Key Points
BK virus–specific CD4 T cells producing Th1 cytokines were detected more frequently than BK virus–specific CD8 T cells.
BK virus–specific CD8 T cells, particularly producing IFN-γ and with cytolytic potential, expand in patients with BK virus disease.
Abstract
Clinical disease caused by BK virus reactivation is a frequent complication of allogeneic hematopoietic cell transplantation (HCT). Because of the lack of effective antiviral agents, BK virus–specific T cells are emerging as a potential therapy for BK virus disease, but the immune response to BK virus after allogeneic HCT has not been well characterized. Our study describes reconstitution of BK virus–specific T-cell immunity in 77 adult patients after HCT. All patients had urinary symptoms, and urine was tested for BK virus replication; 33 patients were positive for BK virus (cases), and 44 were negative (controls). In BK virus cases, the median time to first positive test was 75 days (range, 2-511). BK virus cases had lower CD4 T-cell counts 3 to 9 months after transplant, but CD8 T-cell counts were similar in cases and controls. BK virus–specific T cells were identified by cytokine flow cytometry in cryopreserved samples collected prospectively. BK virus–specific CD4 T cells producing T helper 1 (Th1) cytokines recovered quickly after HCT. BK virus–specific T cells were detected more frequently in patients with BK virus reactivation at most time points, and CD4 T cells producing Th1 cytokines were more frequent than BK virus–specific cytolytic CD8 T cells. Early detection of interferon-γ+ and cytolytic BK virus–specific CD4 T cells was associated with lower rates of hematuria among cases. Overall, our study describes recovery of BK virus–specific T cells after HCT and the distinct roles for BK virus–specific T cells in the development and resolution of clinical symptoms.
Introduction
BK virus is a member of the Polyomaviridae family of viruses, a nonenveloped family of double-stranded DNA viruses. BK virus is highly prevalent in human populations, with seroprevalence studies suggesting that >65% of healthy individuals have detectable BK virus–specific antibodies.1-3 The virus is usually acquired in childhood and establishes latency in the urothelial cells of the kidney and urinary tract.2 Immunosuppression after allogeneic hematopoietic cell transplantation (HCT) results in BK virus reactivation in up to 50% of adult recipients and clinical disease in up to 25%.4-6 With the development of effective prophylactic and preemptive therapies for cytomegalovirus, BK virus has become a prominent cause of clinical viral disease after allogeneic HCT.6,7
BK virus disease manifestations range from mild dysuria to life-threatening hemorrhagic cystitis and renal failure.8-10 Risk factors associated with the development of BK virus disease include cord blood HCT, conditioning regimens that include anti-thymocyte globulin and cyclophosphamide, and severe acute graft-versus-host disease (GVHD).4 A variety of therapeutic approaches, including leflunomide11 and brincidofovir,7 have been evaluated in patients with BK virus disease but have not improved clinical outcomes in affected patients. Considering the lack of effective antiviral agents, efforts have been made to develop BK virus–specific T-cell therapies.12 Infusion of autologous or partially HLA-matched third-party BK virus–specific T cells has been reported to accelerate resolution of BK virus disease,13-15 but the availability of these advanced therapies remains limited.
The potential clinical effectiveness of adoptively transferred BK virus–specific T cells indicates an important role for T-cell immunity in controlling BK virus disease, but the reconstitution of BK virus–specific T cells after HCT remains undefined. To address this knowledge gap, we studied the reconstitution of BK virus–specific T-cell immunity in a cohort of patients with and without BK virus reactivation after allogeneic HCT. This comparison allowed us to describe the normal recovery of BK virus–specific T cells, as well as the impact of BK virus reactivation on this process.
Materials and methods
Patients and sample collection
We analyzed samples from 77 adult allogeneic HCT recipients (Table 1) who underwent allogeneic HCT at the Dana-Farber Cancer Institute.4 All patients had urinary symptoms and were tested for BK virus DNA in urine by polymerase chain reaction as part of standard clinical care. Of these, 33 patients had evidence of BK virus replication (cases), and 44 did not (controls). BK virus disease was defined as evidence of BK virus in urine in association with genitourinary symptoms without other concurrent diagnoses. Hematuria was not required for defining BK virus disease. In HCT recipients with genitourinary symptoms, urine was routinely tested with urinalysis, bacterial culture, adenovirus, and BK virus polymerase chain reaction. Ultrasound and other tests were done only if clinically indicated.
Patient characteristics
| . | Overall (N = 77) . | BKV-negative controls (n = 44) . | BKV positive (n = 33) . | P* . |
|---|---|---|---|---|
| Age at HCT, median (range), y | 53 (20-73) | 55 (20-73) | 51 (20-67) | .077 |
| Sex | .009 | |||
| Male | 47 (61.0) | 21 (47.7) | 26 (78.8) | |
| Female | 30 (39.0) | 23 (52.3) | 7 (21.2) | |
| Diagnosis | .053 | |||
| AML | 33 (42.8) | 25 (56.8) | 8 (24.2) | |
| NHL | 14 (18.2) | 5 (11.4) | 9 (27.3) | |
| MDS/MPD | 10 (13.0) | 4 (9.1) | 6 (18.2) | |
| ALL | 7 (9.1) | 4 (9.1) | 3 (9.1) | |
