Key Points
Efficacy and safety were consistent across frailty subgroups with KRd27, Kd56, and weekly Kd70 in relapsed and/or refractory MM.
Carfilzomib-based regimens should not be restricted based upon frailty status.
Abstract
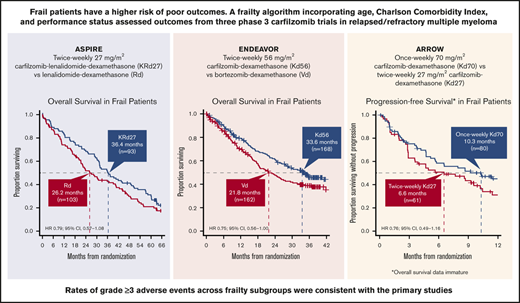
Frailty is most prevalent among elderly multiple myeloma (MM) patients, and frail patients have a higher risk of poor outcomes due to reduced performance status or comorbidities. This post hoc analysis assessed efficacy and safety of carfilzomib combinations in frail patients with relapsed and/or refractory MM from the phase 3 ASPIRE (carfilzomib [27 mg/m2]-lenalidomide-dexamethasone [KRd27] vs lenalidomide-dexamethasone [Rd]), ENDEAVOR (carfilzomib [56 mg/m2]-dexamethasone [Kd56] vs bortezomib-dexamethasone [Vd]), and ARROW (once-weekly carfilzomib [70 mg/m2]-dexamethasone [Kd70] vs carfilzomib [27 mg/m2]-dexamethasone [Kd27]) studies. A frailty algorithm incorporating age, Charlson comorbidity index, and performance status classified patients as fit, intermediate, or frail. Results are presented for frail patients (ASPIRE, n = 196; ENDEAVOR, n = 330; ARROW, n = 141). In ASPIRE, median progression-free survival (PFS) (hazard ratio; 95% confidence interval) was 24.1 (KRd27) vs 15.9 months (Rd) (0.78; 0.54-1.12); median overall survival (OS) was 36.4 vs 26.2 months (0.79; 0.57-1.08). In ENDEAVOR, median PFS was 18.7 (Kd56) vs 6.6 months (Vd) (0.50; 0.36-0.68); median OS was 33.6 vs 21.8 months (0.75; 0.56-1.00). In ARROW, median PFS was 10.3 (once-weekly Kd70) vs 6.6 months (twice-weekly Kd27) (0.76; 0.49-1.16). In all 3 studies, rates of grade ≥3 treatment-emergent adverse events were consistent with those observed in the primary studies. The ASPIRE, ENDEAVOR, and ARROW primary analyses demonstrated favorable benefit-risk profiles with carfilzomib-containing regimens compared with controls. Across clinically relevant subgroups, including those by frailty status, consistent efficacy and safety were observed with KRd27, Kd56, and weekly Kd70, and treatment with these regimens should not be restricted by frailty status.
Introduction
Multiple myeloma (MM) is a disease of the elderly, with a median age at diagnosis of 69 years.1 The incidence and prevalence of MM has increased over time, a phenomenon driven in part by the aging population.2,3 The elderly have an increased prevalence of frailty, a condition characterized by a state of greater vulnerability to stressors coupled with multiple physiological deficits.4 Despite the higher prevalence of frailty, elderly patients comprise a highly heterogenous group of variable fitness.4,5 Frail patients have a higher risk of poor clinical outcomes and are also often excluded from clinical trials due to reduced performance status or comorbidities.4,6 Therefore, there are limited clinical trial data among frail MM patients, further increasing the challenges of managing these patients.
Seeing the need for a comprehensive fitness measure, the International Myeloma Working Group (IMWG) developed a frailty index based on age, comorbidities (Charlson Comorbidity Index [CCI]7 ), and cognitive/physical conditions (Katz Activity of Daily Living [ADL] scale and Lawton Instrumental ADL [IADL] scale8 ).9,10 In a pooled analysis of 869 patients with newly diagnosed MM (NDMM) from 3 trials (median age, 74 years [interquartile range, 70-78]), patients identified as frail according to the IMWG frailty index had the lowest 3-year overall survival (OS) rates and the highest cumulative incidence of grade ≥3 nonhematologic adverse events (AEs) compared with other groups.9 These results, which controlled for International Stage System stage, chromosome abnormalities, and type of treatment, indicated that the IMWG frailty score was able to predict mortality and the risk of toxicity. An analysis of transplant-ineligible patients with NDMM from the FIRST trial, which compared continuous lenalidomide-dexamethasone (Rd) vs melphalan-prednisone-thalidomide, also indicated that patients characterized as frail by the IMWG frailty index had worse outcomes compared with patients characterized as nonfrail.10 In the overall patient population, better fitness was associated with significantly improved OS compared with worse fitness (P < .0001).
Carfilzomib (K) is an irreversible proteasome inhibitor approved for use in combination with lenalidomide plus dexamethasone (KRd27 mg/m2 [twice-weekly carfilzomib at 27 mg/m2]) or dexamethasone (Kd [twice-weekly carfilzomib at 56 mg/m2 or once-weekly carfilzomib at 70 mg/m2]) for the treatment of relapsed and/or refractory MM (RRMM).11 The pivotal phase 3 ASPIRE trial demonstrated significant improvements in progression-free survival (PFS) and OS in patients with relapsed or refractory MM treated with KRd27 mg/m2 vs lenalidomide plus dexamethasone (Rd).12,13 In post hoc analyses of ASPIRE, the treatment effects with KRd27 mg/m2 were consistent across clinically relevant subgroups, including those by patient age.14 Median PFS for patients <70 years old was 28.6 months with KRd27 mg/m2 vs 17.6 months with Rd (HR, 0.70; 95% confidence interval [CI], 0.56-0.88); for patients ≥70 years, median PFS was 23.8 months vs 16.0 months (HR, 0.75; 95% CI, 0.53-1.08).
Two pivotal phase 3 trials, ENDEAVOR and ARROW, investigated Kd in patients with RRMM. In the phase 3 ENDEAVOR trial of patients with RRMM, carfilzomib (56 mg/m2) plus dexamethasone (Kd56 mg/m2) significantly improved PFS and OS vs bortezomib plus dexamethasone (Vd).15,16 Consistent efficacy and safety with Kd56 mg/m2 was observed across patient subgroups, including those by patient age.17 Median PFS for patients aged 65 to 74 years was 15.6 months with Kd56 mg/m2 vs 9.5 months for Vd (HR, 0.53; 95% CI, 0.38-0.73) and median PFS for patients ≥75 years was 18.7 months vs 8.9 months (HR, 0.38; 95% CI, 0.23-0.65). The phase 3 ARROW study demonstrated superior PFS with once-weekly carfilzomib (70 mg/m2) plus dexamethasone (Kd70 mg/m2) vs twice-weekly carfilzomib (27 mg/m2) plus dexamethasone (Kd27 mg/m2) in patients with RRMM.18 Subgroup analyses of the ARROW study showed a general consistency in the treatment effect of Kd70 across subgroups. PFS HRs by age subgroups of the once-weekly Kd70 mg/m2 group over the twice-weekly Kd27 mg/m2 group were 0.60 in patients aged <65 years (95% CI, 0.42-0.86) and 0.84 for patients ≥65 years (95% CI, 0.61-1.15).18
Assessing MM patients for their frailty status is an important consideration in treatment selection. Inadequate assessment can lead to over- or undertreatment, with the potential to negatively impact quality of life and survival.4 In this post hoc analysis, we assessed patient efficacy and safety outcomes in frail patients with RRMM treated with carfilzomib regimens in the phase 3 ASPIRE, ENDEAVOR, and ARROW studies.
