Key Points
VTE incidence for a large cohort in New Orleans, LA, does not differ from previous hospitalized populations matched for acuity.
Noted large subpopulation of dialysis thrombosis may account for high incidence of thrombosis not related to typical VTE.
Abstract
Patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appear to be at increased risk for venous thromboembolism (VTE), especially if they become critically ill with COVID-19. Some centers have reported very high rates of thrombosis despite anticoagulant prophylaxis. The electronic health record (EHR) of a New Orleans–based health system was searched for all patients with polymerase chain reaction–confirmed SARS-CoV-2 infection who were either admitted to hospital or treated and discharged from an emergency department between 1 March 2020 and 1 May 2020. From this cohort, patients with confirmed VTE (either during or after their hospital encounter) were identified by administrative query of the EHR.: Between 1 March 2020 and 1 May 2020, 6153 patients with COVID-19 were identified; 2748 of these patients were admitted, while 3405 received care exclusively through the emergency department. In total, 637 patients required mechanical ventilation and 206 required renal replacement therapy. Within the hospitalized cohort, the overall mortality rate was 24.5% and VTE occurred in 86 patients (3.1%). In the 637 patients who required mechanical ventilation at some point during their hospital stay, 45 developed VTE (7.2%). After a median follow-up of 14.6 days, VTE had been diagnosed in 3 of the 2075 admitted who were discharged alive (0.14%). Among 6153 patients with COVID-19 who were hospitalized or treated in emergency departments, we did not find evidence of unusually high VTE risk. Pending further evidence from prospective, controlled trials, our findings support a traditional approach to primary VTE prevention in patients with COVID-19.
Introduction
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), is a well-described complication of acute medical illness. Among inpatients with an acute medical illness, validated risk-assessment models can identify subgroups at especially high risk to experience VTE.1,2 For example, 1 study suggests that, in the absence of prophylaxis, 11% of medical inpatients with multiple risk factors will be diagnosed with symptomatic VTE.1 The same studies showed that pharmacologic prophylaxis, with low doses of either unfractionated or low-molecular-weight heparin, significantly reduces the risk of VTE; for example, if they received pharmacologic prophylaxis, only 2.2% of patients in the highest-risk stratum of 1 study was diagnosed with VTE within a 90-day follow-up period.1 Among the patients at highest risk, posthospital prophylaxis may further reduce the rate of VTE in persons recovering from acute medical illness.3
There is a widespread perception that VTE is a frequent complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and that this complication may occur despite pharmacologic prophylaxis. Published evidence is equivocal. A report of 184 patients from 3 centers in The Netherlands reported that approximately one-third of patients critically ill with COVID-19 had PE.4 A second center in The Netherlands reported that ∼20% of 198 patients hospitalized for COVID-19 (75 admitted to the intensive care unit [ICU]) had confirmed VTE after a median follow-up of 7 days.5 In contrast, a center in New York City has reported a VTE rate of 3.3% among 393 patients with COVID-19, 130 of whom required mechanical ventilation.6 Authors from a center in northern Italy found no cases of lower-extremity DVT among 388 patients who were admitted to a non-ICU bed and received standard low-molecular-weight heparin prophylaxis, even among the 64-patients subgroup screened for asymptomatic disease.7
To provide additional information about the association of VTE with COVID-19, we present a large retrospective cohort study examining all polymerase chain reaction–confirmed COVID-19 patients admitted to hospitals or treated in emergency departments affiliated with the Ochsner Health System.
Methods
The Ochsner Health System is the largest academic health care system in Louisiana; it encompasses an integrated care delivery network throughout all of Louisiana and portions of southern Mississippi. The Ochsner Health System operates under a single electronic health record (EHR), EPIC (Verona, WI). The use of a single EHR platform permits large-scale data analyses to be performed on a network of >4 million patients from all payer sources. All data presented in this paper were derived using the EPIC “Slicerdicer,” a tool designed to query large data sets in the EPIC EHR.
From the pool of all patients assessed at an Ochsner Health System facility between 1 March 2020 and 1 May 2020, we identified all patients positive for SARS-CoV-2 on polymerase chain reaction–based testing. Patients were seen in an emergency department and/or admitted to an inpatient unit at any of the 14 hospitals affiliated with Ochsner Health. All patients were followed for VTE until death or 21 May 2020. For our analysis, all patients who met our inclusion criteria were assumed to be continuously enrolled in the Ochsner Health System between 1 March 2020 and 21 May 2020. This project was reviewed and approved by the Ochsner Health System Institutional Review Board.