| CLL/SLL/PLL | 7 (9.1) | 3 (6.8) | 4 (12.1) | |
| Aplastic anemia | 2 (2.6) | 0 (0.0) | 2 (6.1) | |
| CML | 2 (2.6) | 2 (4.5) | 0 (0.0) | |
| HD | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| MM | 1 (1.3) | 1 (2.3) | 0 (0.0) | |
| HLA matching† | .958 | |||
| Matched related donor | 22 (28.6) | 12 (27.3) | 10 (30.3) | |
| Matched unrelated donor | 43 (55.8) | 25 (56.8) | 18 (54.5) | |
| Mismatched unrelated donor | 12 (15.6) | 7 (15.9) | 5 (15.2) | |
| Cell source | .887 | |||
| Peripheral blood stem cells | 67 (87.0) | 39 (88.6) | 28 (84.8) | |
| Double cord | 6 (7.8) | 3 (6.8) | 3 (9.1) | |
| Bone marrow | 4 (5.2) | 2 (4.6) | 2 (6.1) | |
| Conditioning regimen | .497 | |||
| Reduced intensity | 41 (53.2) | 25 (56.8) | 16 (48.5) | |
| Myeloablative | 36 (46.8) | 19 (43.2) | 17 (51.5) | |
| Conditioning drugs | .284 | |||
| Bu/Flu | 37 (48.0) | 24 (54.5) | 13 (39.35) | |
| Cy/TBI | 27 (35.1) | 14 (31.8) | 13 (39.35) | |
| Flu/Mel/ATG | 5 (6.5) | 3 (6.8) | 2 (6.1) | |
| Bu/Cy | 3 (3.9) | 1 (2.3) | 2 (6.1) | |
| Bu/Flu/ATG | 2 (2.6) | 2 (4.6) | 0 (0.0) | |
| Flu/Cy/ATG/TBI | 2 (2.6) | 0 (0.0) | 2 (6.1) | |
| Flu/Mel | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| GVHD prophylaxis | .606 | |||
| Tac/MTX | 38 (49.3) | 22 (50.0) | 16 (48.5) | |
| Tac/Sir | 17 (22.1) | 11 (25.0) | 6 (18.2) | |
| Tac/Sir/MTX | 16 (20.8) | 9 (20.4) | 7 (21.2) | |
| Tac/Sir/MMF/MTX | 3 (3.9) | 1 (2.3) | 2 (6.1) | |
| Tac/MMF | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| Tac/MMF/MTX | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| Tac/Sir/MMF | 1 (1.3) | 1 (2.3) | 0 (0.0) | |
| Acute GVHD grades 2-4 | 37 (48.1) | 18 (40.9) | 19 (57.6) | .172 |
| Chronic GVHD | 33 (42.9) | 20 (57.1) | 13 (41.9) | .324 |
| . | Overall (N = 77) . | BKV-negative controls (n = 44) . | BKV positive (n = 33) . | P* . |
|---|---|---|---|---|
| Age at HCT, median (range), y | 53 (20-73) | 55 (20-73) | 51 (20-67) | .077 |
| Sex | .009 | |||
| Male | 47 (61.0) | 21 (47.7) | 26 (78.8) | |
| Female | 30 (39.0) | 23 (52.3) | 7 (21.2) | |
| Diagnosis | .053 | |||
| AML | 33 (42.8) | 25 (56.8) | 8 (24.2) | |
| NHL | 14 (18.2) | 5 (11.4) | 9 (27.3) | |
| MDS/MPD | 10 (13.0) | 4 (9.1) | 6 (18.2) | |
| ALL | 7 (9.1) | 4 (9.1) | 3 (9.1) | |
| CLL/SLL/PLL | 7 (9.1) | 3 (6.8) | 4 (12.1) | |
| Aplastic anemia | 2 (2.6) | 0 (0.0) | 2 (6.1) | |
| CML | 2 (2.6) | 2 (4.5) | 0 (0.0) | |
| HD | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| MM | 1 (1.3) | 1 (2.3) | 0 (0.0) | |
| HLA matching† | .958 | |||
| Matched related donor | 22 (28.6) | 12 (27.3) | 10 (30.3) | |
| Matched unrelated donor | 43 (55.8) | 25 (56.8) | 18 (54.5) | |
| Mismatched unrelated donor | 12 (15.6) | 7 (15.9) | 5 (15.2) | |
| Cell source | .887 | |||
| Peripheral blood stem cells | 67 (87.0) | 39 (88.6) | 28 (84.8) | |
| Double cord | 6 (7.8) | 3 (6.8) | 3 (9.1) | |
| Bone marrow | 4 (5.2) | 2 (4.6) | 2 (6.1) | |
| Conditioning regimen | .497 | |||
| Reduced intensity | 41 (53.2) | 25 (56.8) | 16 (48.5) | |
| Myeloablative | 36 (46.8) | 19 (43.2) | 17 (51.5) | |
| Conditioning drugs | .284 | |||
| Bu/Flu | 37 (48.0) | 24 (54.5) | 13 (39.35) | |
| Cy/TBI | 27 (35.1) | 14 (31.8) | 13 (39.35) | |
| Flu/Mel/ATG | 5 (6.5) | 3 (6.8) | 2 (6.1) | |
| Bu/Cy | 3 (3.9) | 1 (2.3) | 2 (6.1) | |
| Bu/Flu/ATG | 2 (2.6) | 2 (4.6) | 0 (0.0) | |
| Flu/Cy/ATG/TBI | 2 (2.6) | 0 (0.0) | 2 (6.1) | |
| Flu/Mel | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| GVHD prophylaxis | .606 | |||
| Tac/MTX | 38 (49.3) | 22 (50.0) | 16 (48.5) | |
| Tac/Sir | 17 (22.1) | 11 (25.0) | 6 (18.2) | |
| Tac/Sir/MTX | 16 (20.8) | 9 (20.4) | 7 (21.2) | |
| Tac/Sir/MMF/MTX | 3 (3.9) | 1 (2.3) | 2 (6.1) | |
| Tac/MMF | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| Tac/MMF/MTX | 1 (1.3) | 0 (0.0) | 1 (3.0) | |
| Tac/Sir/MMF | 1 (1.3) | 1 (2.3) | 0 (0.0) | |
| Acute GVHD grades 2-4 | 37 (48.1) | 18 (40.9) | 19 (57.6) | .172 |
| Chronic GVHD | 33 (42.9) | 20 (57.1) | 13 (41.9) | .324 |
Unless noted otherwise, data are n (%).
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; BKV, BK virus; Bu, busulfan; CLL/SLL/PLL, chronic lymphocytic leukemia/small lymphocytic lymphoma/prolymphocytic leukemia; CML, chronic myeloid leukemia; Cy, cyclophosphamide; Flu, fludarabine; HD, Hodgkin disease; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; Mel, melphalan; MM, multiple myeloma; MMF, mycophenolate mofetil; MTX, methotrexate; NHL, non-Hodgkin lymphoma; Sir, sirolimus; Tac, tacrolimus; TBI, total body irradiation.
The Mann-Whitney U test was used for quantitative variables, and the Fisher’s exact test and Pearson’s χ2 test were used for qualitative variables.
Matching was done at the 5-loci level (HLA-A/HLA-B/HLA-C/HLA-DRB1/HLA-DQB1).
Prospectively cryopreserved peripheral blood mononuclear cells (PBMCs) were analyzed from each patient at multiple predetermined time points. Quantification of BK virus–specific T cells was performed in 494 PBMC samples, averaging 6.4 samples per patient. Samples were obtained pre-HCT (before conditioning in all but 3 cases) and at 1, 3, 6, 9, 12, 18, and 24 months post-HCT. All samples were assessed using cytokine flow cytometry following adaptation of previously published protocols.16-18 Prospective collection and cryopreservation of blood samples for research studies were approved by the institutional review board of the Dana-Farber/Harvard Cancer Center. Prior written informed consent was obtained from all patients per the Declaration of Helsinki.
