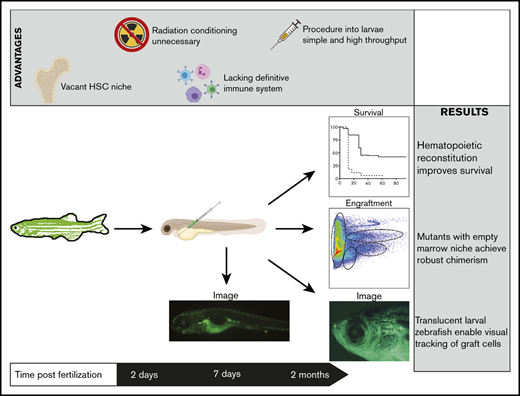
Key Points
Transplantation in HSC-deficient runx1-mutant zebrafish permits robust, multilineage, long-lasting, serially repopulating engraftment.
Engraftment and chimerism are significantly higher in HSC-deficient runx1 mutants than in embryos possessing endogenous HSCs.
Abstract
Transplantation is the most common assay for measuring the in vivo functionality of hematopoietic stem cells (HSCs). Although various HSC transplantation strategies have been developed in zebrafish, they are underutilized because of challenges related to immune matching and preconditioning toxicity. To circumvent these limitations, we developed a simple and robust transplantation model using HSC-deficient hosts. Homozygous runx1W84X mutants are devoid of definitive hematopoietic cells, including HSCs and adaptive immune cells; thus, they require no preconditioning regimen for transplantation. Marrow cell transplantation into runx1-mutant zebrafish 2 days after fertilization significantly improved their survival to adulthood and resulted in robust, multilineage, long-lasting, serially repopulating engraftment. Furthermore, we demonstrated that engraftment into runx1 homozygous mutants was significantly higher than into runx1 heterozygotes, demonstrating that the improved transplantation success is attributable to the empty HSC niche in mutants and not just the embryonic environment. Competitive transplantation of marrow cells into runx1 mutants revealed a stem cell frequency similar to that of murine marrow cells, which demonstrates the utility of this model for quantifying HSC function. The streamlined approach and robustness of this assay will help broaden its feasibility for future high-throughput transplantation experiments in zebrafish and will enable further novel discoveries in the biology of HSCs.
Introduction
The hematopoietic stem cell (HSC) is unique in its extensive abilities to both self-renew and to generate all the differentiated blood cell lineages. These features are best displayed after transplantation when the donor HSCs repopulate the entire blood system of a host. Clinically, HSC transplantation is a standard treatment for malignant and nonmalignant disorders in humans,1-3 and it is a critical laboratory tool for quantifying in vivo HSC function.
The zebrafish is an established model for studying hematopoietic development but also has many advantages that make it an excellent model for studying transplantation biology.4 For example, our ability to use high-resolution whole organism imaging allows a more holistic view of the entire HSC engraftment process.5,6 In addition, the high fecundity, short generation time, and lower cost of husbandry make zebrafish an ideal model for genetic and drug screening to discover novel regulators of HSC transplantation biology.7-9
Several previous HSC transplantation approaches have been developed in zebrafish using both adult and embryonic recipients, with each achieving various degrees of multilineage engraftment.5,6,9-18 From these studies, unique technical challenges were revealed for adult and embryonic zebrafish transplantation strategies. For embryonic recipients, chimerism levels achieved are usually quite low, and the cell dose that can be transplanted is limited by the small size of the embryo.12,15,18 But transplanting adult fish requires irradiation to clear the HSC niche and immune cells, which is not only very toxic to the animals but is also time-consuming and labor-intensive, thus limiting the ease of performing the assay with high-throughput capabilities. Additionally, major histocompatibility complex (MHC) haplotype matching, which is important for preventing graft rejection and graft-versus-host disease, remains incompletely understood in zebrafish.13,19
Drawing on lessons from murine transplantation, we know that genetic models that reduce or eliminate the need for HSC and immune cell clearance can improve transplantation outcomes. Waskow et al20 combined KitW/Wv mice that harbor a mutation that renders endogenous HSCs poorly competitive with Rag2-mutant immunodeficient mice to generate an improved recipient that requires no preconditioning. These animals permit the assessment of HSC function without the complication of the stress caused by irradiation or chemotherapeutic conditioning regimens.
With similar goals in mind, we developed an analogous zebrafish model to obviate the need for preconditioning or haplotype matching. Our radiation-free HSC transplantation approach used runx1W84X-mutant (hereafter runx1-mutant) zebrafish, which are devoid of endogenous definitive hematopoietic cells, including HSCs and adaptive immune cells.21,22 We demonstrated that runx1 mutants are better transplant recipients than embryos with intact definitive hematopoiesis, because they display significantly higher rates of multilineage engraftment and donor-derived chimerism. We showed that runx1 mutants support the engraftment of bona fide HSCs, because the grafts persisted over the long term and were serially transplantable. We then used these runx1-mutant transplants to study HSC function and frequency. Our data suggest that there is some level of HSC heterogeneity, with zebrafish marrow HSCs displaying different lineage outputs toward erythroid, myeloid, lymphoid, and precursor cell production. By using competitive transplants, we revealed a stem cell frequency in zebrafish kidney marrow of ∼1 in 16 000, a number strikingly similar to that found in murine bone marrow. Collectively, these findings delineate the value of this novel transplantation model to assess zebrafish HSC function in a nonablative context and reveal new insights into HSC biology. The streamlined approach and robustness of this assay will help broaden the feasibility for future high-throughput transplantation experiments in zebrafish.
