 4 (100) [58] | 69 (100) [1157] |
| Nausea | |||||||||||||||||
| All grades | 0 | 3 (100) [3] | 2 (67) [2] | 0 | 1 (33)[3] | 4 (80) [7] | 3 (100) [4] | 4 (67) [11] | 1 (33) [1] | 4 (100) [6] | 1 (25) [1] | 4 (67) [9] | 4 (67) [11] | 5 (83) [12] | 5 (83) [14] | 3 (75) [3] | 44 (64) [87] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 2 (50) [2] | 5 (7) [5] |
| Vomiting | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 0 | 0 | 0 | 2 (40) [4] | 1 (33) [1] | 4 (67) [7] | 1 (33) [1] | 4 (100) [6] | 0 | 4 (67) [8] | 3 (50) [10] | 5 (83) [15] | 4 (67) [12] | 3 (75) [3] | 32 (46) [68] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 2 (3) [2] |
| Diarrhea | |||||||||||||||||
| All grades | 2 (50) [2] | 2 (67) [2] | 1 (33) [1] | 1 (33) [2] | 1 (33) [1] | 0 | 1 (33) [3] | 2 (33) [3] | 3 (100) [6] | 2 (50) [4] | 0 | 2 (33) [3] | 3 (50) [6] | 1 (17) [4] | 3 (50) [7] | 1 (25) [1] | 25 (36) [45] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infusion-related reaction | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 0 | 2 (67) [2] | 1 (20) [2] | 1 (33) [1] | 4 (67) [8] | 2 (67) [5] | 1 (25) [4] | 1 (25) [1] | 4 (67) [8] | 3 (50) [4] | 0 | 0 | 0 | 20 (29) [36] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 0 | 0 | 0 | 1(20) [1] | 0 | 0 | 0 | 0 | 1 (25) [2] | 1 (17) [1] | 2 (33) [7] | 4 (67) [12] | 4 (67) [5] | 3 (75) [4] | 17 (25) [33] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 1 (1) [1] |
| Decreased appetite | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 1 (33) [1] | 0 | 0 | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 0 | 1 (25) [1] | 3 (50) [8] | 3 (50) [6] | 2 (33) [8] | 2 (33) [3] | 0 | 15 (22) [30] |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | |||||||||||||||||
| All grades | 0 | 2 (67) [2] | 0 | 0 | 1 (33) [1] | 2 (40) [5] | 2 (67) [3] | 4 (67) [10] | 0 | 1 (25) [1] | 0 | 0 | 3 (50) [4] | 0 | 2 (33) [3] | 1 (25) [1] | 18 (26) [30] |
| Grade ≥3 | 0 | 1 (33) [1] | 0 | 0 | 0 | 1 (20) [2] | 1 (33) [1] | 1 (17) [3] | 0 | 0 | 0 | 0 | 1 (17) [2] | 0 | 0 | 0 | 5 (7) [10] |
| Pyrexia | |||||||||||||||||
| All grades | 0 | 1 (33) [2] | 2 (67) [3] | 1 (33) [3] | 0 | 1 (20) [1] | 0 | 0 | 1 (33) [1] | 1 (25) [1] | 1 (25) [1] | 3 (50) [4] | 2 (33) [3] | 2 (33) [3] | 2 (33) [5] | 0 | 17 (25) [27] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 2 (3) [2] |
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 2 (67) [3] | 3 (60) [8] | 0 | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [2] | 0 | 2 (33) [6] | 1 (25) [1] | 11 (16) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asthenia | |||||||||||||||||
| All grades | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 1 (17) [1] | 4 (67) [7] | 3 (50) [6] | 4 (67) [5] | 1 (25) [1] | 15 (22) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (1) [1] |
| Chills | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 0 | 0 | 1 (20) [1] | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 2 (33) [2] | 0 | 0 | 2 (33) [16] | 0 | 8 (12) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | |||||||||||||||||
| All grades | 1 (25) [1] | 1 (33) [2] | 1 (33) [1] | 1 (33) [1] | 0 | 0 | 0 | 2 (33) [2] | 0 | 0 | 1 (25) [1] | 0 | 2 (33) [2] | 2 (33) [3] | 3 (50) [7] | 0 | 14 (20) [20] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 1 (25) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 3 (4) [3] |
| Dizziness | |||||||||||||||||
| All grades | 3 (75) [3] | 1 (33) [1] | 0 | 1 (33) [1] | 1 (33) [1] | 0 | 2 (67) [2] | 3 (50) [5] | 1 (33) [1] | 0 | 0 | 0 | 2 (33) [2] | 2 (33) [2] | 1 (17) [1] | 0 | 17 (25) [19] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | |||||||||||||||||