Cytokine flow cytometry
PBMCs were stimulated with overlapping 15-mer peptides derived from BK virus LT and VP1 proteins in the presence of Golgi blockers and costimulatory agents. These proteins were chosen because VP1 and LT antigen–specific T cells are detected most frequently in individuals with BK virus.18-20 Results after staining and acquisition were compared with no-PepMix negative controls and staphylococcal enterotoxin B–positive controls. Cryopreserved PBMCs were thawed in media (89% RPMI 1640, 1% l-glutamine, 10% fetal bovine serum) with 0.1% DNase (Sigma-Aldrich). Cells were incubated overnight in media without DNase, counted, and resuspended in media with DNase to a concentration of 15 × 106 cells per milliliter. Thereafter, 96-well plates were used for stimulation assays, with 5 wells per sample: cytokine-negative control, cytokine-PepMix, cytotoxicity-negative control, cytotoxicity-PepMix, and cytokine-positive control.
Media containing DNase at a total volume of 80 µL (100 µL for cytokine-positive control) were placed in all wells, including monensin (BD GolgiStop) and brefeldin A (BD GolgiPlug; both from BD Biosciences), to obtain final concentrations of 2 µM and 10 µg/mL, respectively. Anti-CD28/49d antibodies (BD FastImmune; BD Biosciences) were added to a final concentration of 1 µl/mL (with the exception of positive-control cytokine wells). Twenty microliters of sterile double-distilled water was added to the negative control wells, and 10 µL each of double-distilled water–reconstituted BK virus LT PepMix (PepTivator BKV LT) and BKV VP1 PepMix (PepTivator BKV VP1; both from Miltenyi Biotec) were added to PepMix wells. Cytokine-positive control well mixes also contained staphylococcal enterotoxin B (BT202red; Toxin Technology) at a final concentration of 1 µg/mL. Cells (100 µL) were placed in each well (final volume: 200 µL), and anti-CD107a BV421 (H4A3; BioLegend) was added to cytotoxicity wells. The plate was then incubated for 6 hours at 37°C and 5% CO2.
After stimulation, cells were stained with fluorophore-conjugated antibodies using BD Cytofix/Cytoperm (BD Biosciences). For cytokine wells, the following panel was used: anti-CD3/BV510 (OKT3; BioLegend), anti-CD4/BV711 (SK3; BD Horizon), anti-CD8/BV605 (SK1; BioLegend), anti-CD45RA/PE-CF594 (HI100; BD Horizon), anti-CCR7/BV421 (G043H7; BioLegend), anti–interferon-γ (IFN-γ)/FITC (B27; BD Pharmingen), anti–tumor necrosis factor-α (TNF-α)/APC (MAb11; eBioscience), anti–interleukin-2 (IL-2)/PE (MQ1-17H12; eBioscience), and Fixable Viability Dye eFluor 780 (eBioscience). In addition to anti-CD107a antibody, cytotoxicity wells included anti-CD3/BV510 (OKT3; BioLegend), anti-CD4/BV711 (SK3; BD Horizon), anti-CD8/BV605 (SK1; BioLegend), anti–CD57/PE-CF594 (NK-1; BD Horizon), anti–IFN-γ/FITC (B27; BD Pharmingen), anti–Granzyme B/APC (REA226; Miltenyi Biotec), anti-perforin/PE (B-D4821,22 ; BioLegend), and Fixable Viability Dye eFluor 780 (eBioscience). Samples were acquired using a BD LSRFortessa X-20 flow cytometer (BD Biosciences). Flow cytometry analysis was done using FlowJo X (TreeStar).
The gating strategy is illustrated in supplemental Figure 1. In addition to characterizing memory compartments using CD45RA and CCR7 expression (naive: CD45RA+CCR7+; central memory [CM]: CD45RA−CCR7+; effector memory [EM]: CD45RA−CCR7−; and terminally differentiated EM [TEMRA]: CD45RA+CCR7−), 5 groups of BK virus–specific T cells were described in CD4 and CD8 T cells: IFN-γ+, TNF-α+, IL-2+, cytokine+ (Boolean gating of cells producing IFN-γ, TNF-α, or IL-2), and cytolytic (CD107a+). After quantification of cytokine-producing and degranulating cells, background activation (negative control wells) was subtracted from stimulated activation (PepMix wells) to determine BK virus–specific T cells; any parameter with <5 acquired events was counted as 0. Laboratory assessments were blinded to clinical disease status until after completion of flow cytometry data analysis.
Statistical analysis
Patient characteristics were analyzed descriptively. For group comparisons, the Fisher’s exact test, Pearson’s χ2 test, or the Mann-Whitney U test was used as appropriate. Recursive partitioning (rpart) was used to determine the threshold of positivity for the different parameters analyzed. Time to BK virus–specific T-cell reconstitution was constructed using the Kaplan-Meier method, and the curves were compared using the log-rank test. All tests were 2-sided at the significance level of 0.05, and multiple comparisons were not considered. Statistical analysis was done using IBM SPSS Statistics 22 and R 3.4.1. Additional graphs were made using Microsoft Office Excel 2013 and GraphPad Prism 6.01.
Results
Patient characteristics
Clinical characteristics of the 77 patients are summarized in Table 1. Median age was 53 years (range, 20-73), and 61% were male. There were more males among cases than controls (78.8% vs 47.7%; P = .009). Most patients were transplanted for hematologic malignancies (97.4%); acute myeloid leukemia (43%) was the most common indication. Forty-three patients (55.8%) received transplants from HLA-matched unrelated donors, and 22 patients (28.6%) received transplants from HLA-matched related donors. Sixty-seven patients (87%) received peripheral blood, and 6 patients (7.8%) received cord blood stem cells. Reduced-intensity conditioning regimens were used in 53% of the patients; cyclophosphamide was used in 41.6% and anti-thymocyte globulin was used in 11.7% of all transplants. Tacrolimus was used in all patients; methotrexate and sirolimus were also frequently used for GVHD prophylaxis. Grades 2-acute and chronic GVHD occurred in 48.1% and 42.9% of patients, respectively.