Methods
Study design
Trial design and outcomes of the 3 phase 3, open-label, randomized trials of adult patients (≥18 years) with RRMM (ASPIRE, ENDEAVOR, and ARROW) have been described previously.12,15,18 In ASPIRE (www.clinicaltrials.gov #NCT01080391), patients with 1 to 3 prior regimens and Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 to 2 were randomized (1:1) to KRd27 mg/m2 or Rd in 28-day cycles.12 Carfilzomib (10-minute IV infusion) was administered on days 1, 2, 8, 9, 15, and 16 (starting dose, 20 mg/m2 on days 1 and 2 of cycle 1; target dose, 27 mg/m2 thereafter) during cycles 1 to 12 and on days 1, 2, 15, and 16 during cycles 13 to 18, after which carfilzomib was discontinued. Lenalidomide (25 mg; oral) was administered on days 1 to 21. Dexamethasone (40 mg; oral or IV) was administered on days 1, 8, 15, and 22.
In ENDEAVOR (www.clinicaltrials.gov #NCT01568866), patients with 1 to 3 prior regimens and ECOG PS 0 to 2 were randomized (1:1) to receive Kd56 mg/m2 or Vd.15 The Kd56 mg/m2 group received carfilzomib as an IV infusion over 30 minutes on days 1, 2, 8, 9, 15, and 16 of 28-day cycles. Carfilzomib was given at a dose of 20 mg/m2 on days 1 and 2 of cycle 1, followed by 56 mg/m2 thereafter. Dexamethasone (20 mg; oral or IV) was administered on days 1, 2, 8, 9, 15, 16, 22, and 23. In the Vd group, patients received bortezomib 1.3 mg/m2 as an IV bolus or subcutaneous injection on days 1, 4, 8, and 11 of each 21-day cycle. Dexamethasone (20 mg; oral or IV) was administered on days 1, 2, 4, 5, 8, 9, 11, and 12.
In ARROW (www.clinicaltrials.gov #NCT02412878), patients with 2 or 3 prior regimens, including a proteasome inhibitor and an immunomodulatory agent, and ECOG PS 0 to 1 were included.18 Patients were randomized (1:1) to receive either once-weekly Kd70 mg/m2 or twice-weekly Kd27 mg/m2 in 28-day cycles. The once-weekly Kd70 mg/m2 group received carfilzomib (30-minute IV infusion) on days 1, 8, and 15 of all cycles (20 mg/m2 on day 1, cycle 1; 70 mg/m2 thereafter). The twice-weekly Kd27 mg/m2 group received carfilzomib (10-minute IV infusion) on days 1, 2, 8, 9, 15, and 16 (20 mg/m2 on days 1 and 2 during cycle 1; 27 mg/m2 thereafter). All patients received dexamethasone (40 mg; oral or IV) on days 1, 8, 15 (all cycles), and 22 (cycles 1-9 only).
Eligible patients were required to have adequate hepatic, hematologic, and renal function in all 3 studies, as measured by serum chemistry and hematology panels (supplemental Table 1).12,15,18 The following cardiovascular-related exclusion criteria were reported in these trials: active congestive heart failure (New York Heart Association class III or IV; ASPIRE, ENDEAVOR, and ARROW), myocardial infarction within 4 months (ENDEAVOR and ASPIRE) or 6 months (ARROW) before randomization, and uncontrolled hypertension (ARROW and ASPIRE).12,15,18
Outcomes
The primary end point of the ASPIRE, ENDEAVOR, and ARROW trials was PFS; secondary end points included OS, overall response rate (ORR), and incidence of AEs.12,15,18 Events of interest for carfilzomib that were analyzed included cardiac failure, ischemic heart disease, acute renal failure, and peripheral neuropathy.
Frailty assessment
For each study in this post hoc analysis, patients were categorized into subgroups by an algorithm based on the IMWG frailty index.9,10 This algorithm used a modified CCI for comorbidities and ECOG PS for functional status in lieu of ADL and IADL scales, as ADL/IADL data were not collected for these studies. This algorithm was based on the sum of age (score = 0 if <75 years, score = 1 if 75-80 years, score = 2 if >80 years), modified CCI (score = 0 if CCI ≤1, score = 1 if CCI >1), and ECOG PS (score = 0 if ECOG PS = 0; score = 1 if ECOG PS = 1; score = 2 if ECOG PS = 2) scores. Medical history was coded using the Medical Dictionary of Regulatory Activities versions 20.0 (ASPIRE and ARROW) and 15.1 (ENDEAVOR), and preferred terms for medical history matching those of the CCI were tabulated. Patients without a past medical history were assigned a missing frailty score. Patients with frailty score sums of 0, 1, or ≥2 were classified as fit, intermediate, or frail, respectively. PFS, treatment response, and safety were assessed by randomized treatment arm and frailty score for all 3 studies; OS was assessed only for ASPIRE and ENDEAVOR, as OS data from ARROW was immature at the time of this report. Frailty outcomes for each study were analyzed separately.
Statistical analysis
Efficacy analyses in the frailty subgroups and overall population were conducted in the intent-to-treat population, which consisted of all randomly assigned patients categorized by treatment randomized. Median PFS and OS were estimated using the Kaplan-Meier method, and comparisons between treatment groups were made using the log-rank test. The corresponding HR and its 95% CI were estimated using an unstratified Cox proportional hazards model. Overall response was defined using the IMWG Uniform Response Criteria (URC)19,20 as achieving a best overall response of partial response, very good partial response (VGPR), complete response (CR), or stringent CR (sCR). Overall response assessments were determined by an independent review committee (ASPIRE and ENDEAVOR) or computational algorithm based on IMWG-URC (ARROW). The odds ratio and corresponding 95% CI for the ORR were calculated using the unstratified Cochran-Mantel-Haenszel test. Safety analyses in the frailty subgroups and overall population were based on the safety population, which consisted of all patients who received ≥1 treatment dose.
Ethics
The ethics committee/institutional review board at each participating site approved the study, and the protocol conformed to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. All participants provided informed written consent.