| All grades | 2 (50) [2] | 3 (100) [3] | 2 (67) [2] | 1 (33) [1] | 0 | 0 | 1 (33) [2] | 2 (33) [2] | 2 (67) [2] | 0 | 0 | 2 (33) [2] | 0 | 0 | 1 (17) [1] | 0 | 16 (23) [17] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) [1] |
| Hypophosphatemia | |||||||||||||||||
| All grades | 0 | 0 | 0 | 0 | 0 | 3 (60) [3] | 1 (33) [1] | 2 (33) [4] | 0 | 0 | 1 (25) [3] | 1 (17) [1] | 2 (33) [4] | 1 (17) [1] | 0 | 0 | 11 (16) [17] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 1 (20) [1] | 1 (33) [1] | 0 | 0 | 0 | 1 (25) [1] | 1 (17) [1] | 2 (33) [2] | 0 | 0 | 0 | 6 (8) [7] |
| Constipation | |||||||||||||||||
| All grades | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 2 (33) [2] | 0 | 1 (25) [2] | 0 | 1 (17) [1] | 3 (50) [4] | 1 (17) [1] | 2 (33) [2] | 1 (25) [1] | 13 (19) [15] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 1 (1) [1] |
| Dyspnea | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 1 (33) [1] | 1 (33) [1] | 1 (20) [1] | 2 (67) [2] | 0 | 0 | 0 | 0 | 1 (17) [2] | 2 (33) [2] | 1 (17) [1] | 3 (50) [3] | 1 (25) [1] | 14 (20) [15] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (67) [2] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) [2] |
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 14.1 . | 15 . | All . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients reporting ≥1 TEAE | 4 (100) [20] | 3 (100) [42] | 3 (100) [41] | 3 (100) [32] | 3 (100) [32] | 5 (100) [91] | 3 (100) [52] | 6 (100) [85] | 3 (100) [26] | 4 (100) [53] | 4 (100) [22] | 6 (100) [109] | 6 (100) [183] | 6 (100) [153] | 6 (100) [158] | 4 (100) [58] | 69 (100) [1157] |
| Nausea | |||||||||||||||||
| All grades | 0 | 3 (100) [3] | 2 (67) [2] | 0 | 1 (33)[3] | 4 (80) [7] | 3 (100) [4] | 4 (67) [11] | 1 (33) [1] | 4 (100) [6] | 1 (25) [1] | 4 (67) [9] | 4 (67) [11] | 5 (83) [12] | 5 (83) [14] | 3 (75) [3] | 44 (64) [87] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 2 (50) [2] | 5 (7) [5] |
| Vomiting | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 0 | 0 | 0 | 2 (40) [4] | 1 (33) [1] | 4 (67) [7] | 1 (33) [1] | 4 (100) [6] | 0 | 4 (67) [8] | 3 (50) [10] | 5 (83) [15] | 4 (67) [12] | 3 (75) [3] | 32 (46) [68] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 2 (3) [2] |
| Diarrhea | |||||||||||||||||
| All grades | 2 (50) [2] | 2 (67) [2] | 1 (33) [1] | 1 (33) [2] | 1 (33) [1] | 0 | 1 (33) [3] | 2 (33) [3] | 3 (100) [6] | 2 (50) [4] | 0 | 2 (33) [3] | 3 (50) [6] | 1 (17) [4] | 3 (50) [7] | 1 (25) [1] | 25 (36) [45] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infusion-related reaction | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 0 | 2 (67) [2] | 1 (20) [2] | 1 (33) [1] | 4 (67) [8] | 2 (67) [5] | 1 (25) [4] | 1 (25) [1] | 4 (67) [8] | 3 (50) [4] | 0 | 0 | 0 | 20 (29) [36] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 0 | 0 | 0 | 1(20) [1] | 0 | 0 | 0 | 0 | 1 (25) [2] | 1 (17) [1] | 2 (33) [7] | 4 (67) [12] | 4 (67) [5] | 3 (75) [4] | 17 (25) [33] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 1 (1) [1] |
| Decreased appetite | |||||||||||||||||
| All grades | 0 | 1 (33) [1] | 1 (33) [1] | 0 | 0 | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 0 | 1 (25) [1] | 3 (50) [8] | 3 (50) [6] | 2 (33) [8] | 2 (33) [3] | 0 | 15 (22) [30] |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | |||||||||||||||||
| All grades | 0 | 2 (67) [2] | 0 | 0 | 1 (33) [1] | 2 (40) [5] | 2 (67) [3] | 4 (67) [10] | 0 | 1 (25) [1] | 0 | 0 | 3 (50) [4] | 0 | 2 (33) [3] | 1 (25) [1] | 18 (26) [30] |