Urinary symptoms that prompted testing for BK virus are summarized in Table 2. Median time from HCT to symptom onset was 67 days. Median time from HCT to first BK virus testing was 83 days (range, 0-559). Among patients with BK virus disease, the median time to the first positive test was 75 days (range, 2-511). Total symptom duration, from the onset of symptoms to the resolution of all symptoms (in cases with persistent symptoms) was significantly different between cases and controls: median of 79 days (range, 2-385) vs 7 days (range, 0-432), respectively (P = .009). The incidence of hematuria among patients with BK virus disease was 66.7% compared with 29.5% in patients without documented BK virus reactivation (P = .006). Twenty-six patients (78.8%) with BK virus disease experienced a single episode of viral reactivation, whereas 7 patients (21.2%) experienced persistent or additional episodes of BK virus reactivation, as evidenced by repeated positive testing performed as part of standard clinical care. Sixteen cases were tested for BK viremia; 10 patients (62.5%) were positive. Median urinary viral load in cases at diagnosis was 6.5 × 108 copies per milliliter (range, 1.2 × 103 per milliliter to 2 × 1010 per milliliter); only 2 cases had urinary viral loads < 1 × 104 copies per milliliter (1220 and 6500 copies per milliliter). These levels of viral load in urine are similar to levels reported in patients with BK virus–hemorrhagic cystitis after allogeneic HCT.23 Urinary viral load was similar in cases with different grades of hematuria (supplemental Table 1).
Clinical characteristics of urinary symptoms leading to BK virus testing
| . | Overall (N = 77) . | BKV negative (n = 44) . | BKV positive (n = 33) . | P* . |
|---|---|---|---|---|
| Time to BKV testing, median (range), d | 83 (0-559) | 87 (0-559) | 75 (2-511) | .979 |
| Time to symptom onset, median (range), d | 67 (−3 to 559) | 87 (−3 to 559) | 61 (2-508) | .673 |
| Symptom duration, median (range), d | 44.5 (0-432) | 7 (0-432) | 79 (2-385) | .009 |
| Urinary BK viral load, median (range), copies/mL | 6.5 × 108 (1.2 × 103 to 2 × 1010) | |||
| Maximum grade of hematuria | .006 | |||
| 0 | 42 (54.5) | 31 (70.5) | 11 (33.3) | |
| 1 | 9 (11.7) | 2 (4.5) | 7 (21.2) | |
| 2 | 15 (19.5) | 8 (18.2) | 7 (21.2) | |
| 3 | 8 (10.4) | 3 (6.8) | 5 (15.2) | |
| 4 | 3 (3.9) | 0 (0.0) | 3 (9.1) | |
| Number of BKV episodes | ||||
| 1 | 26 (78.8) | |||
| >1 | 7 (21.2) | |||
| BKV detection in blood | 10/16† (62.5) |
| . | Overall (N = 77) . | BKV negative (n = 44) . | BKV positive (n = 33) . | P* . |
|---|---|---|---|---|
| Time to BKV testing, median (range), d | 83 (0-559) | 87 (0-559) | 75 (2-511) | .979 |
| Time to symptom onset, median (range), d | 67 (−3 to 559) | 87 (−3 to 559) | 61 (2-508) | .673 |
| Symptom duration, median (range), d | 44.5 (0-432) | 7 (0-432) | 79 (2-385) | .009 |
| Urinary BK viral load, median (range), copies/mL | 6.5 × 108 (1.2 × 103 to 2 × 1010) | |||
| Maximum grade of hematuria | .006 | |||
| 0 | 42 (54.5) | 31 (70.5) | 11 (33.3) | |
| 1 | 9 (11.7) | 2 (4.5) | 7 (21.2) | |
| 2 | 15 (19.5) | 8 (18.2) | 7 (21.2) | |
| 3 | 8 (10.4) | 3 (6.8) | 5 (15.2) | |
| 4 | 3 (3.9) | 0 (0.0) | 3 (9.1) | |
| Number of BKV episodes | ||||
| 1 | 26 (78.8) | |||
| >1 | 7 (21.2) | |||
| BKV detection in blood | 10/16† (62.5) |
Unless otherwise noted, data are n (%).
BKV, BK virus.
Mann-Whitney U test was used for quantitative variables, and Pearson’s χ2 test was used for categorical variables.
Only 16 patients were tested for BK virus in blood and urine.
BK virus–specific immune reconstitution
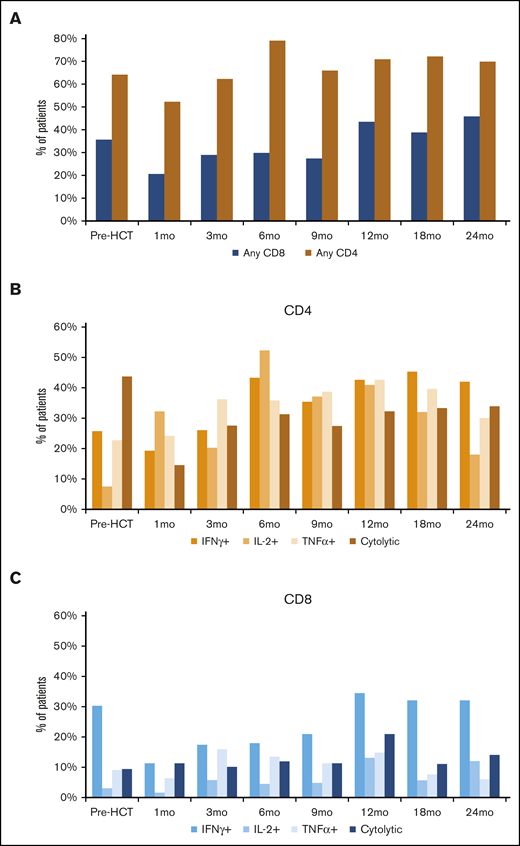
Prior to transplant, 64.2% and 35.8% of patients in the entire cohort had detectable CD4 or CD8 BK virus–specific T cells, respectively (Figure 1A). Following transplant and engraftment with donor cells, CD4 responses recovered quickly and exceeded pre-HCT levels by 6 months post-HCT. CD8 responses recovered more slowly and did not reach pretransplant levels until 12 months after HCT. BK virus–specific CD4 T cells producing T helper 1 (Th1) cytokines recovered quickly after HCT (Figure 1B). A total of 19.4%, 32.3%, and 24.2% of patients had detectable IFN-γ+, IL-2+, and TNF-α+ CD4 T cells 1 month after HCT, respectively, and this increased to >40% of patients for all parameters by 1 year after HCT. BK virus–specific CD8 T cells were detected less frequently (Figure 1C). BK virus–specific CD8 T cells predominantly secreted IFN-γ, and only 11.3% of patients had detectable IFN-γ+ CD8 T cells 1 month after HCT. This level gradually increased to 34.4% of the patients at 1 year after HCT.
Frequency of detection of BK virus–specific T cells across time points in the entire cohort. (A) Any BK virus–specific T cells. (B) BK virus–specific IFN-γ+, TNF-α+, IL-2+, and cytolytic CD4 T cells. (C) BK virus–specific IFN-γ+, TNF-α+, IL-2+, and cytolytic CD8 T cells.