Results
In ASPIRE, 792 eligible patients were enrolled and randomized to the KRd27 mg/m2 group (n = 396) or the Rd group (n = 396). In ENDEAVOR, 929 eligible patients were enrolled and randomized to the Kd56 mg/m2 group (n = 464) or the Vd group (n = 465). In ARROW, 478 eligible patients were enrolled and randomized to once-weekly Kd70 mg/m2 (n = 240) or twice-weekly Kd27 mg/m2 (n = 238). Patient flow diagrams for all 3 studies have been previously published.12,15,18 Baseline characteristics, including patient frailty status (fit, intermediate, or frail), were generally balanced between treatment arms in ASPIRE, ENDEAVOR, and ARROW (Table 1). In ASPIRE, ENDEAVOR, and ARROW, respectively, 196 (25%), 330 (36%), and 141 (29%) of patients were classified as frail. A summary of modified CCI, age, and ECOG PS for frail patients in ASPIRE, ENDEAVOR, and ARROW is shown in supplemental Table 2.
Frailty scores in ASPIRE, ENDEAVOR, and ARROW
| . | ASPIRE . | ENDEAVOR . | ARROW . | |||
|---|---|---|---|---|---|---|
| KRd27 mg/m2 (n = 396) . | Rd (n = 396) . | Kd56 mg/m2 (n = 464) . | Vd (n = 465) . | Once-weekly Kd70 mg/m2 (n = 240) . | Twice-weekly Kd27 mg/m2 (n = 238) . | |
| Age group, n (%), y | ||||||
| <75 | 353 (89) | 343 (87) | 387 (83) | 399 (86) | 194 (81) | 206 (87) |
| 75-80 | 33 (8) | 42 (11) | 60 (13) | 52 (11) | 35 (15) | 29 (12) |
| >80 | 10 (3) | 11 (3) | 17 (4) | 14 (3) | 11 (5) | 3 (1) |
| Modified CCI score, n (%) | ||||||
| ≤1 | 280 (71) | 258 (65) | 225 (48) | 230 (49) | 124 (52) | 138 (58) |
| >1 | 77 (19) | 97 (24) | 221 (48) | 222 (48) | 105 (44) | 92 (39) |
| Missing* | 39 (10) | 41 (10) | 18 (4) | 13 (3) | 11 (5) | 8 (3) |
| ECOG performance status at baseline, n (%) | ||||||
| 0 | 165 (42) | 175 (44) | 221 (48) | 232 (50) | 118 (49) | 118 (50) |
| 1 | 191 (48) | 186 (47) | 210 (45) | 203 (44) | 121 (50) | 120 (50) |
| ≥2 | 40 (10) | 35 (9) | 33 (7) | 30 (6) | 1 (<1) | 0 |
| Frailty score, n (%) | ||||||
| 0 (fit) | 115 (29) | 114 (29) | 110 (24) | 121 (26) | 60 (25) | 66 (28) |
| 1 (intermediate) | 149 (38) | 138 (35) | 168 (36) | 169 (36) | 89 (37) | 103 (43) |
| ≥2 (frail) | 93 (23) | 103 (26) | 168 (36) | 162 (35) | 80 (33) | 61 (26) |
| Missing* | 39 (10) | 41 (10) | 18 (4) | 13 (3) | 11 (5) | 8 (3) |
| . | ASPIRE . | ENDEAVOR . | ARROW . | |||
|---|---|---|---|---|---|---|
| KRd27 mg/m2 (n = 396) . | Rd (n = 396) . | Kd56 mg/m2 (n = 464) . | Vd (n = 465) . | Once-weekly Kd70 mg/m2 (n = 240) . | Twice-weekly Kd27 mg/m2 (n = 238) . | |
| Age group, n (%), y | ||||||
| <75 | 353 (89) | 343 (87) | 387 (83) | 399 (86) | 194 (81) | 206 (87) |
| 75-80 | 33 (8) | 42 (11) | 60 (13) | 52 (11) | 35 (15) | 29 (12) |
| >80 | 10 (3) | 11 (3) | 17 (4) | 14 (3) | 11 (5) | 3 (1) |
| Modified CCI score, n (%) | ||||||
| ≤1 | 280 (71) | 258 (65) | 225 (48) | 230 (49) | 124 (52) | 138 (58) |
| >1 | 77 (19) | 97 (24) | 221 (48) | 222 (48) | 105 (44) | 92 (39) |
| Missing* | 39 (10) | 41 (10) | 18 (4) | 13 (3) | 11 (5) | 8 (3) |
| ECOG performance status at baseline, n (%) | ||||||
| 0 | 165 (42) | 175 (44) | 221 (48) | 232 (50) | 118 (49) | 118 (50) |
| 1 | 191 (48) | 186 (47) | 210 (45) | 203 (44) | 121 (50) | 120 (50) |
| ≥2 | 40 (10) | 35 (9) | 33 (7) | 30 (6) | 1 (<1) | 0 |
| Frailty score, n (%) | ||||||
| 0 (fit) | 115 (29) | 114 (29) | 110 (24) | 121 (26) | 60 (25) | 66 (28) |
| 1 (intermediate) | 149 (38) | 138 (35) | 168 (36) | 169 (36) | 89 (37) | 103 (43) |
| ≥2 (frail) | 93 (23) | 103 (26) | 168 (36) | 162 (35) | 80 (33) | 61 (26) |
| Missing* | 39 (10) | 41 (10) | 18 (4) | 13 (3) | 11 (5) | 8 (3) |
No medical history available.
ASPIRE: efficacy and safety in frail and fit patients
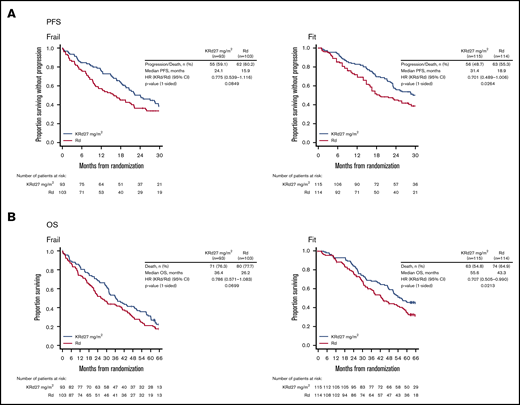
In ASPIRE, median PFS with KRd27 mg/m2 vs Rd in frail patients was 24.1 vs 15.9 months (HR 0.78; 95% CI 0.54-1.12) (Figure 1A). Median OS with KRd27 mg/m2 vs Rd was 36.4 vs 26.2 months (HR 0.79; 95% CI 0.57-1.08) (Figure 1B). ORRs for KRd27 mg/m2 vs Rd were 84% vs 64%; rates of CR or better (CR+) were 31% vs 8%, and rates of VGPR or better (VGPR+) were 69% vs 35% (supplemental Table 3). In fit patients, median PFS with KRd27 mg/m2 vs Rd was 31.4 vs 18.9 months (HR 0.70; 95% CI 0.49-1.01) (Figure 1A); median OS was 55.6 vs 43.3 months (HR, 0.71; 95% CI, 0.51-0.99) (Figure 1B). ORRs for KRd27 mg/m2 vs Rd were 90% vs 75%; rates of CR+ were 34% vs 10%, and rates of VGPR+ were 72% vs 47% (supplemental Table 3).