Methods
Zebrafish
Zebrafish were maintained as described.23 All fish were maintained according to Institutional Animal Care and Use Committee–approved protocols in accordance with Albert Einstein College of Medicine research guidelines. After transplantation, fish were kept at a density of 10 fish per dish or tank and put into the nursery area of the aquatic facility between 5 and 7 days postfertilization (dpf). They were raised in the facility until the time of kidney marrow analysis.
The runx1W84X-mutant strain (acquired from the Liu laboratory) contains a single G>A mutation in exon 3 replacing tryptophan at amino acid position 84 with a premature stop codon, which results in a null allele.22 A small percentage of runx1 mutants survive to adulthood because of compensation during embryogenesis. Genotyping of runx1 mutants was performed using custom-designed TaqMan single nucleotide polymorphism probes with the sequence TCTGCTCCGTCCTGCCGACACACTG[G/A]CGCTGCAACAAGACCCTGCCCATCG. The transgenic (Tg) ubiquitin:green fluorescent protein [Tg(ubi:GFP)] (hereafter ubi:GFP) strain is a stable transgenic line with GFP production driven by the ubiquitin promoter resulting in ubiquitous GFP expression.24 Similarly, the Tg(ubi:mCherry) (hereafter ubi:mCherry) strain expresses mCherry under the ubiquitin promoter.24 All strains used were maintained on an AB genetic background.
HSC donor cell preparation
Donor kidneys were dissected from 4 to 6 euthanized adult (3- to 9-month-old) ubi:GFP or ubi:mCherry fish and placed into fluorescence-activated cell sorting (FACS) buffer (0.9× Dulbecco’s phosphate-buffered saline, 5% fetal bovine serum, and 1% Pen/Strep [Life Technologies]). After trituration, the samples were filtered through a 40-μm cell strainer to remove debris and were pelleted by centrifugation at 2500 rpm for 5 minutes. Cell pellets were resuspended in an appropriate quantity of injection buffer to yield a concentration of 500 leukocytes per nL. The injection buffer consisted of FACS buffer with 500 μM EDTA and 1 μL TurboDNase (Life Technologies) per 20 μL of buffer.
Hematopoietic cell transplantation
At 2 dpf, embryos were manually dechorionated, anesthetized with tricaine, and then placed on an injection plate (1% agarose in a petri dish). A nonfilament microinjection needle was then trimmed to release 5 nL droplets containing ∼2500 leukocytes and loaded with the prepared cell suspension. The needle was inserted into the common cardinal vein by first penetrating through the yolk (Figure 1A). Cells were injected using a pressure of 29 psi. For each experimental day, ∼100 to 200 embryos were transplanted. Both sham-injected and uninjected embryos were used as controls. Sham-injected embryos received only the injection buffer without donor cells. Uninjected embryos were anesthetized in the same way as injected embryos.
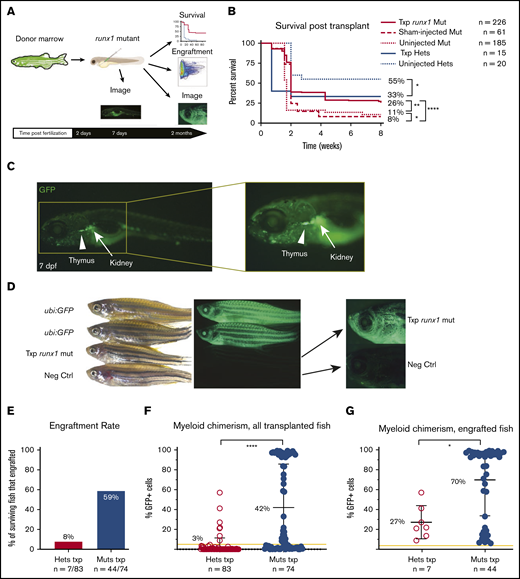
runx1 mutants show robust engraftment after HCT. (A) Experimental schema for hematopoietic cell transplantation (HCT): donor marrow cells from ubi:GFP transgenic adult zebrafish were harvested and transplanted into runx1-mutant embryos at 2 dpf. Survival was assessed for 2 months posttransplant until the time of engraftment analysis. Fluorescent imaging of transplanted fish was also performed to qualitatively assess engraftment. (B) Kaplan-Meier curves showing survival of transplanted runx1 mutants (Txp runx1 mut) in comparison with sham-injected or uninjected mutants as well as transplanted heterozygotes (Txp hets) and uninjected heterozygotes. Survival analysis was performed until 2 months posttransplant. Statistical analysis performed with log-rank (Mantel-Cox) test. (C) Images of transplanted runx1-mutant larvae 5 days posttransplant (7 dpf); the inset highlights the seeding of the developing thymus (arrowhead) and kidney (arrow) with donor-derived GFP+ cells. (D) Bright field (left) and fluorescent (center and right) images of adult zebrafish showing levels of GFP positivity in ubi:GFP donors (top), a transplanted runx1 mutant (middle), and a negative untransplanted wild-type control (Neg Ctrl, bottom). Center images are at 0.63× magnification and lower camera exposure; right images are at 2× magnification and longer exposure times. Note that donor GFP+ cells are detectable in transplanted runx1 mutant adults via fluorescent microscopy with lower GFP intensity compared with ubi:GFP donors because only the blood cells are fluorescent. (E) Donor engraftment rate at 8 weeks posttransplant in the surviving runx1 mutants (Muts txp) compared with heterozygotes (Hets txp). Engraftment was defined as myeloid chimerism ≥5%. Mutants display a higher engraftment rate of 59% compared with 8% in heterozygotes. The result is significant (Fisher’s exact test P < .01). (F) Donor myeloid chimerism at 8 weeks posttransplant for all surviving transplanted fish. Each dot represents the chimerism level of individual transplanted fish. The yellow line indicates 5% chimerism, the cutoff defined as engraftment. Transplanted homozygous runx1 mutants have significantly higher chimerism values than heterozygotes, with a mean of 42% (± 44%) compared with 3% (± 9%), respectively. Statistical analysis was performed with Mann-Whitney U test. (G) Donor myeloid chimerism at 8 weeks posttransplant for all fish that reached the cutoff for engraftment. Each dot represents the chimerism level of individual transplanted fish. The yellow line indicates 5% chimerism, the cutoff defined as engraftment. Engrafted homozygous runx1 mutants have significantly higher chimerism values than engrafted heterozygotes, with a mean of 70% (± 36%) compared with 27% (± 17%), respectively. Error bars are standard deviation. Statistical analysis performed with Mann-Whitney U test. *P = .01-.05; **P = .001-.01; **** P < .0001.