| Grade ≥3 | 0 | 1 (33) [1] | 0 | 0 | 0 | 1 (20) [2] | 1 (33) [1] | 1 (17) [3] | 0 | 0 | 0 | 0 | 1 (17) [2] | 0 | 0 | 0 | 5 (7) [10] |
| Pyrexia | |||||||||||||||||
| All grades | 0 | 1 (33) [2] | 2 (67) [3] | 1 (33) [3] | 0 | 1 (20) [1] | 0 | 0 | 1 (33) [1] | 1 (25) [1] | 1 (25) [1] | 3 (50) [4] | 2 (33) [3] | 2 (33) [3] | 2 (33) [5] | 0 | 17 (25) [27] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 2 (3) [2] |
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 2 (67) [3] | 3 (60) [8] | 0 | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [2] | 0 | 2 (33) [6] | 1 (25) [1] | 11 (16) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asthenia | |||||||||||||||||
| All grades | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 0 | 1 (17) [1] | 4 (67) [7] | 3 (50) [6] | 4 (67) [5] | 1 (25) [1] | 15 (22) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (1) [1] |
| Chills | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 0 | 0 | 1 (20) [1] | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 2 (33) [2] | 0 | 0 | 2 (33) [16] | 0 | 8 (12) [22] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | |||||||||||||||||
| All grades | 1 (25) [1] | 1 (33) [2] | 1 (33) [1] | 1 (33) [1] | 0 | 0 | 0 | 2 (33) [2] | 0 | 0 | 1 (25) [1] | 0 | 2 (33) [2] | 2 (33) [3] | 3 (50) [7] | 0 | 14 (20) [20] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 1 (25) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 3 (4) [3] |
| Dizziness | |||||||||||||||||
| All grades | 3 (75) [3] | 1 (33) [1] | 0 | 1 (33) [1] | 1 (33) [1] | 0 | 2 (67) [2] | 3 (50) [5] | 1 (33) [1] | 0 | 0 | 0 | 2 (33) [2] | 2 (33) [2] | 1 (17) [1] | 0 | 17 (25) [19] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | |||||||||||||||||
| All grades | 2 (50) [2] | 3 (100) [3] | 2 (67) [2] | 1 (33) [1] | 0 | 0 | 1 (33) [2] | 2 (33) [2] | 2 (67) [2] | 0 | 0 | 2 (33) [2] | 0 | 0 | 1 (17) [1] | 0 | 16 (23) [17] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) [1] |
| Hypophosphatemia | |||||||||||||||||
| All grades | 0 | 0 | 0 | 0 | 0 | 3 (60) [3] | 1 (33) [1] | 2 (33) [4] | 0 | 0 | 1 (25) [3] | 1 (17) [1] | 2 (33) [4] | 1 (17) [1] | 0 | 0 | 11 (16) [17] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 1 (20) [1] | 1 (33) [1] | 0 | 0 | 0 | 1 (25) [1] | 1 (17) [1] | 2 (33) [2] | 0 | 0 | 0 | 6 (8) [7] |
| Constipation | |||||||||||||||||
| All grades | 0 | 0 | 0 | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 2 (33) [2] | 0 | 1 (25) [2] | 0 | 1 (17) [1] | 3 (50) [4] | 1 (17) [1] | 2 (33) [2] | 1 (25) [1] | 13 (19) [15] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 1 (1) [1] |
| Dyspnea | |||||||||||||||||
| All grades | 0 | 0 | 1 (33) [1] | 1 (33) [1] | 1 (33) [1] | 1 (20) [1] | 2 (67) [2] | 0 | 0 | 0 | 0 | 1 (17) [2] | 2 (33) [2] | 1 (17) [1] | 3 (50) [3] | 1 (25) [1] | 14 (20) [15] |
| Grade ≥3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (67) [2] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) [2] |
Data are presented as number of patients reporting TEAEs (percentage of patients reporting TEAEs) [number of TEAEs].
Six patients (9%) experienced a total of 7 DLTs (supplemental Table 1). The most common DLT was nausea, reported by 3 patients, 1 each at dose levels of 54, 257 (2-hour infusion), and 334 mg/m2. Other DLTs occurring in 1 patient each were abdominal pain (334 mg/m2), anaphylactic reaction (198 mg/m2), myalgia (334 mg/m2), and rash (153 mg/m2). The MTD was determined to be 257 mg/m2. Six patients were dosed at the MTD with an infusion time of 30 minutes; 6 additional patients were enrolled in the expansion cohort at the MTD with an infusion time of 2 hours to mitigate infusion reactions.