Kaplan-Meier curves for PFS and OS in the frail and fit subgroups in ASPIRE. PFS (A) and OS (B) were assessed in the intent-to-treat population.
Kaplan-Meier curves for PFS and OS in the frail and fit subgroups in ASPIRE. PFS (A) and OS (B) were assessed in the intent-to-treat population.
In the frail subgroup, grade ≥3 treatment-emergent AEs (TEAEs) occurred in 93% of KRd27 mg/m2–treated and 94% of Rd-treated patients; TEAEs leading to treatment discontinuation occurred in 37% of KRd27 mg/m2–treated and 43% of Rd-treated patients. The rate of AEs leading to carfilzomib/lenalidomide dose reduction was 42% in the KRd27 mg/m2 group and 35% in the Rd group. All-grade cardiac failure occurred in 16% of KRd27 mg/m2–treated patients and 4% of Rd-treated patients; grade ≥3 cardiac failure was 10% in the KRd27 mg/m2 arm and 1% in the Rd arm. Treatment discontinuation due to cardiac failure was 2% and 1%, and the rate of cardiac failure leading to carfilzomib/lenalidomide dose reduction was 3% and 0% in the KRd27 mg/m2 and Rd groups, respectively. Rates of grade ≥3 ischemic heart disease were 8% in the KRd27 mg/m2 arm and 4% in the Rd arm; treatment discontinuation rates due to ischemic heart disease were 3% in the KRd27 mg/m2 arm and 0% in the Rd arm. Grade ≥3 acute renal failure occurred in 3% of KRd27 mg/m2–treated patients and 6% of Rd-treated patients; treatment discontinuation rates due to acute renal failure were 2% and 1%, respectively. In the fit subgroup, grade ≥3 TEAEs occurred in 89% of KRd27 mg/m2 –treated and 84% of Rd-treated patients; TEAEs leading to treatment discontinuation occurred in 33% and 30% of patients, respectively. The rate of AEs of interest leading to carfilzomib/lenalidomide dose reduction was 40% in the KRd27 mg/m2 group and 29% in the Rd group. All-grade cardiac failure occurred in 7% of KRd27 mg/m2–treated patients and 4% of Rd-treated patients. Grade ≥3 cardiac failure rates were 4% in the KRd27 mg/m2 arm and 2% in the Rd arm; treatment discontinuation due to cardiac failure did not occur in either arm and the rate of cardiac failure leading to carfilzomib/lenalidomide dose reduction was 1% and 0% in the KRd27 mg/m2 and Rd groups, respectively. Rates of grade ≥3 ischemic heart disease were 3% in both arms; rates of treatment discontinuation due to ischemic heart disease were 1% in KRd27 mg/m2–treated and 3% in Rd-treated patients. Grade ≥3 acute renal failure occurred in 4% of KRd27 mg/m2–treated patients and 3% of Rd-treated patients; treatment discontinuation rates due to acute renal failure were 0% and <1%, respectively. Data for TEAEs, treatment-related AEs (TRAEs), and treatment exposure in ASPIRE are summarized for the frail and fit subgroups in Table 2, and for all frailty subgroups as well as the overall population in supplemental Table 4.
AEs and treatment exposure in the frail and fit subgroups in ASPIRE
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| KRd27 mg/m2, n = 92 . | Rd, n = 100 . | KRd27 mg/m2, n = 115 . | Rd, n = 114 . | |
| Any-grade TEAE, n (%) | 91 (99) | 100 (100) | 114 (99) | 113 (99) |
| Grade ≥3 TEAE, n (%) | 86 (93) | 94 (94) | 102 (89) | 96 (84) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 2 (2) | 5 (5) | 4 (3) | 1 (<1) |
| Acute renal failure | 3 (3) | 6 (6) | 5 (4) | 3 (3) |
| Cardiac failure | 9 (10) | 1 (1) | 5 (4) | 2 (2) |
| Ischemic heart disease | 7 (8) | 4 (4) | 4 (3) | 3 (3) |
| Pulmonary hypertension | 1 (1) | 0 | 0 | 0 |
| Hypertension | 7 (8) | 1 (1) | 11 (10) | 1 (1) |
| TEAEs leading to study treatment discontinuation, n (%) | 34 (37) | 43 (43) | 38 (33) | 34 (30) |
| TEAEs of interest leading to carfilzomib or lenalidomide discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 1 (1) | 2 (2) | 0 |
| Acute renal failure | 2 (2) | 3 (3) | 0 | 1 (<1) |
| Cardiac failure | 2 (2) | 1 (1) | 0 | 0 |
| Ischemic heart disease | 3 (3) | 0 | 1 (<1) | 3 (3) |
| Pulmonary hypertension | 0 | 0 | 0 | 0 |
| TRAEs leading to study treatment discontinuation, n (%) | 17 (18) | 27 (27) | 27 (23) | 22 (19) |
| Median duration of study treatment, weeks | 82.4 | 57.1 | 102.1 | 73.5 |
| Median carfilzomib relative dose intensity, % | 91.2 | N/A | 93.7 | N/A |
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| KRd27 mg/m2, n = 92 . | Rd, n = 100 . | KRd27 mg/m2, n = 115 . | Rd, n = 114 . | |
| Any-grade TEAE, n (%) | 91 (99) | 100 (100) | 114 (99) | 113 (99) |
| Grade ≥3 TEAE, n (%) | 86 (93) | 94 (94) | 102 (89) | 96 (84) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 2 (2) | 5 (5) | 4 (3) | 1 (<1) |
| Acute renal failure | 3 (3) | 6 (6) | 5 (4) | 3 (3) |
| Cardiac failure | 9 (10) | 1 (1) | 5 (4) | 2 (2) |
| Ischemic heart disease | 7 (8) | 4 (4) | 4 (3) | 3 (3) |
| Pulmonary hypertension | 1 (1) | 0 | 0 | 0 |
| Hypertension | 7 (8) | 1 (1) | 11 (10) | 1 (1) |
| TEAEs leading to study treatment discontinuation, n (%) | 34 (37) | 43 (43) | 38 (33) | 34 (30) |
| TEAEs of interest leading to carfilzomib or lenalidomide discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 1 (1) | 2 (2) | 0 |
| Acute renal failure | 2 (2) | 3 (3) | 0 | 1 (<1) |
| Cardiac failure | 2 (2) | 1 (1) | 0 | 0 |
| Ischemic heart disease | 3 (3) | 0 | 1 (<1) | 3 (3) |
| Pulmonary hypertension | 0 | 0 | 0 | 0 |
| TRAEs leading to study treatment discontinuation, n (%) | 17 (18) | 27 (27) | 27 (23) | 22 (19) |
| Median duration of study treatment, weeks | 82.4 | 57.1 | 102.1 | 73.5 |
| Median carfilzomib relative dose intensity, % | 91.2 | N/A | 93.7 | N/A |
AEs were assessed in patients who received ≥1 dose of study drug (safety population).
