Key Points
Children with deficiencies of antithrombin, protein C, or protein S are at high risk of VTE.
This risk is comparable to that reported in adults.
Abstract
Although antithrombin, protein C, and protein S defects are well-recognized inherited risk factors for venous thromboembolism (VTE) in adults, whether they predispose children to these vascular disorders as well is undefined. In a prospective cohort study, we assessed the incidence of spontaneous and risk period–related VTE in children who were family members of adults who, after an episode of symptomatic VTE, had then been identified as carriers of these abnormalities. A total of 134 children from 87 families were enrolled. Seventy (51.5%) of these children were carriers of an inherited defect, and the remaining 64 were not; the mean observation period was 4 years (range, 1-16 years) and 3.9 years (range, 1-13), respectively. Sixteen risk periods were experienced by carriers, and 9 by noncarriers. Six VTE occurred in the 70 carriers during 287 observation-years, accounting for an annual incidence of 2.09% patient-years (95% confidence interval, 0.8-4.5), compared with none in the 64 noncarriers during 248 observation-years. Of the 14 children with thrombophilia who experienced a risk period for thrombosis, 4 (28.6%) developed a VTE episode. The overall incidence of risk-related VTE was 25% per risk period (95% confidence interval, 6.8-64). In conclusion, the thrombotic risk in otherwise healthy children with severe inherited thrombophilia does not seem to differ from that reported for adults with the same defects. Screening for thrombophilia in children who belong to families with these defects seems justified to identify those who may benefit from thromboprophylaxis during risk periods for thrombosis.
Introduction
Venous thromboembolic (VTE) disorders are expected to develop much less frequently in children than in adults.1-6 According to findings from a Canadian registry, the incidence of VTE in childhood has been reported to be as low as 0.07 per 10 000 individuals.3,6 Among children, newborns exhibit the highest risk, with a reported incidence of VTE ranging between 0.24 and 0.51 per 10 000 subjects.4,5 This risk, following a bimodal pattern, is low during childhood and exhibits a second peak during adolescence.7 Most episodes occur in association with underlying neoplasms, congenital heart diseases, systemic lupus erythematosus, renal failure or infective diseases, or they are triggered by central venous lines, major trauma, or surgery.3,6,8-10
Unlike in adults, the role played by congenital prothrombotic abnormalities in the development of VTE complications in pediatric subjects has not yet been fully elucidated. Indeed, in most available family studies of patients with thrombophilia, members aged <18 years were excluded from the evaluation.11-14 It is therefore unclear whether children who are family members of symptomatic probands with thrombophilia have, in turn, an increased risk of spontaneous or risk period–related VTE. Potential benefits coming from the early identification of inherited prothrombotic abnormalities in asymptomatic children depend on valid estimates of such a risk and on whether identified carriers might benefit from some form of thromboprophylaxis when exposed to triggering factors for VTE.
We conducted a prospective long-term follow-up of 134 consecutive children (ie, aged <18 years) belonging to 87 families with prothrombotic abnormalities, of whom 70 were carriers of inherited antithrombin, protein C, or protein S defects. The incidence of both spontaneous and risk period–related thromboembolic complications was assessed during an average 4-year follow-up period in the overall cohort.
Patients and methods
Patients
Consecutive patients referred to the Thrombosis Center of the University of Padua with an episode of objectively proven VTE between 1993 and 2015 were screened for deficiencies of antithrombin and protein C or protein S. All family members of probands who were identified as carriers of these defects were eligible for this investigation, provided they were aged <18 years and had not experienced episodes of VTE. They were enrolled in the study if their parents signed the informed consent. Approval was obtained from the medical Ethical Board of the University of Padua for this study. Informed consent was provided according to the Declaration of Helsinki.
Study design
Before the blood assessment of the thrombophilic abnormalities, a detailed medical history on previous episodes of VTE, risk factors for thrombosis, and anticoagulant treatment was collected for each subject by using a standardized questionnaire. A follow-up visit was performed every 6 months. At each visit, attention was paid to risk periods for VTE (eg, prolonged immobilization, major trauma or surgery, hormonal therapy, the insertion of a central venous line, infectious diseases). In case of clinical signs or symptoms of thromboembolic events, patients were referred to our center, where proper objective tests were performed to confirm or rule out the clinical suspicion.5
The systematic use of antithrombotic drugs was discouraged. The decision as to administer thromboprophylaxis during risk periods for venous thrombosis was left to the discretion of treating physicians. Children left the study when they reached the age of 18 years.