There were 36 cases of infusion-related reactions (Table 3) with mean duration of 6.6 days. Infusion-related reactions reported as transient paresthesia (tingling and burning sensations) radiating from the perineum were clustered in the mid dose range (26-198 mg/m2). Myalgia, which was more frequent, severe, and broader in distribution (back, legs, or whole body), was determined by PK analyses to be correlated with the dose level. In view of the frequency distributions by dose level, it is postulated that these 2 AEs, with onset during or immediately following dosing, may represent different presentations of a drug-induced sensory neuropathy. However, this acute-onset toxicity was managed effectively with analgesia, antihistamines, and/or corticosteroids, as necessary, and resolved completely with no sequelae in all cases. In the expansion cohort, the duration of infusion was lengthened from 30 minutes to 2 hours; while not conclusive, it appeared that the slower drug administration may have partly mitigated myalgia symptoms at the MTD.
Drug-related AEs that were severe or serious were few and mostly observed at the higher dose levels (Table 4). Of note, there was a solitary instance of anaphylactic reaction in a patient at 198 mg/m2. This event manifested as chest discomfort during the first infusion, which progressed to tachyarrhythmia, tachypnea, and hypoxemia associated with other constitutional symptoms; however, the patient responded well to supportive care. He was rechallenged 4 days later with a reduced dose of CWP291 (157 mg/m2) infused over 30 minutes (vs 5 minutes during prior administration), with antihistamine and corticosteroid premedication; no recurrence of the anaphylactic reaction was experienced. However, the fifth dose of CWP291 was associated with further chest discomfort, arrhythmias, and hypoxemia. These allergic reactions also resolved satisfactorily with supportive care, but the patient was withdrawn from the study due to this DLT. As a result of this case, all further patients were required to receive prophylaxis with hydrocortisone and antihistamines to reduce the risk of severe allergic reactions. No further allergic reactions were reported.
Treatment-emergent serious AEs
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 14.1 . | 15 . | All . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (17) [1] | 2 (33) [2] | 1 (25) [1] | 7 (10) [7] |
| Pyrexia | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 5 (7) [5] |
| Cellulitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (33) [2] | 1 (17) [1] | 1 (17) [1] | 1 (25) [2] | 5 (7) [6] |
| Sepsis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50) [3] | 1 (25) [1] | 0 | 1 (17) [1] | 0 | 0 | 0 | 4 (6) [5] |
| Respiratory failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 3 (4) [3] |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (17) [1] | 0 | 2 (3) [2] |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 2 (3) [2] |
| Multiorgan failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 2 (3) [2] |
| Urinary tract infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 2 (3) [2] |
| Cohort . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 14.1 . | 15 . | All . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) [1] | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (17) [1] | 2 (33) [2] | 1 (25) [1] | 7 (10) [7] |
| Pyrexia | 0 | 1 (33) [1] | 0 | 1 (33) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 5 (7) [5] |
| Cellulitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (33) [2] | 1 (17) [1] | 1 (17) [1] | 1 (25) [2] | 5 (7) [6] |
| Sepsis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50) [3] | 1 (25) [1] | 0 | 1 (17) [1] | 0 | 0 | 0 | 4 (6) [5] |
| Respiratory failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 0 | 3 (4) [3] |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 1 (17) [1] | 0 | 2 (3) [2] |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) [1] | 2 (3) [2] |
| Multiorgan failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 1 (17) [1] | 0 | 2 (3) [2] |
| Urinary tract infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 1 (17) [1] | 0 | 0 | 0 | 0 | 2 (3) [2] |
Data are reported as number of patients reporting TEAEs (percentage of patients reporting TEAEs) [number of TEAEs].
The median number of cycles completed was 1 (range, 1-7). The most common reason for withdrawing from the study was disease progression (n = 38, 55%). Five (7.2%) patients withdrew because of AEs that included nausea in 2 patients, myalgia and abdominal pain in 1 patient, and pneumonia and allergic reaction in 1 patient each. Four (5.8%) patients had a dose decrease; 3 were considered DLTs, and 1 was an AE (cerebral hemorrhage for a patient who sustained a head injury when he fell out of bed while asleep). Dose reductions were successful in resolving the DLTs of anaphylactic reaction, nausea, and rash (1 each in 3 patients). Two patients (2.9%) had a dose increase as allowed per protocol.