MedDRA, Medical Dictionary of Regulatory Activities; N/A, not applicable.
Standardized MedDRA Query, narrow scope.
ENDEAVOR: efficacy and safety in frail and fit patients
In ENDEAVOR, median PFS with Kd56 mg/m2 vs Vd in frail patients was 18.7 vs 6.6 months (HR, 0.50; 95% CI, 0.36-0.68) (Figure 2A), and median OS with Kd56 mg/m2 vs Vd was 33.6 vs 21.8 months (HR, 0.75; 95% CI, 0.56-1.00) (Figure 2B). ORRs for Kd56 mg/m2 vs Vd were 76% vs 54%; rates of CR+ were 6% vs 6%, and rates of VGPR+ were 52% vs 26% (supplemental Table 5). In fit patients, median PFS with Kd56 mg/m2 vs Vd was not estimable vs 12.1 months (HR, 0.51; 95% CI, 0.33-0.79) (Figure 2A); median OS was not estimable vs 42.2 months (HR, 0.65; 95% CI, 0.40-1.06) (Figure 2B). ORRs for Kd56 mg/m2 vs Vd were 78% vs 70%; rates of CR+ were 18% vs 7%, and rates of VGPR+ were 54% vs 28% (supplemental Table 5).
Kaplan-Meier curves for PFS and OS in the frail and fit subgroups in ENDEAVOR. PFS (A) and OS (B) were assessed in the intent-to-treat population. NE, not estimable.
Kaplan-Meier curves for PFS and OS in the frail and fit subgroups in ENDEAVOR. PFS (A) and OS (B) were assessed in the intent-to-treat population. NE, not estimable.
In the frail subgroup, grade ≥3 TEAEs occurred in 85% of Kd56 mg/m2–treated and 79% of Vd-treated patients; TEAEs leading to treatment discontinuation occurred in 33% of Kd56 mg/m2–treated and 30% of Vd-treated patients. The rate of AEs of interest leading to carfilzomib/bortezomib dose reduction was 34% in the Kd56 mg/m2 group and 48% in the Vd group. All-grade cardiac failure occurred in 15% of Kd56 mg/m2–treated patients and 10% of Vd-treated patients. Rates of grade ≥3 cardiac failure were 9% (Kd56 mg/m2) and 4% (Vd); treatment discontinuation rates due to cardiac failure were 4% and 1%, and the rate of cardiac failure leading to carfilzomib/bortezomib dose reduction was 2% and 0% in the KRd27 mg/m2 and Rd groups, respectively. Rates of grade ≥3 ischemic heart disease were 5% in the Kd56 mg/m2 arm and 4% in the Vd arm, and treatment discontinuation due to ischemic heart disease occurred in 2% of Kd56 mg/m2–treated and 2% of Vd-treated patients. Grade ≥3 acute renal failure occurred in 9% of Kd56 mg/m2–treated patients and 4% of Vd-treated patients; treatment discontinuation rates due to acute renal failure were <1% and 0%, respectively. In the fit subgroup, grade ≥3 TEAEs occurred in 83% of Kd56 mg/m2–treated and 64% of Vd-treated patients; TEAEs leading to treatment discontinuation occurred in 26% and 29% of patients, respectively. The rate of AEs of interest leading to carfilzomib/bortezomib dose reduction was 40% in the Kd56 mg/m2 group and 55% in the Vd group. All-grade cardiac failure occurred in 10% of Kd56 mg/m2–treated patients and 3% of Vd-treated patients. Grade ≥3 cardiac failure rates were 4% with Kd56 mg/m2 and 2% with Vd; rates of treatment discontinuation due to cardiac failure were 2% and 1%, and the rate of cardiac failure leading to carfilzomib/bortezomib dose reduction was 2% and 0%, in the Kd56 mg/m2 and Vd groups, respectively. Rates of grade ≥3 ischemic heart disease were 2% with Kd56 mg/m2 and 1% with Vd; treatment discontinuation due to ischemic heart disease did not occur in either arm. Grade ≥3 acute renal failure occurred in 4% of Kd56 mg/m2–treated patients and 2% of Vd-treated patients; treatment discontinuation rates due to acute renal failure were 2% and <1%, respectively. Data for TEAEs, TRAEs, and treatment exposure in ENDEAVOR are summarized for the frail and fit subgroups in Table 3 and for all frailty subgroups as well as the overall population in supplemental Table 6.
AEs and treatment exposure in frail and fit subgroups in ENDEAVOR
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| . | Kd56 mg/m2, n = 168 . | Vd, n = 159 . | Kd56 mg/m2, n = 110 . | Vd, n = 119 . |
| Any-grade TEAE, n (%) | 168 (100) | 157 (99) | 110 (100) | 118 (99) |
| Grade ≥3 TEAE, n (%) | 142 (85) | 125 (79) | 91 (83) | 76 (64) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 4 (2) | 15 (9) | 3 (3) | 12 (10) |
| Acute renal failure | 15 (9) | 7 (4) | 4 (4) | 2 (2) |
| Cardiac failure | 15 (9) | 7 (4) | 4 (4) | 2 (2) |
| Ischemic heart disease | 8 (5) | 6 (4) | 2 (2) | 1 (<1) |
| Pulmonary hypertension | 0 | 1 (<1) | 3 (3) | 0 |
| Hypertension | 27 (16) | 8 (5) | 19 (17) | 2 (2) |
| TEAEs leading to study treatment discontinuation, n (%) | 55 (33) | 48 (30) | 29 (26) | 34 (29) |
| TEAEs of interest leading to carfilzomib/bortezomib discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 15 (9) | 1 (<1) | 12 (10) |
| Acute renal failure | 1 (<1) | 0 | 2 (2) | 1 (<1) |
| Cardiac failure | 7 (4) | 2 (1) | 2 (2) | 1 (<1) |
| Ischemic heart disease | 3 (2) | 3 (2) | 0 | 0 |
| Pulmonary hypertension | 1 (<1) | 1 (<1) | 1 (<1) | 0 |
| TRAEs leading to study treatment discontinuation, n (%) | 35 (21) | 34 (21) | 23 (21) | 32 (27) |
| Median duration of study treatment, wk | 36.0 | 22.0 | 63.4 | 32.9 |
| Median carfilzomib/bortezomib relative dose intensity, % | 89.9 | 84.7 | 88.8 | 81.7 |
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| . | Kd56 mg/m2, n = 168 . | Vd, n = 159 . | Kd56 mg/m2, n = 110 . | Vd, n = 119 . |
| Any-grade TEAE, n (%) | 168 (100) | 157 (99) | 110 (100) | 118 (99) |
| Grade ≥3 TEAE, n (%) | 142 (85) | 125 (79) | 91 (83) | 76 (64) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 4 (2) | 15 (9) | 3 (3) | 12 (10) |
| Acute renal failure | 15 (9) | 7 (4) | 4 (4) | 2 (2) |
| Cardiac failure | 15 (9) | 7 (4) | 4 (4) | 2 (2) |
| Ischemic heart disease | 8 (5) | 6 (4) | 2 (2) | 1 (<1) |
| Pulmonary hypertension | 0 | 1 (<1) | 3 (3) | 0 |
| Hypertension | 27 (16) | 8 (5) | 19 (17) | 2 (2) |
| TEAEs leading to study treatment discontinuation, n (%) | 55 (33) | 48 (30) | 29 (26) | 34 (29) |
| TEAEs of interest leading to carfilzomib/bortezomib discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 15 (9) | 1 (<1) | 12 (10) |
| Acute renal failure | 1 (<1) | 0 | 2 (2) | 1 (<1) |
| Cardiac failure | 7 (4) | 2 (1) | 2 (2) | 1 (<1) |
| Ischemic heart disease | 3 (2) | 3 (2) | 0 | 0 |
| Pulmonary hypertension | 1 (<1) | 1 (<1) | 1 (<1) | 0 |
| TRAEs leading to study treatment discontinuation, n (%) | 35 (21) | 34 (21) | 23 (21) | 32 (27) |
| Median duration of study treatment, wk | 36.0 | 22.0 | 63.4 | 32.9 |
| Median carfilzomib/bortezomib relative dose intensity, % | 89.9 | 84.7 | 88.8 | 81.7 |
AEs were assessed in patients who received ≥1 dose of study drug (safety population).