runx1 mutants show robust engraftment after HCT. (A) Experimental schema for hematopoietic cell transplantation (HCT): donor marrow cells from ubi:GFP transgenic adult zebrafish were harvested and transplanted into runx1-mutant embryos at 2 dpf. Survival was assessed for 2 months posttransplant until the time of engraftment analysis. Fluorescent imaging of transplanted fish was also performed to qualitatively assess engraftment. (B) Kaplan-Meier curves showing survival of transplanted runx1 mutants (Txp runx1 mut) in comparison with sham-injected or uninjected mutants as well as transplanted heterozygotes (Txp hets) and uninjected heterozygotes. Survival analysis was performed until 2 months posttransplant. Statistical analysis performed with log-rank (Mantel-Cox) test. (C) Images of transplanted runx1-mutant larvae 5 days posttransplant (7 dpf); the inset highlights the seeding of the developing thymus (arrowhead) and kidney (arrow) with donor-derived GFP+ cells. (D) Bright field (left) and fluorescent (center and right) images of adult zebrafish showing levels of GFP positivity in ubi:GFP donors (top), a transplanted runx1 mutant (middle), and a negative untransplanted wild-type control (Neg Ctrl, bottom). Center images are at 0.63× magnification and lower camera exposure; right images are at 2× magnification and longer exposure times. Note that donor GFP+ cells are detectable in transplanted runx1 mutant adults via fluorescent microscopy with lower GFP intensity compared with ubi:GFP donors because only the blood cells are fluorescent. (E) Donor engraftment rate at 8 weeks posttransplant in the surviving runx1 mutants (Muts txp) compared with heterozygotes (Hets txp). Engraftment was defined as myeloid chimerism ≥5%. Mutants display a higher engraftment rate of 59% compared with 8% in heterozygotes. The result is significant (Fisher’s exact test P < .01). (F) Donor myeloid chimerism at 8 weeks posttransplant for all surviving transplanted fish. Each dot represents the chimerism level of individual transplanted fish. The yellow line indicates 5% chimerism, the cutoff defined as engraftment. Transplanted homozygous runx1 mutants have significantly higher chimerism values than heterozygotes, with a mean of 42% (± 44%) compared with 3% (± 9%), respectively. Statistical analysis was performed with Mann-Whitney U test. (G) Donor myeloid chimerism at 8 weeks posttransplant for all fish that reached the cutoff for engraftment. Each dot represents the chimerism level of individual transplanted fish. The yellow line indicates 5% chimerism, the cutoff defined as engraftment. Engrafted homozygous runx1 mutants have significantly higher chimerism values than engrafted heterozygotes, with a mean of 70% (± 36%) compared with 27% (± 17%), respectively. Error bars are standard deviation. Statistical analysis performed with Mann-Whitney U test. *P = .01-.05; **P = .001-.01; **** P < .0001.
Assessment of engraftment
Surviving fish were euthanized at 2 months posttransplantation, and kidney marrow cells from individual recipients were prepared as described above. 4′,6-Diamidino-2-phenylindole was added to a final concentration of 1 μg/mL to facilitate exclusion of dead cells from the analysis. At the same time as kidney harvest, tail clips were obtained for genotyping. After trituration, kidney samples were filtered through a 40-μm strainer into FACS tubes and were analyzed on a BD LSRII flow cytometer. Gating for hematopoietic cell populations was performed as previously described and was validated with May-Grünwald-Giemsa staining by Traver et al.12 To quantify chimerism (as shown in Figures 1F-G, 2C-F, and 3B; supplemental Figure 2), we first gated on the hematopoietic lineage of interest (eg, precursor, erythroid, myeloid, and lymphoid) and then determined the percentage of GFP+ cells within the lineage-restricted gate (supplemental Figure 1A-B). For assessment of the lineage contribution among all GFP+ donor-derived cells (as shown in Figures 2G and 3C), we first gated on all GFP+ cells and then determined the percentage of cells within each hematopoietic lineage population (supplemental Figure 1C).