Eleven patients died during the study period; none of the deaths were considered to be related to the study drug. Four deaths were due to serious AEs, including pneumonia (n = 2) and sepsis (n = 2). Six patients died due to disease progression, and 1 died of an unknown cause.
PK
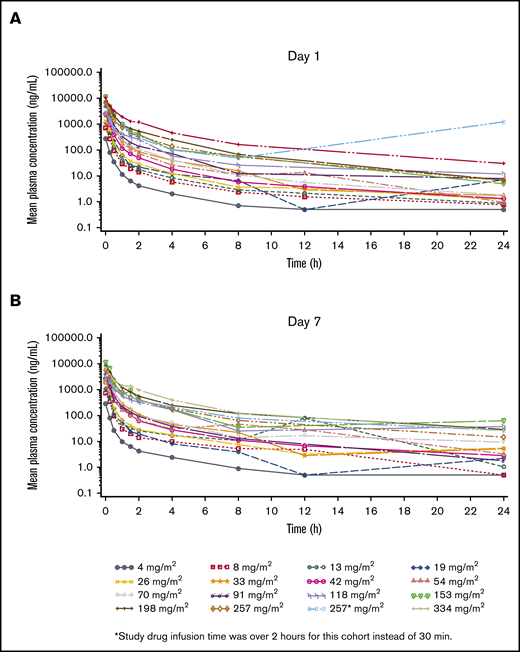
All 69 patients in this study were evaluable for PK. CWP291 disappeared rapidly from the plasma after IV administration; concentrations were universally lower for CWP291 than for its key metabolite, CWP232204. Plasma concentrations of CWP232204 decreased in a multi-exponential manner following administration (Figure 1; supplemental Table 2), and showed PK linearity (dose proportionality) on both days 1 and 7 for area under the curve, whereas maximum plasma concentration (Cmax) increased in a slightly less than dose-proportional manner. The terminal half-life (t1/2) of CWP232204 when dosed with CWP291 at 257 mg/m2 was ∼12 hours. A modest amount of accumulation was seen after 7 days of dosing; the median area under the curve was 22% higher on day 7 relative to day 1, although Cmax was only 6% higher on day 7. Urinary CWP232204 excretion was low, ranging from <1% to 5% of the administered dose in the 6 patients with sufficient data for analysis.
Profile of CWP232204 over time in patients. PK of CWP232204 on day 1 (A) and steady state (day 7) (B).
Profile of CWP232204 over time in patients. PK of CWP232204 on day 1 (A) and steady state (day 7) (B).
Efficacy
In the intent-to-treat population, a complete response (CR) for AML was achieved in 1 out of 64 patients (1.56%) and a partial response (PR) was achieved in 1 out of 64 patients (1.56%), with mean (standard deviation) overall survival of 1.58 (1.48) months; MDS patients had no response (n = 5), with mean (standard deviation) overall survival of 2.53 (1.65) months. The protocol specified efficacy-evaluable patients as those who received ≥5 of 7 CWP291 doses in the first cycle and had ≥1 evaluation of response or were withdrawn from the study early due to disease progression. Sixty-one of 69 patients (88%) were efficacy evaluable per protocol. Seven patients with AML were unevaluable, among whom 3 patients met the 5 to 7 dose requirement but had no response assessment because of death (n = 1), the patient did not undergo bone marrow biopsy (n = 1), or an unknown reason (n = 1). For the 57 evaluable patients (93%) with AML, the median duration of overall survival was 1.12 months (range, 0.3-7.0 months). Fifty-four of the 57 AML patients were assessed for response; 2 patients had no assessment of response and were classified as response unknown, and 1 other patient withdrew early. Of the 54 response-evaluable AML patients, 1 patient experienced a CR (153 mg/m2 dose cohort), and 1 patient had a PR (198 mg/m2 dose cohort). Responses for these patients occurred on cycle 3 day 1 and cycle 5 day 1, respectively, with bone marrow blast cells reduced from 58.3% to 3.5% (CR) and 15.0% to 4.2% (PR), respectively. Duration of response for both responders was 1.5 months. The patient with a CR was a 75-year-old Asian female with de novo AML disease with multilineage dysplasia. Her prior therapy was idarubicin. Her baseline karyotype was 48, XX, +4, +8[5]/46, XX[18], FLT3(−), NPM1(−), and CEBPA was not done. Her immunophenotype profile was cMPO+, CD13 dim+, CD33 dim+, CD14 dim+, CD117+, CD123+, CD34+, HLA-DR+, and CD38+. The platelets fully recovered. A graph depicting peripheral blood counts vs time has been added as supplemental Figure 1.