Laboratory assays
Laboratory tests for antithrombin, protein C, and protein S were performed according to previously described methods.11,14 The following reference values were used: antithrombin antigen concentration, 70% to 120%; antithrombin activity, 70% to 120%; protein C antigen concentration, 65% to 130%; protein C activity, 65% to 130%; total protein S concentration, 60% to 120%; free protein S concentration, 60% to 108%; and protein S activity, 70% to 120% .
Type I defects (concomitant reduction of antigen and activity to ∼50%) were considered for antithrombin and protein C deficiency. Type I (concomitant reduction of total and free protein S antigen and protein S activity to ∼40%-50%) and type III (normal total protein S antigen, reduced free protein S antigen, and protein S activity to ∼40%-50%) defects were considered for protein S deficiency.
Those subjects who were found to be deficient on 2 consecutive determinations and had at least 1 of the investigated relatives with the same defect were labeled as being carriers of the defect. Thrombophilic tests were performed in children at least 2 years of age, or earlier in the case of a thrombotic event. The tests were repeated annually until the age of 15 years.
The DNA analysis for Factor V Leiden mutation and G20210A prothrombin variant was performed by using previously described methods.14
Outcomes
The primary outcome was the occurrence of an objectively documented thromboembolic event, including deep vein thrombosis of the limbs, pulmonary embolism, and venous thrombosis in unusual sites (ie, retinal, splanchnic, cerebral, renal vein thrombosis). Thrombotic events were categorized as spontaneous if occurring without a predisposing risk period and secondary if occurring during or within 3 months after a risk period for thrombosis.
Analysis
The incidence of thromboembolic events, both spontaneous and risk period–related, and its 95% confidence interval (CI) was calculated in carriers and noncarriers of thrombophilia. The relative risk for the development of both spontaneous and risk period–related VTE was calculated by dividing the incidence rate of VTE in carriers by that found in noncarriers. The cumulative incidence of VTE and its 95% CI was also calculated, and the Kaplan-Meier method was used for both the time-dependent analysis (assuming as baseline the time at the enrollment of patients in the study) and the age-dependent analysis. A log-rank test was conducted to determine if there were differences in the VTE cumulative incidence between groups.
Results
Patients
A total of 137 children, who were family members of 87 probands with a single recognized prothrombotic disorder, were eligible for the current investigation. Of these, 3 (all of them carriers of a deficiency of natural anticoagulants) were excluded because of a history of thrombotic events (Figure 1). Accordingly, we recruited 134 family members, including 70 with inherited defects (22 of antithrombin, 29 of protein C, and 19 of protein S deficiencies) and 64 free from defects. The main characteristics of the enrolled children are shown in Table 1.
Family cohorts with hereditary antithrombin, protein C, or protein S deficiencies. *Deep vein thrombosis at 14 years of age after a posttraumatic fracture of the lower limbs (antithrombotic prophylaxis was not used in this circumstance). †Idiopathic VTE event at 8 years of age. ‡Thrombosis of the cerebral venous sinuses after birth.
Family cohorts with hereditary antithrombin, protein C, or protein S deficiencies. *Deep vein thrombosis at 14 years of age after a posttraumatic fracture of the lower limbs (antithrombotic prophylaxis was not used in this circumstance). †Idiopathic VTE event at 8 years of age. ‡Thrombosis of the cerebral venous sinuses after birth.
Characteristics of the study population
| Characteristic . | Total cohort . | Antithrombin cohort . | Protein C cohort . | Protein S cohort . | ||||
|---|---|---|---|---|---|---|---|---|
| Deficient . | Nondeficient . | Deficient . | Nondeficient . | Deficient . | Nondeficient . | Deficient . | Nondeficient . | |
| Subject, n | 70 | 64 | 22 | 16* | 29 | 22† | 19‡ | 26§ |
| Male sex, n (%) | 35 (50) | 34 (51) | 12 (54) | 7 (43.7) | 16 (55) | 13 (59) | 6 (32) | 14 (50) |
| Median age at enrollment (range), y | 12 (1-17) | 13 (5-17) | 12 (5-16) | 13 (8-17) | 12 (2-17) | 14 (5-16) | 12 (6-17) | 14 (5-17) |
| Observation years | 287 | 248 | ||||||
| Mean length of follow-up (range), y | 4 (1-16) | 3.9 (1-13) | ||||||
| Characteristic . | Total cohort . | Antithrombin cohort . | Protein C cohort . | Protein S cohort . | ||||
|---|---|---|---|---|---|---|---|---|
| Deficient . | Nondeficient . | Deficient . | Nondeficient . | Deficient . | Nondeficient . | Deficient . | Nondeficient . | |
| Subject, n | 70 | 64 | 22 | 16* | 29 | 22† | 19‡ | 26§ |
| Male sex, n (%) | 35 (50) | 34 (51) | 12 (54) | 7 (43.7) | 16 (55) | 13 (59) | 6 (32) | 14 (50) |
| Median age at enrollment (range), y | 12 (1-17) | 13 (5-17) | 12 (5-16) | 13 (8-17) | 12 (2-17) | 14 (5-16) | 12 (6-17) | 14 (5-17) |
| Observation years | 287 | 248 | ||||||
| Mean length of follow-up (range), y | 4 (1-16) | 3.9 (1-13) | ||||||
Two with Factor V Leiden mutation.