Standardized MedDRA Query, narrow scope.
ARROW: efficacy and safety in frail and fit patients
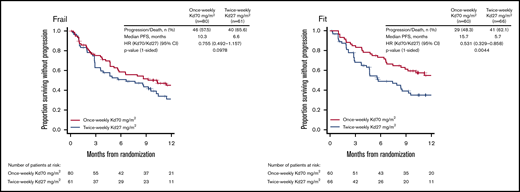
In ARROW, median PFS with once-weekly Kd70 mg/m2 vs twice-weekly Kd27 mg/m2 in frail patients was 10.3 vs 6.6 months (HR, 0.76; 95% CI, 0.49-1.16) (Figure 3). OS data for ARROW were immature at the time of this study and therefore not included in this analysis. ORRs with once-weekly Kd70 mg/m2 vs twice-weekly Kd27 mg/m2 were 56% vs 41%; rates of CR+ were 4% vs 0%, and rates of VGPR+ were 29% vs 15% (supplemental Table 7). In fit patients, median PFS with once-weekly Kd70 mg/m2 vs twice-weekly Kd27 was 15.7 vs 5.7 months (HR, 0.53; 95% CI, 0.33-0.86) (Figure 3). ORRs with once-weekly Kd70 mg/m2 vs twice-weekly Kd27 mg/m2 were 67% vs 29%; rates of CR+ were 10% vs 3%, and rates of VGPR+ were 40% vs 9% (supplemental Table 7).
Kaplan-Meier curves for PFS in the fit and frail subgroups in ARROW. PFS was assessed in the intent-to-treat population.
Kaplan-Meier curves for PFS in the fit and frail subgroups in ARROW. PFS was assessed in the intent-to-treat population.
In the frail subgroup, grade ≥3 TEAEs occurred more frequently in those treated with once-weekly Kd70 mg/m2 vs twice-weekly Kd27 mg/m2 (81% vs 70%), with minimal impact on rates of treatment discontinuations. TEAEs leading to carfilzomib discontinuation were comparable between the 2 arms (20% for once-weekly Kd70 mg/m2 and 18% in twice-weekly Kd27 mg/m2). Patients treated with once-weekly Kd70 mg/m2 experienced fewer all-grade and grade ≥ 3 cardiac failure events (4% for both) than those treated with twice-weekly Kd27 mg/m2 (8% for both). Rates of carfilzomib discontinuation due to cardiac failure occurred in 4% of Kd70 mg/m2–treated patients and 5% of Kd27 mg/m2–treated patients, and cardiac failure did not lead to carfilzomib dose reduction in either treatment group. Rates of grade ≥3 ischemic heart disease were 0% in the Kd70 mg/m2 arm and 2% in the Kd27 mg/m2 arm. Ischemic heart disease did not lead to carfilzomib discontinuation in either arm. Grade ≥3 acute renal failure occurred in 4% of Kd70 mg/m2–treated patients and 7% of Kd27 mg/m2–treated patients; treatment discontinuation rates due to acute renal failure were 4% and 2%, respectively. In the fit subgroup, grade ≥3 TEAEs occurred in 55% of once-weekly Kd70 mg/m2–treated and 62% of twice-weekly Kd27 mg/m2–treated patients; TEAEs leading to carfilzomib discontinuation occurred in 3% and 8% of patients, respectively. All-grade cardiac failure occurred in 2% of patients treated with once-weekly Kd70 mg/m2 and 2% of patients treated with twice-weekly Kd27 mg/m2. Grade ≥3 cardiac failure rates were 2% in both arms; rates of treatment discontinuation due to cardiac failure were 2% and 0%, respectively, and cardiac failure did not lead to carfilzomib dose reduction in either treatment group. Rates of grade ≥3 ischemic heart disease were 2% with Kd70 mg/m2 and 0% with Kd27 mg/m2; treatment discontinuation due to ischemic heart disease did not occur in either arm. Grade ≥3 acute renal failure occurred in 0% of Kd70 mg/m2–treated patients and 5% of Kd27 mg/m2–treated patients; treatment discontinuation rates due to acute renal failure were 0% and 2%, respectively. Data for TEAEs, TRAEs, and treatment exposure in ARROW are summarized for the frail and fit subgroups in Table 4 and for all frailty subgroups as well as the overall population in supplemental Table 8.