Frequency of detection of BK virus–specific T cells across time points in the entire cohort. (A) Any BK virus–specific T cells. (B) BK virus–specific IFN-γ+, TNF-α+, IL-2+, and cytolytic CD4 T cells. (C) BK virus–specific IFN-γ+, TNF-α+, IL-2+, and cytolytic CD8 T cells.
Phenotypic analysis of BK virus–specific T cells
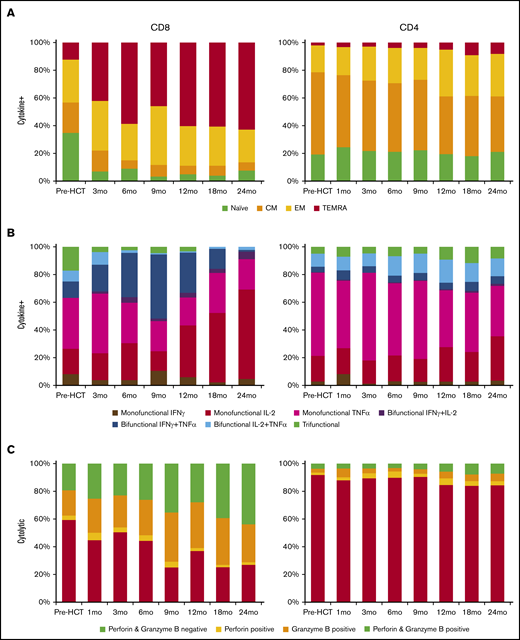
Very few CD8 BK virus–specific cytokine-secreting cells had a naive or CM phenotype after HCT. Cytokine secretion by BK virus–specific CD8 T cells was not reliably detected 1 month after HCT; subsequently, BK virus–specific cytokine-positive CD8 T cells gradually became increasingly differentiated after HCT, with a predominance of TEMRA cells in the CD8 T-cell compartment (42.1–62.8%) by 1 year after HCT (Figure 2A). In contrast, the majority of BK virus–specific cytokine-secreting CD4 T cells were naive and CM cells. There was also a gradual differentiation of CD4 BK virus–specific cytokine-secreting T cells that became evident 1 year after HCT. Still, only 32.8% of BK virus–specific cytokine-positive CD4 T cells had an effector memory or TEMRA phenotype 2 years after HCT.
Functional characterization of BK virus–specific T cells. (A) Mean memory compartment distribution of BK virus–specific cytokine-positive T cells across time points. (B) Mean functional characterization of BK virus–specific cytokine-positive T cells. (C) Mean cytotoxic granule content of BK virus–specific cytolytic T cells.
Functional characterization of BK virus–specific T cells. (A) Mean memory compartment distribution of BK virus–specific cytokine-positive T cells across time points. (B) Mean functional characterization of BK virus–specific cytokine-positive T cells. (C) Mean cytotoxic granule content of BK virus–specific cytolytic T cells.
Within CD8 BK virus–specific cytokine-positive T cells, the majority of cells secreted IL-2, TNF-α, IFN-γ, or IFN-γ + TNF-α; there were few trifunctional cells or cells that secreted IFN-γ + IL-2 (Figure 2B). As T-cell function reconstituted, there was a gradual increase in the polyfunctionality of BK virus–specific cytokine-positive CD8 T cells until 9 months after HCT, with 49% of cells producing 2 Th1 cytokines and 4.5% of cells producing all 3 cytokines by that time. Subsequently, there was a large expansion of primarily IL-2–producing CD8 T cells, which became the predominant subset of BK virus–specific cytokine-positive cells. Most BK virus–specific cytokine-producing CD4 T cells secreted 1 cytokine (at 18 months only 21.2% were bifunctional and 11.4% were trifunctional), but this restriction gradually shifted from primarily TNF-α+ cells (61.7% at 3 months and 36% at 24 months) to IL-2+ cells at later time points (16.3% at 3 months and 31.6% at 24 months).
In our study, cytolytic T cells were defined by their ability to degranulate after stimulation and express CD107a (Figure 2C). In the first 6 months posttransplant, ∼50% of BK virus–specific cytolytic CD8 T cells also expressed perforin, granzyme B, or both. This percentage gradually increased to >70% by 18 months posttransplant. At 3 months, 22.9% of cells were positive for perforin and granzyme B; that number increased to 43.1% at 24 months. Relatively large numbers of BK virus–specific CD4 T cells expressed CD107a after stimulation, but very few of these cells expressed perforin or granzyme B; this scenario remained stable at all time points after transplant. BK virus–specific cytolytic CD8 T cells coexpressed IFN-γ at varying percentages after HCT, with a peak of 33.1% at 9 months, whereas CD4 cytolytic cells coexpressed IFN-γ less frequently (6.1% at 3 months and 15.3% at 12 months) (supplemental Figure 2).
Lymphocyte recovery and association with BK virus disease
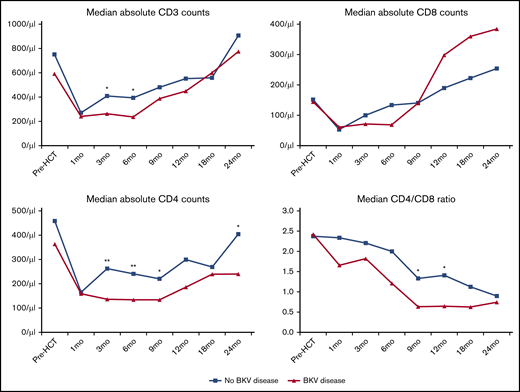
Serial measurements demonstrated differences in T-cell recovery between cases and controls (Figure 3). Prior to transplant and 1 month after transplant, T-cell counts were similar in cases and controls. The median time to detection of BK virus reactivation in cases was 75 days post-HCT. Following this, patients with BK virus disease had significantly lower absolute CD3 T-cell counts at 3 and 6 months. This was largely due to significantly lower CD4 counts in cases at 3, 6, 9, and 24 months. There were no significant differences in CD8 T-cell recovery. As a result, a lower CD4/CD8 ratio in patients with BK virus disease was noted at all time points after HSCT; however, these differences were only statistically significant at 9 and 12 months.
T-cell recovery and CD4/CD8 ratios after HCT. *P < .05, **P < .01, independent samples Mann-Whitney U test.
T-cell recovery and CD4/CD8 ratios after HCT. *P < .05, **P < .01, independent samples Mann-Whitney U test.