Four with Factor V Leiden mutation.
One with G20210A prothrombin variant.
One with Factor V Leiden mutation.
Laboratory results
Children recognized as carriers of antithrombin, protein C, and protein S deficiencies exhibited antigen and activity levels <50% (mean value, ∼40%). These levels were comparable with those of adult family members recognized as carriers of the same defects (Table 2).
Mean range levels for antithrombin, protein C, and protein S deficiencies in the study population
| Deficiency . | No. of subjects . | Antigen, mean ± SD, % . | Activity, mean ± SD, % . | Average age (range), y . |
|---|---|---|---|---|
| Antithrombin | 22 | 48.8 ± 12.3 | 45.6 ± 10.2 | 12 (3-17) |
| Protein C | 29 | 46.8 ± 12.2 | 43.6 ± 5.8 | 13 (1-17) |
| Protein S | 19 | 48.5 ± 16.4,* 46.9 ± 5.8† | 40.1 ± 12.8 | 12 (6-17) |
| Deficiency . | No. of subjects . | Antigen, mean ± SD, % . | Activity, mean ± SD, % . | Average age (range), y . |
|---|---|---|---|---|
| Antithrombin | 22 | 48.8 ± 12.3 | 45.6 ± 10.2 | 12 (3-17) |
| Protein C | 29 | 46.8 ± 12.2 | 43.6 ± 5.8 | 13 (1-17) |
| Protein S | 19 | 48.5 ± 16.4,* 46.9 ± 5.8† | 40.1 ± 12.8 | 12 (6-17) |
Protein S total antigen.
Protein S free antigen.
Follow-up and risk periods
There were 287 observation-years in carriers, and 248 in noncarriers. The mean observation time in each group was 4 years (Table 1). Of the 70 carriers of a thrombophilia, 14 experienced 16 risk periods for thrombosis: 10 major trauma, 2 surgical operation, 1 lung infection, 1 positioning of an indwelling central venous line, and 2 oral contraceptive treatment. Nine risk periods for thrombosis occurred in the 64 noncarriers: 5 major trauma, 2 surgical operation, 1 urinary infection, and 1 hormonal treatment. Antithrombotic prophylaxis with low-molecular-weight heparin was used in 1 child with deficiency of antithrombin soon after a trauma with a plaster cast.
VTE events
During the study period, 6 (8.6%) of the 70 carriers developed a VTE episode (isolated deep vein thrombosis in 2, deep vein thrombosis associated with pulmonary embolism in 2, cerebral vein thrombosis in 1, and isolated pulmonary embolism in 1), leading to an overall annual incidence of VTE of 2.09% (95% CI, 0.8-4.5).
Of the 6 thrombotic events, 2 were spontaneous, and the remaining 4 occurred in the 14 children (28.6%) who experienced a transient risk period for thrombosis. The annual incidence for spontaneous VTE and the incidence for risk period–related VTE was 0.7% (95% CI, 0.08-2.5) and 25% (95% CI, 6.8-64), respectively (Table 3). In all the thrombotic events, concomitant anatomic, neoplastic, or rheumatologic causes have been ruled out.