AEs and treatment exposure in frail and fit subgroups in ARROW
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| . | Once-weekly Kd70 mg/m2, n = 79 . | Twice-weekly Kd27 mg/m2, n = 60 . | Once-weekly Kd70 mg/m2, n = 60 . | Twice-weekly Kd27 mg/m2, n = 66 . |
| Any-grade TEAE, n (%) | 78 (99) | 60 (100) | 57 (95) | 66 (100) |
| Grade ≥3 TEAEs, n (%) | 64 (81) | 42 (70) | 33 (55) | 41 (62) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 0 | 0 | 0 | 1 (2) |
| Acute renal failure | 3 (4) | 4 (7) | 0 | 3 (5) |
| Cardiac failure | 3 (4) | 5 (8) | 1 (2) | 1 (2) |
| Ischemic heart disease | 0 | 1 (2) | 1 (2) | 0 |
| Pulmonary hypertension | 0 | 1 (2) | 0 | 0 |
| Hypertension | 8 (10) | 4 (7) | 1 (2) | 5 (8) |
| TEAEs leading to carfilzomib discontinuation, % | 16 (20) | 11 (18) | 2 (3) | 5 (8) |
| TEAEs of interest leading to carfilzomib discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 0 | 0 | 0 |
| Acute renal failure | 3 (4) | 1 (2) | 0 | 1 (2) |
| Cardiac failure | 3 (4) | 3 (5) | 1 (2) | 0 |
| Ischemic heart disease | 0 | 0 | 0 | 0 |
| Pulmonary hypertension | 0 | 0 | 0 | 0 |
| TRAEs leading to carfilzomib discontinuation, n (%) | 7 (9) | 6 (10) | 2 (3.3) | 0 |
| Median duration of carfilzomib treatment, wk | 36.1 | 29.1 | 45.4 | 24.4 |
| Median carfilzomib relative dose intensity, % | 94.7 | 96.3 | 96.5 | 96.0 |
| . | Frail . | Fit . | ||
|---|---|---|---|---|
| . | Once-weekly Kd70 mg/m2, n = 79 . | Twice-weekly Kd27 mg/m2, n = 60 . | Once-weekly Kd70 mg/m2, n = 60 . | Twice-weekly Kd27 mg/m2, n = 66 . |
| Any-grade TEAE, n (%) | 78 (99) | 60 (100) | 57 (95) | 66 (100) |
| Grade ≥3 TEAEs, n (%) | 64 (81) | 42 (70) | 33 (55) | 41 (62) |
| Grade ≥3 TEAEs of interest, n (%)* | ||||
| Peripheral neuropathy | 0 | 0 | 0 | 1 (2) |
| Acute renal failure | 3 (4) | 4 (7) | 0 | 3 (5) |
| Cardiac failure | 3 (4) | 5 (8) | 1 (2) | 1 (2) |
| Ischemic heart disease | 0 | 1 (2) | 1 (2) | 0 |
| Pulmonary hypertension | 0 | 1 (2) | 0 | 0 |
| Hypertension | 8 (10) | 4 (7) | 1 (2) | 5 (8) |
| TEAEs leading to carfilzomib discontinuation, % | 16 (20) | 11 (18) | 2 (3) | 5 (8) |
| TEAEs of interest leading to carfilzomib discontinuation, n (%)* | ||||
| Peripheral neuropathy | 0 | 0 | 0 | 0 |
| Acute renal failure | 3 (4) | 1 (2) | 0 | 1 (2) |
| Cardiac failure | 3 (4) | 3 (5) | 1 (2) | 0 |
| Ischemic heart disease | 0 | 0 | 0 | 0 |
| Pulmonary hypertension | 0 | 0 | 0 | 0 |
| TRAEs leading to carfilzomib discontinuation, n (%) | 7 (9) | 6 (10) | 2 (3.3) | 0 |
| Median duration of carfilzomib treatment, wk | 36.1 | 29.1 | 45.4 | 24.4 |
| Median carfilzomib relative dose intensity, % | 94.7 | 96.3 | 96.5 | 96.0 |
AEs were assessed in patients who received ≥1 dose of study drug (safety population).
Standardized MedDRA Query, narrow scope.
Discussion
Primary analyses of the ASPIRE, ENDEAVOR, and ARROW studies demonstrated significant improvements in PFS and OS for carfilzomib-containing regimens vs controls in the treatment of patients with RRMM.12,13,15,16,18 Previous post hoc analyses, including those by patient age, consistently showed that the PFS and OS effects observed in the primary studies were generally maintained across clinically relevant subgroups. This post hoc analysis by frailty status provides further evidence that treatment with KRd27, Kd56, and weekly Kd70 confers generally consistent efficacy and safety across a range of subgroups and that carfilzomib-containing regimens should not be restricted by patient frailty status.
In previous ASPIRE and ENDEAVOR analyses by age subgroups,14,17 PFS results for older age groups (ASPIRE, ≥70 years; ENDEAVOR, ≥75 years) were similar to the PFS data for frail patients from these trials in our analysis. As previously described, the elderly population is at increased risk of frailty4 and may follow similar patterns as frail patients with regard to treatment efficacy.
In ASPIRE and ENDEAVOR, among patients treated with carfilzomib-containing regimens, rates of grade ≥3 cardiac failure and ischemic heart disease were higher among frail patients than in fit patients (Tables 2,3-4). Although rates of cardiac failure were higher with carfilzomib-containing regimens compared with controls among frail patients in ASPIRE and ENDEAVOR, treatment discontinuations related to cardiac AEs were relatively low across treatment groups regardless of frailty status. In addition, rates of grade ≥3 ischemic heart disease were <8% across treatment groups in the frail subgroups of all 3 studies. The cardiac risks associated with carfilzomib, as well as risk factor management strategies and appropriate carfilzomib administration, should be discussed with patients prior to treatment. It should be noted that our results were obtained in a clinical trial population and may not fully reflect patient populations in a real-world setting.
Frail patients with MM can be challenging to treat, as they are potentially less able to tolerate therapy, are more vulnerable to side effects, and have compromised physiological function.21 These challenges may be further compounded in the setting of relapsed and/or refractory disease, where exposure to prior therapy and development of comorbidities may reduce tolerability of treatments, potentially diminishing efficacy. In all 3 studies investigated in our analysis, patients had RRMM and were exposed to ≥1 treatment regimen. In ASPIRE and ENDEAVOR, patients were required to have RRMM and 1 to 3 prior treatment regimens12,15 ; in ARROW, these criteria were even more stringent, with patients needing to have RRMM with 2 or 3 prior treatment regimens and previous exposure to both a proteasome inhibitor and an immunomodulatory agent.18 Despite these requirements, carfilzomib-based regimens and once-weekly Kd70 mg/m2 conferred consistent efficacy across clinically relevant subgroups.
Although there have been advances in identifying frail patients with NDMM, data on the assessment of frailty status in patients with RRMM has been lacking. As frail patients have been identified in the NDMM population to be at risk of poorer outcomes, it is important to identify these patients in the RRMM population and explore how their efficacy and safety outcomes compare with those of other patients. Furthermore, there is a need to understand the extent to which treatment may drive these poorer outcomes and measure the impact of specific treatments on efficacy and AEs. Consistent with results from a pooled NDMM analysis using the IMWG frailty index,9 in our analysis of carfilzomib-containing regimens, rates of grade ≥3 AEs were higher and efficacy lower in frail patients relative to the overall populations. These data suggest that the proxy algorithm for frailty applied to patients with RRMM in our study was able to identify the frail group, a population that is vulnerable and difficult to treat.