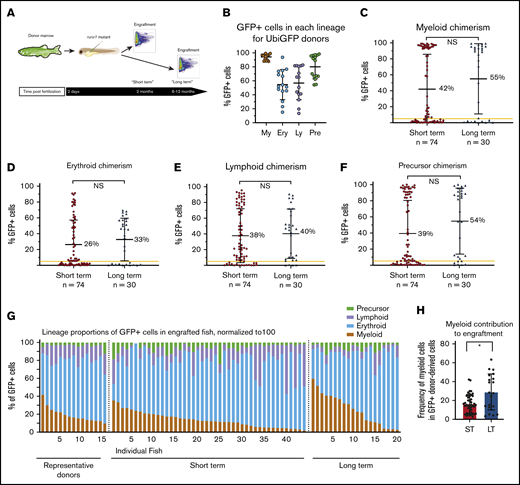
Engraftment in runx1 mutants is multilineage and persistent. (A) Experimental schema: donor marrow was harvested from ubi:GFP adult fish and transplanted into runx1-mutant embryos at 2 dpf. Multilineage engraftment was assessed at 2 months (short term) and 6 to 12 months (long term). (B) Dot plot demonstrating the percentages of myeloid (My), erythroid (Ery), lymphoid (Ly), and precursor (Pre) cells that are GFP+ in 15 different ubi:GFP donor marrows. The vast majority of myeloid cells are GFP+, but there is much more variable GFP expression in the other lineages. (C-F) Myeloid (C), erythroid (D), lymphoid (E), and precursor (F) chimerism values for individual transplanted runx1-mutant fish, as assessed by percent GFP+ cells within each lineage at 2 months (short term) or 6 to 12 months (long term) posttransplant. The yellow line indicates 5% chimerism cutoff for engraftment. Comparison between the 2 groups is not significant by Mann-Whitney U test for any of the 4 blood lineages. (G) Lineage distribution of donor-derived GFP+ cells in individual engrafted runx1-mutant fish at 2 months (short term) or 6 to 12 months (long term) posttransplant, as well as in representative ubi:GFP donors. (H) The percentage of GFP+ donor-derived cells from the myeloid lineage in transplanted recipients at short-term (ST) and long-term (LT) time points posttransplantation. Error bars are standard deviation. Statistical analysis was performed via Mann-Whitney U test. *P < .05. NS, not significant.
Engraftment in runx1 mutants is multilineage and persistent. (A) Experimental schema: donor marrow was harvested from ubi:GFP adult fish and transplanted into runx1-mutant embryos at 2 dpf. Multilineage engraftment was assessed at 2 months (short term) and 6 to 12 months (long term). (B) Dot plot demonstrating the percentages of myeloid (My), erythroid (Ery), lymphoid (Ly), and precursor (Pre) cells that are GFP+ in 15 different ubi:GFP donor marrows. The vast majority of myeloid cells are GFP+, but there is much more variable GFP expression in the other lineages. (C-F) Myeloid (C), erythroid (D), lymphoid (E), and precursor (F) chimerism values for individual transplanted runx1-mutant fish, as assessed by percent GFP+ cells within each lineage at 2 months (short term) or 6 to 12 months (long term) posttransplant. The yellow line indicates 5% chimerism cutoff for engraftment. Comparison between the 2 groups is not significant by Mann-Whitney U test for any of the 4 blood lineages. (G) Lineage distribution of donor-derived GFP+ cells in individual engrafted runx1-mutant fish at 2 months (short term) or 6 to 12 months (long term) posttransplant, as well as in representative ubi:GFP donors. (H) The percentage of GFP+ donor-derived cells from the myeloid lineage in transplanted recipients at short-term (ST) and long-term (LT) time points posttransplantation. Error bars are standard deviation. Statistical analysis was performed via Mann-Whitney U test. *P < .05. NS, not significant.
Cells engrafted in runx1 mutants have capacity for serially repopulating. (A) Experimental schema: donor marrow was harvested from 6-month-old highly engrafted (>70% myeloid chimerism) runx1 primary transplanted fish (1° txp), and secondary transplants (2° txp) were performed into runx1-mutant embryos 2 dpf. Chimerism in secondary transplant recipients was assessed at 2 months posttransplant by flow cytometry. Tertiary transplants (3° txp) were performed with the same procedure using 6-month-old secondary recipients as donors. (B) Multilineage chimerism (myeloid [My], erythroid [Ery], lymphoid [Ly], and precursor [Pre]) in runx1-mutant recipients after secondary and tertiary transplant measured at 2 months posttransplant. The yellow line indicates 5% chimerism cutoff for engraftment. Thirty-one of 32 surviving transplanted runx1 mutants demonstrated high levels of multilineage engraftment after secondary transplantation, with 9 of 47 showing high-level engraftment after tertiary transplantation. (C) Lineage distribution of donor-derived GFP+ cells in individual primary and secondary transplanted donors as well as secondary and tertiary engrafted runx1-mutant recipients at 2 months posttransplant. (D) The percentage of donor-derived cells of myeloid lineage in recipients of primary, secondary, and tertiary transplantation. Secondary transplants demonstrated a significantly higher contribution from the myeloid lineage than primary recipients, but this effect was not seen in tertiary transplants. Error bars are standard deviation. Statistical analysis was performed via Mann-Whitney U test. **P < .001; ****P < .0001.