Among the 5 patients (7%) with MDS, 4 were evaluable for response. Three of these patients had stable disease (1 treated at 198 mg/m2 and 2 at 257 mg/m2), and 1 patient had treatment failure. Median overall survival for the 4 MDS evaluable patients was 3.3 months (range, 0.9-4.3 months). Of the patients with stable disease, 2 patients withdrew consent during treatment cycle 4 and 5, respectively, and 1 patient died on day 129 due to pneumonia unrelated to the study drug.
Pharmacodynamics
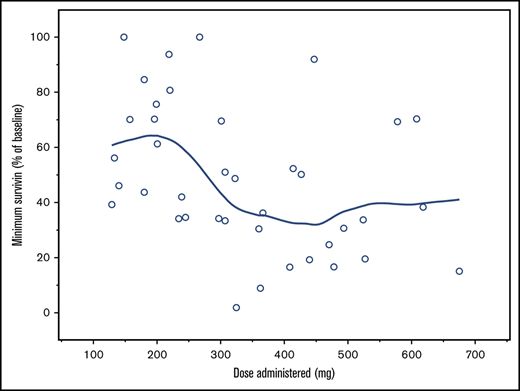
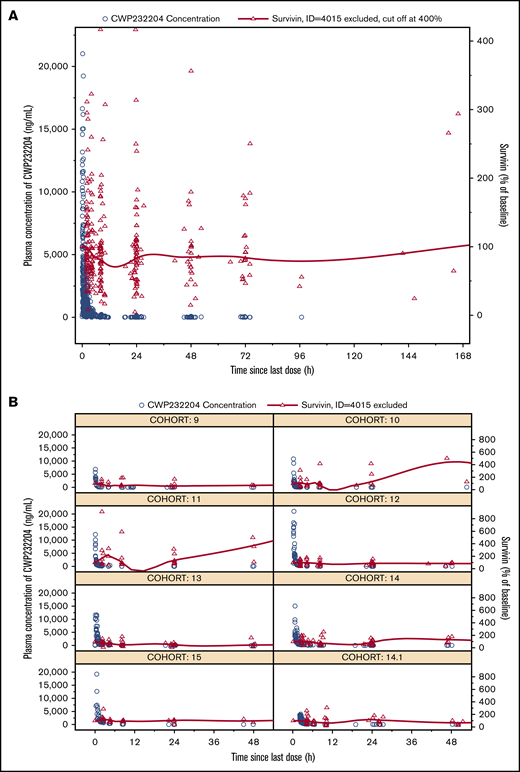
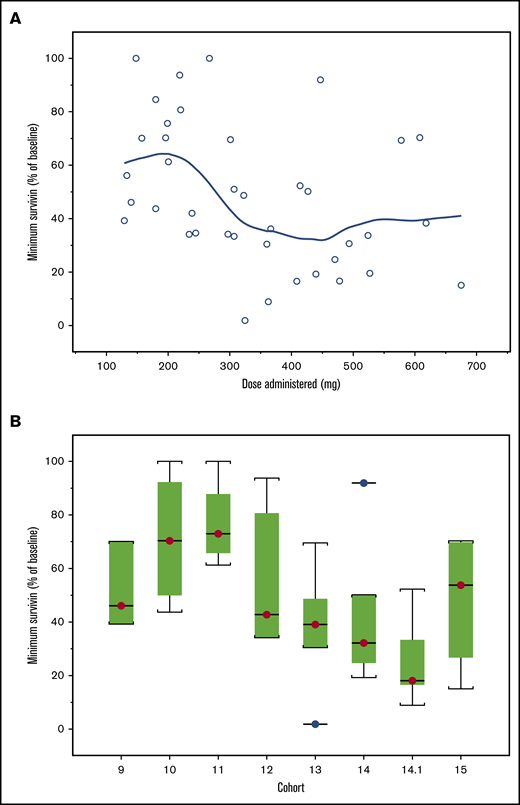
Patients in cohorts 9 to 15 (n = 39) analyzed for changes from baseline in plasma survivin showed high interindividual variability. There was lack of a consistent trend in plasma survivin in relationship to dose levels. One patient who showed a >3500% increase was considered an outlier. In general, the mean plasma survivin level started to decrease 2 to 4 hours postdosing and reached nadir at 12 to 16 hours, returning to baseline value by 24 hours postdose (Figure 2). While biomarker studies were exploratory and hypothesis generating due to the small number of patients, higher doses showed a trend for association with a greater decrease from baseline in plasma survivin, particularly for cohorts 12 to 14.1 (Figure 3). In cohort 15, the minimum plasma survivin after treatment showed a slight increase, which may have been affected by the 2 patients who dropped out early due to DLTs. Polymerase chain reaction data for survivin (supplemental Figure 2) did not show any trend in relation to plasma CWP291 levels.