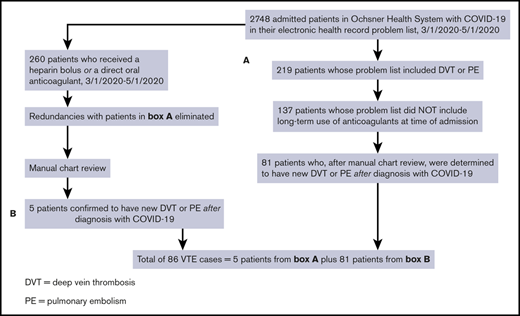
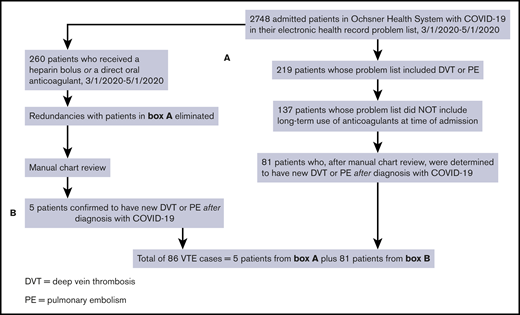
We identified cases of new VTE on hospitalized inpatients and those who were discharged by first searching for all patients with COVID-19 who had a diagnosis of DVT or PE in their problem list. We then removed patients if the International Classification of Diseases, Tenth Revision code for “long-term current use of anticoagulants” was present at the time of the positive SARS-CoV-2 test. We performed 2 further queries to identify patients with DVT or PE who may have been missed by the first search algorithm. The first query identified all patients with COVID-19 who received apixaban, rivaroxaban, or dabigatran, excluding those with atrial fibrillation, those with stroke, and those on long-term anticoagulation. The second additional query identified patients with COVID-19 who received an unfractionated heparin bolus, excluding patients with atrial fibrillation, patients with non–ST-elevation myocardial infarction, patients with ischemic stroke, and those on chronic anticoagulation. Unless they also had objectively confirmed VTE based on chart review, we excluded patients whose indication for heparin bolus was thrombosis of an extracorporeal circuit (eg, renal replacement therapy or extracorporeal membrane oxygenation). We manually reviewed the charts of all cases flagged by the query for VTE for objective documentation of thrombosis. Confirmation of thrombosis required presence of a noncompressible deep venous segment on ultrasound, or evidence of an otherwise unexplained intraluminal filling defect on contrast examination, such as computerized tomographic pulmonary angiography. In 1 case, V/Q scanning was used to establish the diagnosis. All examinations were performed as a component of clinical care, and as such, both radiology department, and rarely, bedside ultrasonographic scans were included in our analysis. A flow diagram summarizing the search method is shown in Figure 1. All data were stored in a secure database, and only deidentified data were uploaded to Microsoft Excel. The patients with COVID-19 found to have new VTE by this strategy were further evaluated by chart review.
Flow diagram showing the strategies used to identify cases of new VTE among hospitalized patients with SARS-CoV-2 infection (COVID-19).
Flow diagram showing the strategies used to identify cases of new VTE among hospitalized patients with SARS-CoV-2 infection (COVID-19).
All medical inpatients admitted to the Ochsner Health System are assessed for VTE risk by the Padua risk score,1 a widely accepted risk-stratification scheme that classifies patients with a score of 4 or greater as high risk for VTE and, unless the risk for VTE is low, they receive mechanical or pharmacologic VTE prophylaxis. Routine pharmacologic prophylaxis consists of low-dose low-molecular-weight heparin (enoxaparin, 40 mg daily, or 30 mg daily for CrCl < 30 or 40 mg twice daily for body mass index [BMI] > 40) or unfractionated heparin (5000 U every 8 hours for BMI < 40 and 7500 U every 8 hours for BMI > 40). Mechanical prophylaxis includes elastic compression stockings or sequential compression devices and is used if the patient has a contraindication for pharmacologic prophylaxis. In the cohort used to derive the Padua risk score, patients with a score of 4 or greater had a 90-day VTE rate of 2.2% with thromboprophylaxis and 11% without prophylaxis. Neither screening for asymptomatic VTE nor postdischarge VTE prophylaxis was routinely undertaken within the Ochsner Health System during the time described in this report.