Reconstitution of BK virus–specific T cells in cases and controls
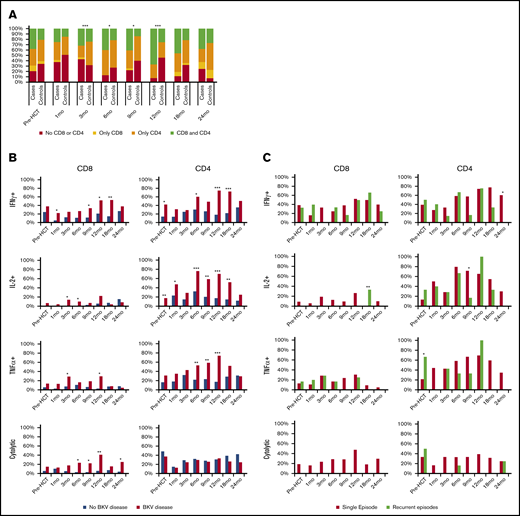
Prior to transplant, CD4 or CD8 BK virus–specific T cells were detected in 71.6% of patients (79.3% of cases and 65.8% of controls) (Figure 4A). In patients with BK virus–specific T cells, 39.5% had CD4 and CD8 T cells, 50% had only CD4 T cells, and 10.4% had only CD8 T cells. At 3 months after transplant, BK virus–specific T cells were detected less frequently in cases than in controls. Between 6 and 12 months, BK virus–specific T cells were detected more frequently in cases than controls. No significant differences were seen after 12 months. The most consistent changes in the first 12 months post-HCT were the gradual increase in the fraction of cases that developed both CD4 and CD8 T-cell responses and the gradual decrease in the fraction of patients without a CD4 or CD8 T-cell response among those with documented BK virus disease. By 12 months post-HCT, 66.7% of cases had CD4 and CD8 responses, 25.9% had only CD4 responses, and only 7.4% had no evidence of CD4 or CD8 responses to BK virus (P < .001). In contrast, BK virus–specific T cells were detected in only 54.3% of controls. At later time points after transplant (18–24 months), there were no significant differences between cases and controls. In the first year post-HCT, almost all patients (both cases and controls) with CD8 BK virus responses also had BK virus–specific CD4 T cells. In contrast, 38.6% to 63.7% of patients with CD4 BK virus responses did not have detectable CD8 responses. In the second year post-HCT, the fraction of patients with both CD4 and CD8 responses decreased in cases and controls, and the fraction of patients with only CD8 responses also increased.
Frequency of detection of BK virus–specific CD8 and CD4 T cells across time points. (A) Any BK virus–specific T cells, in cases and controls. (B) BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic T cells, in cases and controls. (C) Frequency of detection of BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T cells across time points, in cases with a single episode of BK virus reactivation and cases with recurrent symptoms. *P < .05, **P < .01, ***P < .001, Pearson’s χ2 test (A) and independent-samples Mann-Whitney U test (B-C).
Frequency of detection of BK virus–specific CD8 and CD4 T cells across time points. (A) Any BK virus–specific T cells, in cases and controls. (B) BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic T cells, in cases and controls. (C) Frequency of detection of BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T cells across time points, in cases with a single episode of BK virus reactivation and cases with recurrent symptoms. *P < .05, **P < .01, ***P < .001, Pearson’s χ2 test (A) and independent-samples Mann-Whitney U test (B-C).
The functional activity of BK virus–specific CD4 and CD8 T cells in cases and controls is shown in Figure 4B. BK virus–specific CD8 T cells secreting IFN-γ and cytolytic CD8 T cells were detected more frequently in cases than in controls at almost all time points. Notably, IFN-γ+ CD8 T cells could be detected as early as 1 month post-HCT (21.7% in cases vs 5.1% in controls; P = .046). These differences persisted at 18 months, by which time almost all cases of BK disease had resolved. In contrast, BK virus–specific cytolytic CD8 T cells were rarely detected in patients without BK virus reactivation (controls). BK virus–specific CD4 T cells secreting Th1 cytokines were also detected more frequently in cases compared with controls. Similar to CD8 T cells, the percentage of patients with BK virus–specific CD4 T cells secreting IFN-γ and IL-2 was significantly higher in cases, and this difference persisted for 18 months post-HCT. The percentage of patients with BK virus–specific IL-2–producing CD4 T cells peaked at 6 months, whereas the fraction of patients with IFN-γ+ and TNF-α+ BK virus–specific CD4 T cells peaked at 12 months. In contrast, the percentage of patients with cytolytic BK virus–specific CD4 T cells increased slowly after HCT, and there were no significant differences between the 2 cohorts.
Seven patients with BK virus disease had persistent or recurrent genitourinary symptoms. Figure 4C compares BK virus–specific T-cell responses in these 7 cases vs patients with only a single episode of urinary symptoms. Although the number of patients with recurrent or persistent urinary symptoms was small, none of these patients developed BK virus–specific CD8 T cells secreting IL-2 or BK virus–specific cytolytic CD8 T cells in the first year posttransplant.
Time to detection of BK virus–specific T cells
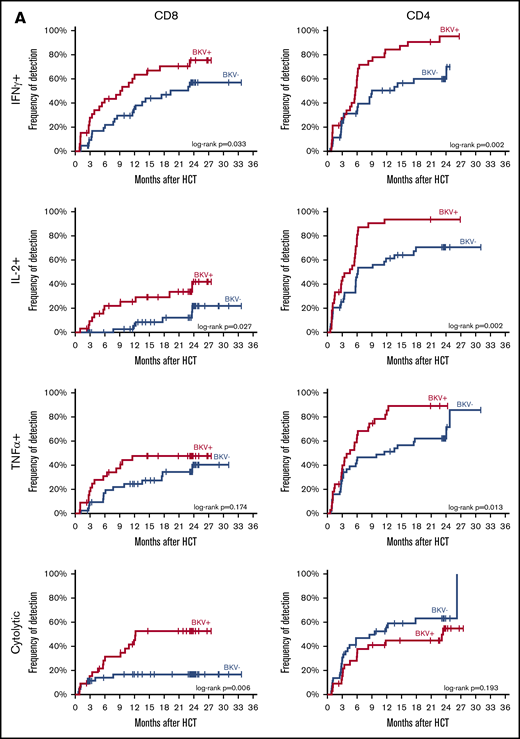
Clinical BK virus disease primarily occurred in the first 6 months after HCT; only 1 case was diagnosed >1 year post-HCT (supplemental Figure 4). Figure 5A shows time to first detection of BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T cells. BK virus–specific CD4 T cells secreting IFN-γ, IL-2, or TNF-α recovered rapidly in the first 6 months post-HCT. During this period, recovery was similar in patients who developed BK virus disease and in patients who did not develop BK viruria. Subsequently, BK virus–specific CD4 T cells secreting IFN-γ, IL-2, or TNF-α were detected in 84.2%, 93.7%, and 85.6% of patients with BK virus disease by 1-year post HCT, respectively, but these cells were detected less frequently in patients without BK virus disease (50.4%, P = .002; 61.3%, P = .002; 51.4%, P = .013). BK virus–specific cytolytic CD4 T cells had equal recovery in cases and controls and were detected in 44.7% of cases and in 55.6% of controls by 1 year post-HCT (P = .193).