Spontaneous and risk period–related VTE in carriers and noncarriers of severe thrombophilia
| Variable . | Carriers . | Noncarriers . |
|---|---|---|
| Subject, n | 70 | 64 |
| Overall VTE | 6 | 0 |
| OAI, 2.09 (95% CI, 0.8-4.5) | OAI, 0 (95% CI, 0-1.4) | |
| Spontaneous VTE | 2 | 0 |
| AI, 0.7 (95% CI, 0.08-2.5) | ||
| Risk period–related VTE | 4 | 0 |
| Incidence for risk period–related VTE, 25% (95% CI, 6.8-64) | ||
| 10 traumas* | ||
| 2 operation | 5 traumas | |
| 2 OCT | 2 operations | |
| 1 CVL | 1 OCT | |
| 1 lung infection | 1 urinary infection |
| Variable . | Carriers . | Noncarriers . |
|---|---|---|
| Subject, n | 70 | 64 |
| Overall VTE | 6 | 0 |
| OAI, 2.09 (95% CI, 0.8-4.5) | OAI, 0 (95% CI, 0-1.4) | |
| Spontaneous VTE | 2 | 0 |
| AI, 0.7 (95% CI, 0.08-2.5) | ||
| Risk period–related VTE | 4 | 0 |
| Incidence for risk period–related VTE, 25% (95% CI, 6.8-64) | ||
| 10 traumas* | ||
| 2 operation | 5 traumas | |
| 2 OCT | 2 operations | |
| 1 CVL | 1 OCT | |
| 1 lung infection | 1 urinary infection |
AI, annual incidence; CVL, central venous line; OAI, overall annual incidence; OCT, oral contraceptive treatment.
One with prophylaxis.
No thromboembolic events occurred in the group of 64 noncarriers, leading to an overall annual incidence of VTE of 0% (95% CI, 0-1.4). The lack of thrombotic events in the group of noncarriers of defect did not allow the calculation of the relative risk.
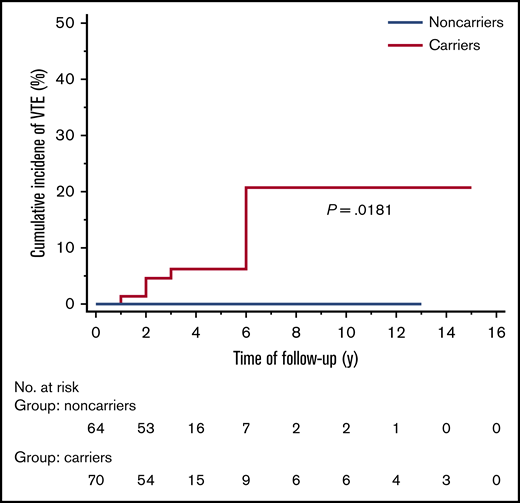
The overall cumulative incidence of VTE in carriers during follow-up was 8.57% (95% CI, 3.15-18.65), and the difference with the group of noncarriers was statistically significant (P = .0181) (Figure 2).
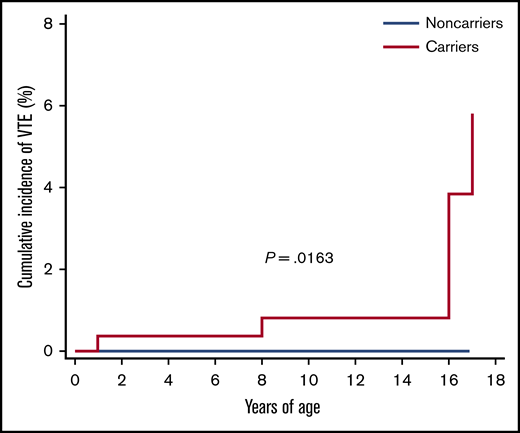
Furthermore, taking into consideration that the thrombotic risk in children is related to age, an analysis was conducted that took into account the age groups of the children. Four age groups were analyzed: 0 to 4 years, 5 to 8 years, 9 to 13 years, and 14 to 18 years. The overall, spontaneous, and risk period–related incidence of thrombotic events occurring in the 4 groups are reported in Table 4 and illustrated in Figure 3. The overall cumulative incidence of VTE according to years of age was 2.23% (95% CI, 1.05-4.88). The Kaplan-Meier curve is shown in Figure 4.