Cells engrafted in runx1 mutants have capacity for serially repopulating. (A) Experimental schema: donor marrow was harvested from 6-month-old highly engrafted (>70% myeloid chimerism) runx1 primary transplanted fish (1° txp), and secondary transplants (2° txp) were performed into runx1-mutant embryos 2 dpf. Chimerism in secondary transplant recipients was assessed at 2 months posttransplant by flow cytometry. Tertiary transplants (3° txp) were performed with the same procedure using 6-month-old secondary recipients as donors. (B) Multilineage chimerism (myeloid [My], erythroid [Ery], lymphoid [Ly], and precursor [Pre]) in runx1-mutant recipients after secondary and tertiary transplant measured at 2 months posttransplant. The yellow line indicates 5% chimerism cutoff for engraftment. Thirty-one of 32 surviving transplanted runx1 mutants demonstrated high levels of multilineage engraftment after secondary transplantation, with 9 of 47 showing high-level engraftment after tertiary transplantation. (C) Lineage distribution of donor-derived GFP+ cells in individual primary and secondary transplanted donors as well as secondary and tertiary engrafted runx1-mutant recipients at 2 months posttransplant. (D) The percentage of donor-derived cells of myeloid lineage in recipients of primary, secondary, and tertiary transplantation. Secondary transplants demonstrated a significantly higher contribution from the myeloid lineage than primary recipients, but this effect was not seen in tertiary transplants. Error bars are standard deviation. Statistical analysis was performed via Mann-Whitney U test. **P < .001; ****P < .0001.
Statistics
The Mann-Whitney U test was performed to compare chimerism values between different groups. The log-rank (Mantel-Cox) test was used to compare survival between different groups. Levene’s test was used to compare variances between groups.
Results
runx1 mutants show robust engraftment after HSC transplantation
Homozygous runx1 mutants fail to develop a definitive blood system and thus should have an empty HSC niche and lack the adaptive immune cells that normally facilitate graft failure and rejection.22 On the basis of these characteristics, we hypothesized that runx1 mutants would be excellent HSC transplantation recipients. To test this hypothesis, we transplanted 2 dpf runx1-mutant embryos with adult marrow cells and then assessed survival and engraftment rates (Figure 1A). Specifically, we harvested whole kidney marrows from adult ubi:GFP zebrafish24 to track donor-derived cells and then injected the cells into the common cardinal vein of runx1-mutant embryos (Figure 1A). Uninjected and sham-injected embryos served as controls.
Most homozygous runx1 mutants die within the first 2 weeks of life because they lack a definitive blood system.21 If hematopoietic cell transplantation (HCT) was successful in replacing the faulty blood system, we posited that survival of runx1 mutant animals would improve. Survival was measured and compared among uninjected, sham-injected, and transplanted runx1 mutants and heterozygotes (Figure 1B). The sham-injected mutants had poorer survival compared with the uninjected embryos, which indicated some procedure-related mortality. Consistent with this observation, transplanted runx1 heterozgyotes had poorer survival than uninjected animals. In contrast, survival of runx1 mutants was significantly improved to 26% after HCT compared with uninjected (11%) or sham-injected (8%) mice. These results suggest successful hematopoietic engraftment into runx1 mutants.
To directly assess donor cell engraftment, we assessed the presence of ubi:GFP+ cells in transplanted zebrafish by using microscopy and flow cytometry (Figure 1A). Immediately after the transplantation procedure, GFP+ cells can be seen circulating around the embryo by using fluorescent microscopy. By 7 dpf, which is 5 days posttransplant, GFP+ cells are visible throughout the entire circulatory system and are concentrated in the developing thymus and kidney, 2 major sites of hematopoiesis (Figure 1C). GFP+ cells are also readily detected throughout transplanted zebrafish at 2 months posttransplantation (Figure 1D). Their fluorescence is significantly less than that of ubi:GFP donors that express GFP in all cells in their bodies because the transplanted runx1 mutants have only GFP+ hematopoietic cells.
To gain a more quantitative view of engraftment, hematopoietic chimerism in whole kidney marrows of 2-month-old transplant recipients was assessed by flow cytometry (Figure 1A). To determine whether engraftment into runx1 mutants is superior to transplantation into animals with an intact definitive hematopoietic system, we compared engraftment between transplanted runx1 mutants and heterozygotes. Because myeloid cells display the fastest turnover of all blood cells, we first determined that donor-derived chimerism in myeloid cells is a more sensitive metric of hematopoietic stem or progenitor cell activity. Myeloid cells were gated on the basis of forward and side scatter parameters, and then the percentage of GFP+ cells was determined (supplemental Figure 1A). Zebrafish with ≥5% donor-derived myeloid chimerism were defined as engrafted. On the basis of this metric, 59% of surviving transplanted runx1 mutants met the threshold for engraftment at 2 months after transplant (Figure 1E). This was significantly higher than the 8% of engrafted runx1 heterozygotes. Consistent with higher engraftment, runx1 mutants also displayed higher myeloid chimerism with an average of 42% (± 44%) GFP+ cells compared with only 3% (± 9%) in heterozygotes for all transplanted fish (Figure 1F), and 70% (± 36%) for engrafted mutants vs 27% (± 17%) for heterozygotes (Figure 1G). Many of the mutants displayed >90% chimerism, which suggests an almost complete replacement of the blood system.
Engraftment in runx1 mutants is multilineage and persistent and serially repopulates
Robust myeloid chimerism at 2 months after transplant is suggestive of stem cell engraftment. To further test this, we analyzed additional characteristics that designate long-term HSC engraftment: multilineage contribution, long-term engraftment, and serial repopulating capacity (Figures 2 and 3).