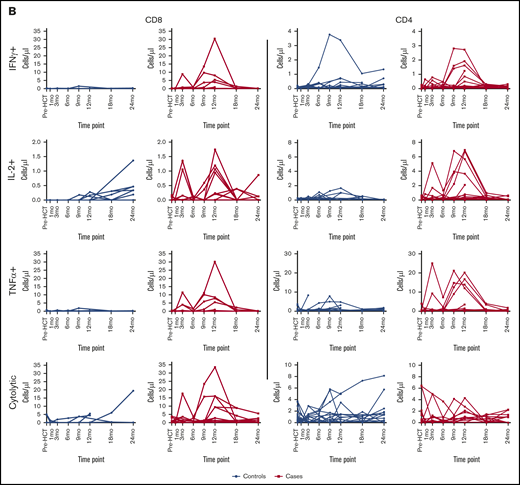
BK virus–specific T-cell reconstitution patterns in cases and controls. (A) Time to first detection of BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T cells in BK virus-positive cases and BK virus-negative controls. Differences between curves were assessed by the log-rank test. (B) Absolute individual patient IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T-cell counts over time, in patients with detectable cells at any time point.
BK virus–specific T-cell reconstitution patterns in cases and controls. (A) Time to first detection of BK virus–specific IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T cells in BK virus-positive cases and BK virus-negative controls. Differences between curves were assessed by the log-rank test. (B) Absolute individual patient IFN-γ+, IL-2+, TNF-α+, and cytolytic CD8 and CD4 T-cell counts over time, in patients with detectable cells at any time point.
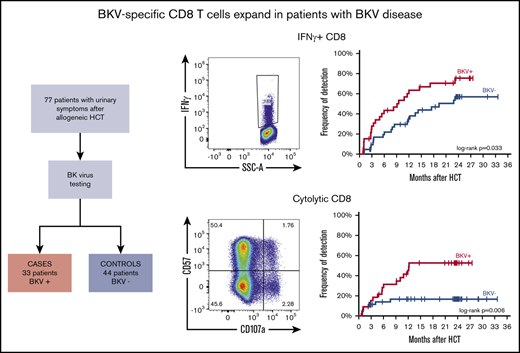
In contrast to CD4 T cells, BK virus–specific CD8 T cells recovered more rapidly in BK virus cases than controls. At 1 year, IFN-γ+, IL-2+ and TNF-α+ CD8 T cells were detected in 63.4%, 25.4% and 47.7% of BK virus+ cases, respectively, and in 37.9% (P = .033), 5.5% (P = .027) and 24.5% (P = .174) of BK virus- controls, respectively. At this time, cytolytic BK virus–specific CD8 T cells were only detected in 45.2% of BK virus cases, but were rarely detected in patients without BK virus reactivation, appearing in only 16.7% of controls (P = .006). Cytokine-producing BK virus–specific CD8 T cells also recovered more slowly and in a smaller fraction of patients without BK virus reactivation. In patients with BK virus disease, the detection of IFN-γ+ and cytolytic CD4 T cells at 1 month after HCT was significantly associated with lower absolute rates of hematuria: 42.9% vs 87.5% (P = .045) and 0% vs 80% (P = .02), respectively.
Serial measurements of BK virus–specific T cells by cytokine flow cytometry also quantifies the magnitude of specific T-cell responses. Figure 5B tracks the levels of CD8 and CD4 T cells in peripheral blood specifically capable of responding to BK virus peptides in individual cases and controls. In controls without evidence of BK virus reactivation, specific responses are generally only detectable at low levels. In contrast, higher levels of BK virus–specific CD8 and CD4 T cells are detected in patients with evidence of BK virus reactivation. In these patients, peaks of circulating T cells appear in the first year after transplant and revert to baseline by 2 years after HCT.
Discussion
Although BK virus disease has become a major cause of morbidity in patients undergoing allogeneic HCT, few studies have examined the role of T-cell immunity in the response to BK virus reactivation. Expanding on previous studies by Saliba et al,24 who described recovery of BK virus–specific T cells after umbilical cord blood transplants, we examined a large cohort of adult patients who received primarily peripheral blood stem cell grafts and conventional GVHD prophylaxis. All patients in our study developed urinary symptoms that prompted testing for BK virus. Testing identified 33 patients with documented BK virus reactivation and no other reason for clinical symptoms, as well as 44 controls with urinary symptoms without evidence of BK virus reactivation. These patients may have developed hematuria as a result of cyclophosphamide toxicity or urinary tract damage in the context of thrombocytopenia. All patients had been enrolled in a prospective posttransplant tissue banking protocol; therefore, cryopreserved PBMCs were available for detailed analysis of BK virus–specific T-cell immunity. In patients without documented BK virus reactivation, recovery of BK virus–specific T-cell immunity presumably reflects the normal pattern of immune reconstitution in the context of a highly prevalent DNA virus. This is derived, in part, from expansion of BK virus–specific T cells present in the donor graft, as well as responses to endogenous viral epitopes in the recipient. In patients with urinary symptoms and BK virus reactivation, the emergence of BK virus–specific T cells also reflects the effects of additional exposure to this immunogenic virus.