Biomarker analysis for Wnt signaling pathway inhibition. Survivin protein concentrations in plasma vs time and CWP291 concentrations in plasma vs time. (A) Data pooled for the entire study. (B) Data for each cohort. (Note: A LOESS smoothing line is overlaid for survivin. Concentration-time profiles of CWP232204 were also shown for comparison with survivin. Patient 4015 was excluded.)
Biomarker analysis for Wnt signaling pathway inhibition. Survivin protein concentrations in plasma vs time and CWP291 concentrations in plasma vs time. (A) Data pooled for the entire study. (B) Data for each cohort. (Note: A LOESS smoothing line is overlaid for survivin. Concentration-time profiles of CWP232204 were also shown for comparison with survivin. Patient 4015 was excluded.)
Survivin profile. (A) Minimum survivin protein levels achieved (percentage of baseline levels) for dose administered and cohort. Note: A LOESS smoothing line is overlaid. (B) For the box-and-whisker plot, the line across each box, the top edge, and the bottom edge represent the median, the first quartile, and the third quartile, respectively. The horizontal lines connected with the whiskers extending from the box denote the minimum and the maximum values, respectively. Empty circles (○) indicate an outlier, defined as a value less than the first quartile minus 1.5 times interquartile range or a value greater than the third quartile plus 1.5 times interquartile range.
Survivin profile. (A) Minimum survivin protein levels achieved (percentage of baseline levels) for dose administered and cohort. Note: A LOESS smoothing line is overlaid. (B) For the box-and-whisker plot, the line across each box, the top edge, and the bottom edge represent the median, the first quartile, and the third quartile, respectively. The horizontal lines connected with the whiskers extending from the box denote the minimum and the maximum values, respectively. Empty circles (○) indicate an outlier, defined as a value less than the first quartile minus 1.5 times interquartile range or a value greater than the third quartile plus 1.5 times interquartile range.
Discussion
The primary objective of the study was to establish the MTD for CWP291. When administered as an IV infusion spanning a period of ≤2 hours per day for 7 consecutive days in 21-day cycles, the MTD for CWP291 was determined to be 257 mg/m2 and was considered to be appropriate for this schedule for evaluation in future clinical studies. CWP291 was found to be safe and well tolerated. AEs were manageable, with the majority reflective of the underlying disease and patient comorbidity. While Wnt/β-catenin networks are present in cancer stem cells3 as well as in nonmalignant HSCs,1 another CWP drug, CWP232228, was shown to achieve in vivo selective targeting of AML patient–derived cancer stem cells.19 Our clinical study with CWP291 was in agreement with that observation; myelosuppression was absent in patients dosed with CWP291. The most frequent TEAEs considered by investigators to be related to study drug were gastrointestinal (nausea, vomiting, and diarrhea), infusion-related reactions, and myalgia.
Gastrointestinal toxicities were the most common study drug–related TEAEs and were observed at all dose levels and showed no clear dose-related trends, although a correlation with vomiting was suggested by PK analyses. CWP291 was thus considered to be mildly or moderately emetogenic but effectively managed with standard-of-care antiemetics and routine prophylaxis.20
Patients who were withdrawn from the study due to AEs saw their AEs resolve, except for some instances of pneumonia, which was considered not related to the study drug. The absence of significant organ toxicity (including renal, liver, and cardiac) and a mechanism of action that does not rely on or is associated with nonspecific myelosuppression make CWP291 potentially amenable for combination with other drugs, including those that exhibit these toxicities. Overall, the safety profile of CWP291 at all dose levels, and especially at those doses at which clinical efficacy was observed, was considered acceptable for the population of patients studied.
CWP232204 was the active metabolite of CWP291 and showed dose-proportional linear PK on days 1 and 7. The mean t1/2 of CWP232204 was 12.6 hours, supporting a once-daily dosing schedule. Urinary excretion of CWP232204 was very low (<5%). The lack of renal elimination may be an advantage for patients with hematological malignancies where renal function is compromised.