To measure engraftment in other lineages, mature erythroid and lymphoid cells as well as precursors were gated based on forward and side scatter parameters, and then the percentage of GFP+ cells was determined (supplemental Figure 1B). The frequency of GFP expression in erythroid, myeloid, lymphoid, and precursor cells was determined in donors (Figure 2B). All runx1 mutants that displayed myeloid engraftment also showed appreciable erythroid, lymphoid, and precursor engraftment (Figure 2C-F). We observed substantially lower erythroid, lymphoid, and precursor chimerism compared with myeloid chimerism, but this can be explained by the lower and more heterogeneous expression of the ubi:GFP transgene in donor erythroid, lymphoid, and precursor cells compared with the nearly 100% GFP expression in the donor myeloid cells (Figure 2B). Similar to myeloid chimerism, the donor-derived contribution to erythroid, lymphoid, and precursor cells was significantly higher in runx1 mutants compared with runx1 heterozygotes (supplemental Figure 2). Multilineage chimerism in runx1 mutants remained high at long-term time points (>6 months) (Figure 2C-F), which indicates that mutants are capable of supporting long-term multilineage engraftment.
Not all HSCs are the same. Numerous HSC subtypes with different lineage output preferences have been defined in mammalian hematopoiesis in recent years.25 To evaluate the HSC subtypes supported in runx1 mutants, we analyzed lineage output in all transplant recipients (Figure 2G; supplemental Figure 1C). Examination of the relative erythroid, myeloid, lymphoid, and precursor cell contributions within the GFP+ cells revealed extensive heterogeneity across recipients. Although there is variation of ubi:GFP transgene expression in erythroid, lymphoid, and precursor cells that could also be contributing to this heterogeneity, the variance in recipients of transplants at long-term time points is greater than expected from the donor heterogeneity alone (P < .001 by Levene’s test). These data suggest that both balanced and lineage-skewed HSCs exist in zebrafish marrow.
To more robustly evaluate long-term multilineage repopulating capacity, we performed serial transplantation. The marrow cells from highly engrafted (>70% donor chimerism) runx1-mutant primary transplant recipients at least 6 months after transplant were transplanted into secondary runx1-mutant embryonic recipients with subsequent tertiary transplantation of the marrow cells from these animals (Figure 3A). Recipients of secondary and tertiary transplantation were assayed for multilineage chimerism at 2 months after transplantation, as described above. We observed excellent multilineage reconstitution in 31 of 32 secondary recipients, with mean chimerism values of 66% (± 21%) for erythroid, 91% (± 19%) for myeloid, 71% (± 22%) for lymphoid, and 86% (± 21%) for precursor cells (Figure 3B). In tertiary recipients, we observed 10% (± 21%) erythroid, 14% (± 31%) myeloid, 14% (± 26%) lymphoid, and 12% (± 27%) precursor mean chimerism (Figure 3B). These data indicate that runx1 mutants sustain multilineage serially repopulating HSCs.
To investigate lineage distribution, we examined the relative erythroid, myeloid, lymphoid, and precursor contributions to the GFP+ cells of secondary and tertiary transplanted runx1 mutants (Figure 3C). Interestingly, the donor-derived contribution in engrafted secondary transplant recipients was significantly more myeloid skewed than that in primary transplant recipients, although this was no longer seen in tertiary transplants (Figure 3D).
HSC frequency can be estimated by competitive repopulation in runx1 mutants
Competitive repopulation assays are commonly used to estimate HSC frequencies and to compare hematopoietic reconstitution capacities between different experimental groups.26 We tested whether runx1-mutant HSC transplantation could be a valuable competitive repopulation system. Donor marrow cells were harvested from adult ubi:GFP and ubi:mCherry donors and then transplanted into runx1 mutants at 2 dpf at various ratios (Figure 4A). To determine the frequency of competitive repopulating units in the ubi:GFP donor cells, we measured GFP and mCherry chimerism in recipients 2 months after transplant and determined engraftment frequency (defined as fish with ≥5% GFP+ myeloid chimerism) for each limited-dilution experimental group (Figure 4B). We used the Extreme Limiting Dilution Analysis calculator,27 which used the engraftment success rates to calculate a stem cell frequency of 1 in 15 826 cells (confidence interval: 8773-28 552). This number is in line with previous work that estimated stem cell frequency in murine bone marrow with competitive repopulation.28
Competitive repopulation in runx1 mutants reveals a high stem cell frequency. (A) Experimental schema: adult ubi:GFP and ubi:mCherry donor marrow was harvested and transplanted at different ratios into runx1-mutant embryos at 2 dpf. Donor-derived GFP+ and mCherry+ chimerism was assessed 2 months posttransplant. (B) Percentage of transplanted runx1 mutants engrafted with GFP+ donor cells after transplantation with varying proportions of mixed GFP and mCherry cells. Engraftment was defined as ≥5% GFP+ myeloid cells. (C) Stem cell frequency was calculated by using the Extreme Limiting Dilution Analysis calculator.27 The tested subjects included all transplanted runx1-mutant larvae, and subjects with a response were fish that met the 5% myeloid chimerism threshold.
Competitive repopulation in runx1 mutants reveals a high stem cell frequency. (A) Experimental schema: adult ubi:GFP and ubi:mCherry donor marrow was harvested and transplanted at different ratios into runx1-mutant embryos at 2 dpf. Donor-derived GFP+ and mCherry+ chimerism was assessed 2 months posttransplant. (B) Percentage of transplanted runx1 mutants engrafted with GFP+ donor cells after transplantation with varying proportions of mixed GFP and mCherry cells. Engraftment was defined as ≥5% GFP+ myeloid cells. (C) Stem cell frequency was calculated by using the Extreme Limiting Dilution Analysis calculator.27 The tested subjects included all transplanted runx1-mutant larvae, and subjects with a response were fish that met the 5% myeloid chimerism threshold.