In our cohort, BK virus–specific T cells reconstituted rapidly in the first 3 to 6 months after HCT, with 79% of patients having detectable CD4 BK virus–specific T cells and 30% of patients having CD8 BK virus–specific T cells by 6 months posttransplant. BK virus–specific CD4 T cells are predominantly cytokine-producing cells, but cytolytic CD4 T cells are also present. The BK virus–specific CD4 T-cell response was diverse, with IFN-γ, TNF-α, and IL-2–producing cells recovering concurrently. Polyfunctional cytokine-producing CD4 T cells were also observed, and these cells gradually increased in the first year after transplant; however, only 30% of cytokine–secreting CD4 T cells were polyfunctional 12 months after transplant. BK virus–specific cytolytic CD4 T cells were unusual in that they demonstrated antigen-specific degranulation, but very few of these cells were also positive for perforin or granzyme. The BK virus–specific CD8 T-cell response was predominantly IFN-γ producing and cytolytic. In contrast to cytolytic CD4 T cells, cytolytic CD8 T cells often expressed perforin and/or granzyme. In CD4 and CD8 T-cell responses, BK virus–specific cells became increasingly differentiated as time post-HCT increased. This coincided with a gradual increase in IL-2 production in the polyfunctional cytokine-positive subsets, reflecting the role of IL-2 in antigen-specific T-cell maturation.25,26
Absolute T-cell counts were lower in patients with BK virus reactivation at 3 and 6 months after HCT. This was due entirely to delayed recovery of CD4 T cells at 3, 6, and 9 months after transplant. This may be an unappreciated consequence of BK virus reactivation; alternatively, delayed recovery of CD4 T cells may enhance susceptibility to BK virus reactivation. Although BK virus–specific CD4 T cells were detected more frequently than CD8 T cells, patients with BK virus disease had lower total CD4 counts for up to 2 years compared with patients who did not experience BK virus reactivation. CD8 T-cell counts in peripheral blood after transplant were similar in patients with and without BK virus reactivation. Despite having fewer circulating CD4 T cells, the fraction of patients who developed BK virus–specific CD4 T cells in the first 6 months after transplant was similar in patients with and without evidence of viral reactivation. This encompasses the period when most patients experience BK virus reactivation. Subsequently, cytokine-secreting BK virus–specific CD4 T cells developed in more patients who had BK virus reactivation, and the magnitude of response detected in peripheral blood is greater in many of these patients. In contrast, BK virus–specific CD8 T cells recovered earlier and more frequently in patients with BK virus disease. By 1 year after transplant, when BK virus disease was controlled in most patients, levels of BK virus–specific T cells were similar in cases and controls.
Of interest, patients with early (1 month after HCT) reconstitution of BK virus–specific IFN-γ+ and cytolytic CD4 T cells who developed BK virus reactivation had less severe disease, which was manifested by lower rates of hematuria, compared with those with late reconstitution. This suggests that patients who develop BK virus reactivation can initiate a specific immunologic response to BK virus prior to the onset of clinical symptoms. These results are consistent with those observed in kidney transplant patients, in whom higher frequencies of polyfunctional BK virus–specific CD4 T cells were associated with faster viral clearance.27
Our data also provide additional evidence to support the use of in vitro–expanded BK virus–specific T cells for treatment of hemorrhagic cystitis due to BK virus reactivation in HCT recipients. In our cases, BK virus reactivation led to the expansion of CD4 and CD8 BK virus–specific T cells. Although only 7 patients had persistent or recurrent disease, these patients appeared to have delayed recovery of BK virus–specific cytolytic CD8 and CD4 T cells. The vast majority of cases (78.8%) did not have recurrence of symptoms, suggesting that CD8 and CD4 responses effectively prevented further reactivation in most patients. Because early recovery of BK virus–specific CD4 T cells appears to modulate the severity of BK virus disease, these observations suggest that cellular therapies should include both major subsets of BK virus–specific T cells.
All patients in our study developed urinary symptoms. The duration of urinary symptoms was significantly longer in cases than in controls, and significantly fewer controls developed hematuria.10 These clinical findings validate our presumption that urinary symptoms were not related to BK virus in our control patients. Nevertheless, some of these patients may have had transient BK virus reactivation prior to the onset of symptoms or even after resolution of symptoms. Therefore, our control group may not be entirely reflective of patients without BK virus reactivation. Because BK virus reactivation can occur without urinary symptoms, our cohort does not include all HCT patients who developed BK viruria or viremia during the study period. Furthermore, PBMC sampling was obtained at fixed time points, which limited our ability to characterize T-cell responses during specific phases of BK virus disease in a more refined manner. Because immune control is exerted in the local tissue environment, results obtained from peripheral blood may not accurately reflect immune responses occurring at sites of BK virus reactivation. Lastly, we did not assess BK virus–specific antibodies or BK virus genotype and cannot comment on the potential impact of these factors on BK virus reactivation or immune reconstitution. Without knowledge of BK virus donor and recipient serostatus, it is impossible to distinguish de novo immune response to endogenous BK virus from expansion of donor BK virus–specific T cells present in the stem cell graft. To address these limitations, prospective studies that include patients who do not develop urinary symptoms are needed to further expand our understanding of BK virus–specific T-cell immune reconstitution after allogeneic HCT.
Data sharing requests should be sent to Jerome Ritz (jerome_ritz@dfci.harvard.edu).
Acknowledgments
This work was supported by a Collaborative Research Grant from the Harvard Medical School–Portugal Program in Translational Research HMSP-ICT/0001/201, National Institutes of Health, National Cancer Institute grants CA183559, CA183560, and CA229092, and the Pasquarello Tissue Bank in Hematologic Malignancies.
E.E. is a PhD candidate at Universidade de Lisboa, and this work is submitted in partial fulfillment of the requirement for a PhD and was supported by a grant for medical fellows enrolled in a PhD program (Subsídios aos Internos Doutorandos–SINTD) from Fundação para a Ciência e Tecnologia, number SFRH/SINTD/135312/2017.
Authorship
Contribution: E.E. designed and performed research, analyzed data, and wrote the paper; M.P.C. and H.T.K. analyzed data and wrote the paper; A.E.W., E.F., M.V.D.S., J.F.L., S.N., M.G., R.R., E.P.A., P.A., C.S.C., V.T.H., J.K., J.H.A., and R.J.S. contributed vital new reagents or analytical tools; J.I.A. performed research; and F.M.M. and J.R. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: E.E. has received consulting fees from MSD. P.A. has received research funding from Adaptive, Affimed, Bristol-Myers Squibb, Genentech, IGM, Merck, Otsuka, Roche, Sigma Tau, and Tensha Therapeutics; consulting fees from Adaptive, ADC Therapeutics, Affimed, BMS, C4, Celgene, Daiichi Sankyo, Genmab, Infinity, Merck, Miltenyi Biotec, MorphoSys, Pfizer, and Tessa; and honoraria from Bristol-Myers Squibb and Merck. F.M.M. has received research funding from Ansun BioPharma, Chimerix, Cidara Therapeutics, F2G, Gilead, Merck, SCYNEXIS, Shire, and WHISCON and consulting fees from AlloVir, Amplyx, Janssen, KYORIN Pharmaceutical, Merck, and Regeneron. J.R. has received research funding from Amgen, Equillium, and Kite Pharma and consulting fees from Aleta Biotherapeutics, AVROBIO, Celgene, Falcon Therapeutics, LifeVault Bio, Rheos Medicines, Talaris Therapeutics, and TScan Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jerome Ritz, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jerome_ritz@dfci.harvard.edu.
References
Author notes
The full-text version of this article contains a data supplement.