Spontaneous and risk period–related VTE according to age group
| Age group . | 0-4 y . | 5-8 y . | 9-13 y . | 14-18 y . |
|---|---|---|---|---|
| No. of subjects | 6 | 11 | 19 | 60 |
| Overall VTE | 1 | 1 | 0 | 4 |
| AI, 12.5 (95% CI, 0.32-69.6) | AI, 3.2 (95% CI, 0.82-18) | AI, 0 (95% CI, 0-6.2) | AI, 2.1 (95% CI, 0.6-5.4) | |
| Spontaneous VTE | 1 | 0 | 0 | 1 |
| AI, 12.5 (95% CI, 0.32-69.6) | AI, 0 (95% CI, 0-12) | AI, 0 (95% CI, 0-6.2) | AI, 0.53 (95% CI, 0.013-2.9 | |
| Risk period–related VTE | 0 | 1 | 0 | 3 |
| 0% per risk period (95% CI, 0-369) | 25% per risk period (95% CI, 0.63-139) | 0% per risk period (95% CI, 0-74) | 50% per risk period (95% CI, 10.3-146) | |
| 1 lung infection | 3 traumas | 1 operation | 1 operation | |
| 1 CVL | 4 traumas | 3 traumas* | ||
| 2 OCT |
| Age group . | 0-4 y . | 5-8 y . | 9-13 y . | 14-18 y . |
|---|---|---|---|---|
| No. of subjects | 6 | 11 | 19 | 60 |
| Overall VTE | 1 | 1 | 0 | 4 |
| AI, 12.5 (95% CI, 0.32-69.6) | AI, 3.2 (95% CI, 0.82-18) | AI, 0 (95% CI, 0-6.2) | AI, 2.1 (95% CI, 0.6-5.4) | |
| Spontaneous VTE | 1 | 0 | 0 | 1 |
| AI, 12.5 (95% CI, 0.32-69.6) | AI, 0 (95% CI, 0-12) | AI, 0 (95% CI, 0-6.2) | AI, 0.53 (95% CI, 0.013-2.9 | |
| Risk period–related VTE | 0 | 1 | 0 | 3 |
| 0% per risk period (95% CI, 0-369) | 25% per risk period (95% CI, 0.63-139) | 0% per risk period (95% CI, 0-74) | 50% per risk period (95% CI, 10.3-146) | |
| 1 lung infection | 3 traumas | 1 operation | 1 operation | |
| 1 CVL | 4 traumas | 3 traumas* | ||
| 2 OCT |
Patients contributed to multiple age groups.
One with prophylaxis.
The cumulative incidence of VTE, according to years of age, between carriers and noncarriers.
The cumulative incidence of VTE, according to years of age, between carriers and noncarriers.
The main characteristics of the subjects with a VTE event during the study are shown in Table 5, which also illustrates the type of event and its anatomic location.
Characteristics of the subjects with a VTE event during the study, the relationship with a risk period, and the localization
| Patient no. . | Sex/age . | Deficiency . | Risk period . | VTE localization . |
|---|---|---|---|---|
| 1 | Male/17 y | Antithrombin | None | Femoro-popliteal thrombosis + PE |
| 2 | Female/1 y | Protein C* | None | Inferior vena cava thrombosis |
| 3 | Male/8 y | Protein C | CVL | Iliac-femoral thrombosis† |
| 4 | Male/17 y | Protein C* | Trauma | Femoral thrombosis + PE |
| 5 | Female/15 y | Protein S | OCT | CSVT |
| 6 | Female/17 y | Protein S | OCT | Isolated PE |
| Patient no. . | Sex/age . | Deficiency . | Risk period . | VTE localization . |
|---|---|---|---|---|
| 1 | Male/17 y | Antithrombin | None | Femoro-popliteal thrombosis + PE |
| 2 | Female/1 y | Protein C* | None | Inferior vena cava thrombosis |
| 3 | Male/8 y | Protein C | CVL | Iliac-femoral thrombosis† |
| 4 | Male/17 y | Protein C* | Trauma | Femoral thrombosis + PE |
| 5 | Female/15 y | Protein S | OCT | CSVT |
| 6 | Female/17 y | Protein S | OCT | Isolated PE |
CSVT, cerebral sinus venous thrombosis; PE, pulmonary embolism.
Plus Factor V Leiden mutation.
Following the placement of a central venous access during hospitalization for corrective surgery in Crouzon syndrome.
Discussion
Information on the true risk of VTE in children who are carriers of inherited deficiencies of antithrombin, protein C, or protein S is at present scarce and inconclusive. We therefore designed a prospective cohort study aimed at estimating the age-related risk of both spontaneous and risk-related VTE in a broad number of healthy subjects aged <18 years who were family members of probands with inherited deficiencies of antithrombin, protein C, or protein S.
The rate of VTE events that was recorded during a mean follow-up of 4 years in the 70 carriers of these abnormalities was remarkably high (6 of 70 carriers, 2.09% patient-years) vs the 64 noncarriers (0 of 64 noncarriers, 0% patient-years). Unfortunately, the lack of thrombotic events in the group of noncarriers of defect did not allow calculation of the relative risk, but the difference in the cumulative incidence of thrombotic events in the group of carriers vs noncarriers was statistically significant (P = .0181).