Discussion
HCT assays are critical tools in the study of HSC biology, because they are the gold standard for assessing both self-renewal and differentiation capacity. Our novel transplantation system provides a relatively simple method for testing HSC function in zebrafish. We demonstrated that runx1 mutants serve as excellent transplantation recipients capable of sustaining robust donor-derived multilineage, long-term, serially repopulating hematopoiesis.
The lack of definitive hematopoietic development in runx1 mutants precludes the need for any myeloablation, so HSC function can be assessed without the massive cytokine release that normally accompanies irradiation- or chemotherapy-based preconditioning. Studying the process of stem cell homing and donor-host cell interactions within the niche will benefit greatly from examination within an undamaged microenvironment. In addition, the lack of an adaptive immune system means that graft rejection should be largely mitigated. Although MHC typing in zebrafish has greatly improved in recent years,13,19 the full characterization of immune compatibility across MHC loci is still lacking. Thus, obviating the need for immune matching to prevent graft rejection is a major advance of our transplantation model.
Although HSC transplantation into embryos was previously performed by a few groups, the chimerism and engraftment rates were considerably lower than that observed in our system. Actually, the first published report of HCT in zebrafish was transplant into wild-type and gata1-mutant embryos.12 In that study, successful engraftment of whole kidney marrow cells was demonstrated via fluorescent microscopy imaging of circulating fluorescent blood cells in the vasculature of recipients 8 weeks after transplant, but a quantitative multilineage chimerism assessment was not performed.12 More recently, Tamplin et al15 performed transplantation of purified runx1+23:mCherry HSCs into wild-type embryos and demonstrated multilineage hematopoietic repopulation. The chimerism levels in that study were generally low (<1%), which could reflect the potency of the HSC fraction or recipient factors. Our data demonstrating a significant difference in chimerism levels between runx1 heterozygotes (<3%) and runx1 mutants (>40%) suggest that the presence of endogenous functional HSCs greatly diminishes donor HSC competition and results in diminished engraftment. Although our studies were performed with unfractionated whole kidney marrow cells, the results suggest that the low-level chimerism of the runx1+23:mCherry cells transplanted into wild-type embryos was more likely a reflection of the transplantation system than the potency of the purified population. We transplanted into embryos at 2 dpf, a developmental time point when the caudal hematopoietic tissue is the major hematopoietic niche. Our data, which demonstrates a difference in engraftment rates and chimerism levels between the HSC-competent runx1 heterozygous animals and HSC-deficient runx1 mutants, suggest that even at this early time point, endogenous HSC-niche interactions must be robust and relatively resistant to displacement by donor-derived HSCs. Future studies of endogenous vs donor HSC interaction with distinct developmental HSC depots will provide more insight into this important component of transplant biology.
Immune-deficient strains such as rag2, jak3, and prkdc knockouts have been used successfully for marrow or leukemic cell transplantation without preconditioning.29,30 These strains show great promise for tumor transplantation, but the existence of endogenous HSCs within these animals sets up a competitive environment for transplanted HSCs that could limit their utility for studying normal HSC biology. In addition, high-throughput capability in these models remains limited because some strains must be bred as heterozygotes with the homozygous mutants at significantly higher risk of infection and thus early mortality. Our approach to transplanting into 2 dpf embryos provides a fully functional immune system to the fragile runx1 mutants.
Similar to runx1 mutants, c-mybt25127 mutants lack definitive hematopoiesis and have been used to perform radiation-free HSC transplantation.16 Transplantation of whole kidney marrow cells into adult c-myb mutants resulted in multilineage and serially repopulating engraftment. Although this is a useful model, it has some practical drawbacks for large-scale HSC transplantation in zebrafish. Unlike the ∼10% of zebrafish with a runx1 mutation that survive, those with loss of c-myb universally succumb by 10 to 11 weeks after fertilization. Generation of c-myb homozygous mutants for transplantation is therefore accomplished by breeding heterozygotes. In practice, this necessitates the generation of 4 times more animals than will be used in any transplantation experiments and genotyping of all animals before transplantation, which could lead to additional lethality from injury and increased risk of infection from fin clipping. In contrast, runx1 homozygous mutants can be bred to obtain all-mutant clutches of embryos, such that all transplanted animals will be informative. This advantage could permit more rapid and large-scale screening for transplantation modifiers compared with previous studies,9 although the variable escaper phenotype means that nontransplanted, sham-injected controls must be obtained and considered for each transplantation experiment. Means of suppressing HSC restoration in escapers during embryogenesis could alleviate this caveat.
We observed heterogeneity in lineage output in primary runx1-mutant recipients, suggesting that whole kidney marrow cells contain HSCs with different lineage preferences. One caveat to our findings is that we used pooled donor cells that could have varied ubi:GFP transgene expression (as observed in Figure 2B). Thus, the extent of HSC heterogeneity in zebrafish marrow HSCs should be further explored using single donors with known GFP expression levels across each lineage. This lineage heterogeneity in GFP fluorescence in ubi:GFP donors could also lead to an underestimate of donor chimerism in erythroid, lymphoid, and precursor populations. Assessment of donor and recipient cell contribution by quantitative polymerase chain reaction genotyping of donor (wild-type) and recipient (runx1-mutant) alleles in sorted cells of each lineage could be more accurate because it circumvents heterogeneous transgene expression, but this comes with a higher cost in time and resources to sort cells from each recipient. In contrast, the ease of flow cytometry assessment provides a higher throughput and simpler approach for measuring donor cell chimerism, but conclusions regarding heterogeneous lineage contribution must be made with caution. In secondary transplant recipients, we noted a more universal predilection for myeloid lineage output, suggestive of the myeloid skewing observed in aged HSCs in mice,25 although this was not seen in tertiary transplants likely because of HSC exhaustion. Further studies using the runx1-mutant model can be used to more fully explore potential effects of age on lineage output by using single donors of various ages and also potentially using the nearly isogenic homozygous diploid CG1 and CG2 animals as donors.31,32
Lineage-biased HSCs in mice can be purified by differential marker expression, such that their similarities and differences can be studied. Functional testing of diverse HSC populations such as those marked by cd41:gfp,14 runx1+23:mCherry,15 and the more recently described gata2a:gfp+;runx1+23:mCherry+33 using the runx1-mutant transplantation system could help identify equivalent lineage-biased HSCs in zebrafish. Once defined, the origins and regulation of the various HSC populations can be examined in this genetically malleable model.