For clinical practice, it is important to consider the clinical characteristics of patients deemed frail in our analysis. In our analysis, 10%, 9%, and 10% of frail patients in the ASPIRE, ENDEAVOR, and ARROW groups, respectively, were >80 years of age, and 34%, 19%, and 1% had an ECOG PS of ≥2, whereas in Facon et al, 25% of patients were >80 years of age, and 43% had an ECOG PS of 2.10 Since the frailty algorithm used in our analysis is similar to that used in Facon et al, differences in these clinical characteristics likely reflect differences in the overall study populations. Baseline characteristics in ASPIRE, ENDEAVOR, and ARROW studies are consistent with another recent phase 3 MM study.22 Thus, differences in frail patient characteristics between Facon et al and our study are also likely due to differences in the transplant-ineligible NDMM population compared with the RRMM population, highlighting the need to validate the modified algorithm in an RRMM population.
Frail, elderly patients often receive suboptimal therapies.23 There exist concerns about prescribing full doses in frail patients who may experience adverse reactions without additional efficacy benefits. However, these concerns should be balanced by the potential for enhanced efficacy with higher treatment doses. In a real-world study of patients with MM treated with Kd regimens, patients receiving the recommended carfilzomib dosing had significantly improved OS and time to next treatment compared with those who received reduced dosing.24 This observation is confirmed in our study, as shown by the consistent benefit across the optimal carfilzomib dosing regimens in each study. Therefore, carfilzomib doses of twice-weekly 56 mg/m2 or weekly 70 mg/m2 should also be considered as treatment options for frail patients with RRMM.
This post hoc analysis has several limitations. The ASPIRE, ENDEAVOR, and ARROW studies were not originally designed to assess outcomes by frailty status, as these were not prespecified subgroups in the primary analyses. The analysis of RRMM patients used a proxy algorithm to determine frailty scores, which differed from the IMWG frailty index for NDMM patients. ECOG PS was used in lieu of ADL or IADL scales, which may have affected the number of patients classified as frail in this analysis, as ECOG PS reflects overall disease burden as well as frailty status. Although ECOG PS was used as a variable to assess frailty, patients with ECOG PS 3 to 4 were excluded from all 3 trials in this analysis. However, other studies have taken a similar approach and used measures of performance in lieu of ADL and IADL scales to determine the effect of treatment by frailty status.25,26 Furthermore, in a post hoc analysis of outcomes based on frailty for patients with NDMM from the FIRST trial, Facon et al used the same frailty algorithm as in our study.10 Similar to the IMWG frailty scale,9 and consistent with our analyses, the modified frailty scale used in the Facon et al study was able to predict efficacy outcomes by frailty status, with frail patients experiencing a worse PFS and OS compared with nonfrail patients.10 The method of deriving CCI score is another potential limitation of our analysis. As medical coding rules were used to define CCI scores from past medical history, changes in these rules could impact CCI scores. Furthermore, the available medical history depends heavily upon the information provided by a patient or site and may have been incomplete or missing. Data on detailed cardiac evaluations before, during, and after treatment, particularly in frail patients and in those >80 years, were not available. However, in our analysis, the trends toward lower efficacy and higher toxicity in the frail groups compared with the fit groups suggest that the frailty classification used in our analysis was able to identify the frail subgroup.
In this first reported analysis employing a frailty algorithm in RRMM, our results continue to show that the efficacy and safety of carfilzomib-containing regimens observed in the primary ASPIRE, ENDEAVOR, and ARROW studies is consistently maintained across subgroups. Regardless of frailty status, carfilzomib-based regimens should be considered as viable treatment options for patients with RRMM, whether lenalidomide containing (KRd27 mg/m2) or lenalidomide sparing (twice-weekly Kd56 mg/m2 or once-weekly Kd70 mg/m2). Because frail patients may be at greater risk for cardiac AEs, a baseline medical assessment should be conducted before starting carfilzomib. Appropriate carfilzomib administration and strategies to manage cardiac risks should be discussed with patients, and carfilzomib should be used based on a benefit-risk assessment. Collectively, these data indicate that carfilzomib-based regimens should not be restricted based upon frailty status.
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Acknowledgments
This work was supported by Onyx Pharmaceuticals, Inc., a subsidiary of Amgen, Inc. (the ENDEAVOR and ASPIRE studies) and Amgen, Inc. (the ARROW study). Medical writing assistance was provided by Sachi Yim and Andrew Gomes (BlueMomentum, an Ashfield company, part of UDG Healthcare PLC) and was funded by Amgen, Inc.
Authorship
Contribution: T.F., R.N., M.-V.M., D.S., K. Weisel, H.L., and M.A.D. were involved in the conception and design of the study, patient data collection/acquisition of data, and analysis and interpretation of data; C.R., S.B., P.J.H., S.K., K. Wang, M.O., Z.Y., Z.K., K.M., A.G., and C.T. were involved in the analysis and interpretation of data; and in collaboration with the medical writers, all authors participated in the writing and review of the first and subsequent versions of the manuscript, and all authors approved the final version before submission.
Conflict-of-interest disclosure: T.F. served as a consultant or advisor for Janssen, Celgene, Amgen, Takeda, Karyopharm, and Oncopeptides and served on the speakers’ bureau for Janssen, Celgene, and Takeda. R.N. served as a consultant for Celgene, Amgen, Takeda, Janssen, and BMS. M.-V.M. received honoraria from Celgene, Janssen, Takeda, Amgen, and AbbVie. D.S. received honoraria and consulting/advisory role fees for Celgene, Amgen, Merck, Janssen, BMS, Takeda, and Karyopharm; participated in the speakers’ bureau for Celgene, Amgen, Merck, Janssen, BMS, and Takeda; and received research funding from Celgene. C.R. received honoraria from, and had travel/accommodations/other expenses paid by, Celgene and BMS. S.B. served as a consultant for Takeda; received honoraria from Amgen, Takeda, Janssen, Celgene, and BMS; and served as a member of the board of directors/advisory committees for Janssen and Celgene. K. Weisel received honoraria from Amgen, Celgene, BMS, Janssen, and Takeda; received research funding from Amgen, Celgene, Janssen, and Sanofi; and served as a consultant for Amgen, Adaptive Biotech, Sanofi, Celgene, BMS, Juno, Janssen, Takeda. P.J.H. received an honorarium from Janssen-Cilag for an advisory board. H.L. served as a consultant or advisor for PharmaMar; served on the speakers’ bureau for Takeda, Amgen, BMS, Janssen, and Celgene; and received research funding from Takeda and Amgen. S.K. reports non-paid consulting and advisory board roles for AbbVie, Celgene, Janssen, Kite Pharma, Merck, and Takeda. K. Wang, M.O., Z.Y., Z.K., K.M., A.G., and C.T. are employees of and own stock in Amgen, Inc. M.A.D. received honoraria from Celgene, BMS, Janssen, Takeda, and Amgen.
Correspondence: Thierry Facon, Hôpital Claude Huriez, Rue Michel Polonowski, 59037 Lille, France; e-mail: thierry.facon@chru-lille.fr.
References
Author notes
The full-text version of this article contains a data supplement.