The potential benefits of this drug and its mechanism of action likely require a combination regimen and sustained therapy. In preclinical studies, others have demonstrated that the downregulation of β-catenin in combination with target drugs augmented apoptosis of malignant cells.21 Other agents, including venetoclax and glasdegib, which are more directed to stem cell targets, have similarly shown very modest activity as single agents but more clear benefits as combination therapy.22,23 Venetoclax with a backbone therapy (hypomethylators and low-dose cytarabine) is thought to partially work through a novel mechanism that targets the leukemia stem cell population and has shown promise in older, untreated patients with AML.24,25 Glasdegib prolongs survival when combined with low-dose cytarabine.26
CWP291 exposure is expected to lead to β-catenin degradation, thereby inhibiting the expression of cell cycle and antiapoptotic genes such as CCND1 that encodes the cyclin D1 protein and BIRC5 that encodes the survivin protein.8,27 Survivin, a member of the inhibitor of apoptosis family, causes negative regulation of apoptosis or programmed cell death functions by inhibiting caspase activation.28 We found survivin to decrease within hours after dosing with CWP291, while levels returned to baseline within 24 hours. This suggested that there was significant correlation of downstream signaling of the Wnt signaling pathway with CWP291 drug administration at levels expected to potentially result in achieving efficacy in future combination studies; these results were more apparent in the higher dose cohorts. Due to the heterogeneity of cancer cells, the efficacy of Wnt signaling pathway blockade may be limited when used as monotherapy but cumulative when used as part of combination therapy with drugs that target other pathways. Cross-talk between pathways and chemoresistance in cancer can involve survivin.29 Furthermore, the expected efficacy of Wnt signaling blockade may be dependent on the degree of activation of malignant Wnt signaling in each patient. The development of predictive biomarkers and concomitant research for ensuring efficacy may provide knowledge for exploiting the blockade of this pathway in future studies.
In conclusion, the primary objective of the study to establish the MTD for CWP291 was met, and CWP291 was considered to be well tolerated, with favorable PK. While overall efficacy was minimal/modest in the trial as a single agent, CWP291 may be efficacious in combination with other agents widely used in the salvage setting. Being that the mechanism of action of CWP291 is more relevant for disrupting early cells in the HSC hierarchy, this drug may potentially be suited for eradicating leukemia stem cells.3,19 CWP291 could potentially be synergistic with other agents, including chemotherapy30 and targeted therapy. Further studies would be required to determine optimal combinations and schedules.
Inquiries regarding protocol and data can be submitted to the corresponding author, Jorge E. Cortes, at jorge.cortes@augusta.edu.
Acknowledgments
The authors thank the patients and their families for volunteering to be part of this clinical trial. CWP291 is being developed by JW Pharmaceutical (Seoul, Korea). Writing and editorial support was funded by JW Pharmaceuticals and provided by A. Daisy Goodrich.
This study was funded by JW Pharmaceutical Corporation.
Authorship
Contribution: J.-H.L., J.M.P., C.W.J., J.C., K.L., and J.E.C. contributed to conception and design; J.-H.L., S.-S.Y., H.L., and K.L. developed methodology; J.-H.L., S.F., J.M.P., C.W.J., S.-S.Y., A.D.P., P.S.B., and J.E.C. acquired data; J.-H.L., J.M.P., C.W.J., S.-S.Y., P.S.B., H.L., K.L., M.K., and J.E.C. analyzed and interpreted data; all authors wrote, reviewed, and/or revised the manuscript; J.-H.L., J.C., M.K., and J.E.C. provided administrative, technical, or material support; J.-H.L., C.W.J., P.S.B., J.C., and J.E.C. supervised the study; and all authors provided consent for authorship and approval of the finalized manuscript.
Conflict-of-interest disclosure: S.F. is an employee and stockholder of Jazz Pharmaceuticals. J.M.P. was a consultant for Actinium Pharmaceuticals. S.-S.Y. was on advisory committees at Janssen, Celgene, and Amgen. P.S.B. received research funding form AbbVie, Amgen, Bristol-Myers Squibb, JW Pharmaceutical, Novartis, Pfizer, Glycomimetics, Trovagene, Invivoscribe, Aptose Biosciences, and Trethera; performed consulting for CVS Caremark and McKesson; and received honoraria from Physician Education Resource and The France Foundation. J.C., K.L., and M.K. are employees of JW Pharmaceutical. J.E.C. received research support (to institution) from JW Pharmaceutical, BMS, Novartis, Astellas, Daiichi, Jazz, Pfizer, Amphivena, and Merus; and consulting fees from Novartis, Pfizer, Jazz, and Daiichi. The remaining authors declare no competing financial interests.
Correspondence: Jorge E. Cortes, Georgia Cancer Center, 1410 Laney Walker Rd, CN2222, Augusta, GA 30912; e-mail: jorge.cortes@augusta.edu.
References
Author notes
J.-H.L. and S.F. contributed equally to this study.
The full-text version of this article contains a data supplement.