Of utmost importance, of the 14 children who experienced a situation at risk (eg, trauma, surgery, hormonal treatment, the positioning of indwelling venous catheters), 4 (28.6%) developed a symptomatic VTE event, a rate that is consistent with that expected in adults. One of 10 who remained free from complications had received thromboprophylaxis. The absolute risk of VTE found in these circumstances seems to be strong enough to warrant protection against thrombotic complications. None of the 9 noncarriers who experienced a situation at risk developed VTE.
Our findings are plausible. Indeed, the incidence of VTE found in our cohort of children who were carriers of major thrombophilia overlaps that reported in adults under the same circumstances.11-15 In our previous prospective study,14 the incidence of spontaneous thrombosis in adults was found to be 0.8% (95% CI, 0.3-1.9), and the incidence related to risk periods was 10% (95% CI, 2.8-23.7); in our most recent prospective observation,15 these findings were 1.2% (95% CI, 0.9-1.7) and 21.2% (95% CI, 13.1-32.4), respectively.
In addition, our findings are consistent with those reported from recent case-control studies, which suggest that children with thrombophilia have a higher risk of thromboembolic complications than matched control subjects without thrombophilia.16-25 Indeed, in a recent meta-analysis, the risk of developing a first thrombotic event was found to be 8.73 (95% CI, 3.12-24.42) for the antithrombin defect, 5.77 (95% CI, 3.07-10.85) for the protein S defect, and 7.75 (95% CI, 4.48-13.38) for the protein C defect.25
The strength of the current study lies in its prospective design, in the recruitment of a broad number of children who were family members of symptomatic carriers of these abnormalities, in the adoption of rigorously predefined criteria for the end point adjudication, and in a reasonably long follow-up that was conducted with an identical approach both in carriers and in noncarriers. Moreover, the absolute rate of events occurring in circumstantial risk factors during the study period was remarkably high and mirrors that expected in adults.11-15
In contrast, because of the relatively low number of events occurring outside circumstantial factors, their interpretation requires caution. Furthermore, because no thrombotic events occurred in the neonatal age, the study cannot provide information on the thrombotic risk in this age group.
Considering the age at which thrombotic events occurred, the results of our study confirm that puberty represents the age of the second peak of thrombotic risk in pediatric age after infancy. In addition, our findings also show that the thrombotic risk increases according to the severity of the thrombophilic condition. For example, thrombotic events developed in 2 of the 4 children with double defects.
What are the implications of our findings? Essentially, there are 2 questions that need to be answered. First, should all children who are family members of probands with defects of inherited deficiencies of antithrombin, protein C, or protein S be screened for these abnormalities? Based on our study results, they should be, as early as possible. Indeed, the detection of the abnormality has the potential to identify those who are likely to benefit from some form of thromboprophylaxis. Second, once the defect has been detected, should carriers receive a systematic long-term pharmacologic protection? Our results strongly suggest that this is the case when children, irrespective of their age, experience those circumstantial situations that are well-known risk factors for thrombosis in adults, the duration being variable, depending on the length of the risk. Outside these circumstances, the extent by which they remain at risk of spontaneous complications in the absence of thromboprophylaxis, although unavoidably higher than in noncarriers, should be balanced against the risks and the inconveniences of a life-long pharmacologic prophylaxis. We would not recommend it.
In conclusion, the thrombotic risk in otherwise healthy children with an identified major thrombophilic defect does not seem to differ from that expected in adults with the same pattern. Children who are carriers of deficiencies of antithrombin, protein C, or protein S, and are family members of probands with symptomatic VTE, should receive a proper thromboprophylaxis at least when they are exposed to circumstantial risk factors. This implies the need for a systematic screening of the index defect.
Requests for data sharing may be submitted to the corresponding author (Daniela Tormene; e-mail: daniela.tormene@unipd.it).
Authorship
Contribution: D.T. designed the study, enrolled patients, analyzed data, and wrote the manuscript; E.C. analyzed data; C.S., G.T., M.M., and A.P. enrolled and conducted follow-up with patients; C.M.R. performed laboratory analyses; P.P. analyzed data and wrote the manuscript; and P.S. designed the study, analyzed data, and wrote the manuscript.
Conflicts-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniela Tormene, Department of Medicine, Thrombotic and Haemorrhagic Diseases Unit, University of Padua Medical School, Via Giustiniani 2, 35128 Padua, Italy; e-mail: daniela.tormene@unipd.it.