In summary, we present here a novel method for HSC transplantation that can be used to measure self-renewal and differentiation capacity in zebrafish. Visualizing host-donor cell interaction within an unperturbed niche is greatly facilitated by this model. Combined with the existence of numerous transgenic and mutant lines and the ability to rapidly generate others using CRISPR/Cas9, the runx1 transplantation system can be used as a powerful platform for discovering new regulation of HSC functionality.
Please send data sharing requests via e-mail to the corresponding author, Teresa V. Bowman, at teresa.bowman@einsteinmed.org.
Acknowledgments
The authors thank Kathryn Potts, Kira Gritsman, David Loeb, Kerry Morrone, and Sofia de Oliveira for their critical questions, comments, and assistance with the conceptual development of this project; Paul Liu and Erica Bresciani for sharing the runx1 mutants and helpful discussions on maintaining the line; and Clinton dePaolo for logistical assistance and Kaitlin Strumph for help with statistical analysis.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (P30CA013330) (Analytical Imaging Facility [AIF] and flow cytometry facilities), National Institute of Diabetes and Digestive and Kidney Diseases (1R56DK121738-01) (T.V.B.), and National Institute of General Medical Sciences (R25-GM104547) (Postbaccalaureate Research Education Program at Albert Einstein College of Medicine) (M.F.N.); the American Cancer Society (RSG-129527-DDC) (T.V.B.); the US Department of Defense (BM180109) (T.V.B.); and the Edward P. Evans Foundation.
Experimental schema images were created with BioRender.com. Confocal imaging was performed at the Albert Einstein College of Medicine AIF with the assistance of Hillary Guzik, and flow cytometry was performed at the Albert Einstein College of Medicine flow cytometry facility.
Authorship
Contribution: E.F. designed and performed experiments, analyzed the data, and drafted the manuscript; M.F.N. performed experiments; and T.V.B. designed experiments, supervised the project, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.F. is Department of Pediatrics (Stem Cell Transplantation and Cellular Therapies), Memorial Sloan Kettering Cancer Center, New York, NY.
Correspondence: Teresa V. Bowman, Albert Einstein College of Medicine and Montefiore Medical Center, 1300 Morris Park Ave, Chanin 501, Bronx, NY 10461; e-mail: teresa.bowman@einsteinmed.org.
References
Author notes
The full-text version of this article contains a data supplement.

![Cells engrafted in runx1 mutants have capacity for serially repopulating. (A) Experimental schema: donor marrow was harvested from 6-month-old highly engrafted (>70% myeloid chimerism) runx1 primary transplanted fish (1° txp), and secondary transplants (2° txp) were performed into runx1-mutant embryos 2 dpf. Chimerism in secondary transplant recipients was assessed at 2 months posttransplant by flow cytometry. Tertiary transplants (3° txp) were performed with the same procedure using 6-month-old secondary recipients as donors. (B) Multilineage chimerism (myeloid [My], erythroid [Ery], lymphoid [Ly], and precursor [Pre]) in runx1-mutant recipients after secondary and tertiary transplant measured at 2 months posttransplant. The yellow line indicates 5% chimerism cutoff for engraftment. Thirty-one of 32 surviving transplanted runx1 mutants demonstrated high levels of multilineage engraftment after secondary transplantation, with 9 of 47 showing high-level engraftment after tertiary transplantation. (C) Lineage distribution of donor-derived GFP+ cells in individual primary and secondary transplanted donors as well as secondary and tertiary engrafted runx1-mutant recipients at 2 months posttransplant. (D) The percentage of donor-derived cells of myeloid lineage in recipients of primary, secondary, and tertiary transplantation. Secondary transplants demonstrated a significantly higher contribution from the myeloid lineage than primary recipients, but this effect was not seen in tertiary transplants. Error bars are standard deviation. Statistical analysis was performed via Mann-Whitney U test. **P < .001; ****P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/24/10.1182_bloodadvances.2020002424/2/m_advancesadv2020002424f3.png?Expires=1765892423&Signature=rm95TiKP47C3Eni~d79-byWfvCiOkBohgnt3fhgTeYLhXh~PBvHefQSnGD1WF-mm3aWPf0IdVcLtQYQP4X8STiZVOnzDaS2yhmJ3WuOL3I6GOavkIUHQdtl3hShp7TlYn5PYlm1C55jyUsqQV44VKBfB3q2ywS1wTYFbO2uuIbK3olCraDgSOZezQg8MxiBvOR41zgnYh3BRIF1jRrKKgAvWEJ-XrHi668lOZHXBk~hGgg1bPMzP-j8kC04S2wXfVm59om5KzJ2RtT3rGE2O7NbdsAI~wpo36u5U8Pw7uM3HKwJb6xWHXzYa6tjHxs08TUMuJJE2Zjx32rFAE6tTzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