To estimate the rate of failure of VTE prophylaxis among COVID-19–positive hospitalized patients, we defined failure as any DVT or PE event diagnosed ≥3 days after the admission of a patient who had received ≥2 days of evidence-based mechanical or pharmacologic VTE prophylaxis immediately prior. Evidence-based VTE prophylaxis was defined as sequential compression devices (alone or in combination with elastic compression stockings), or low-molecular-weight heparin or unfractionated heparin administered at usual prophylactic doses.
Results
Between 1 March 2020 and 1 May 2020, 6153 patients with COVID-19 were admitted or evaluated in an emergency department; 2748 of these patients were admitted to the hospital, whereas 3405 received care exclusively through the emergency department. Patient characteristics are presented in Table 1. In total, 86 VTE events were diagnosed during a hospital stay; an additional 3 cases were diagnosed and discharged from the emergency department. Of the 2075 hospitalized patients who survived to be discharged between 1 March 2020 and 1 May 2020, 3 had experienced postdischarge VTE on or before 21 May 2020. An additional single patient who had been evaluated and discharged was later diagnosed with (and hospitalized for) VTE. Of the 86 inpatient VTE events, 42 (48%) were diagnosed with DVT and 35 (40.6%) were diagnosed with PE. Nine (10.4%) patients did not have confirmatory studies and were deemed to have VTE based on clinical impression of the care team. There were various reasons for the absence of diagnostic testing, including rapid death of 3 patients within 24 hours of admission, right heart strain and elevated D-dimer on 2 patients, contrast allergy in 2 patients, and morbid obesity preventing proper DVT imaging in 2 patients. Of the other 77 events, all were confirmed by standard definitions of appropriate objective testing. In addition, 84 of the 86 patients received venous thromboprophylaxis, and the 2 who received no thromboprophylaxis were both categorized by the PADUA scale as being low risk for VTE. There were 7 patients identified with arterial thrombus: 3 being embolic strokes, 3 occurring in patients with concomitant cancer, and one an iliac artery thrombosis that proceeded to hospice care precluding further investigation.
Characteristics of the 86 hospitalized patients with COVID-19 and VTE
| Characteristics | Data |
| Age, mean; SD | 61; 15.4 |
| Male sex, n (%) | 42 (48) |
| Active cancer,* n (%) | 3 (3.4) |
| INR >1.5 during admission,† n (%) | 4 (4.6) |
| Renal replacement therapy during admission, n (%) | 26 (30.2) |
| Peak D-dimer, median (IQR), μg/mL | 7.59 (3.07-32.24) |
| Mechanical ventilation, n (%) | 45 (52.3) |
| High risk, by Padua score, n (%) | 77 (89) |
| Low risk, by Padua score, n (%) | 9 (11) |
| Characteristics | Data |
| Age, mean; SD | 61; 15.4 |
| Male sex, n (%) | 42 (48) |
| Active cancer,* n (%) | 3 (3.4) |
| INR >1.5 during admission,† n (%) | 4 (4.6) |
| Renal replacement therapy during admission, n (%) | 26 (30.2) |
| Peak D-dimer, median (IQR), μg/mL | 7.59 (3.07-32.24) |
| Mechanical ventilation, n (%) | 45 (52.3) |
| High risk, by Padua score, n (%) | 77 (89) |
| Low risk, by Padua score, n (%) | 9 (11) |
INR, international normalized ratio; IQR, interquartile range; SD, standard deviation.
*Received at least 1 treatment of cancer (radiation, chemotherapy, or surgery) in prior 12 mo.
†Coagulopathy was defined as INR >1.5 prior to anticoagulation.
The overall proportion of patients with VTE within the hospitalized cohort was 86/2748 (3.1%). Considering only the subgroup of patients with COVID-19 who required mechanical ventilation at some point during their hospital stay, the proportion who developed VTE was 45/637 (7.2%). Of 89 episodes of VTE that occurred within the entire 6153-patient cohort, 82 were identified during a hospital admission, and 4 were diagnosed after a prior hospital admission or emergency department visit. The remaining 3 patients with VTE were diagnosed and treated exclusively during a single emergency department encounter. For patients who were discharged alive, the mean duration of follow-up after hospital discharge was 21.3 days (median 14.6 days, interquartile range 7.2 to 36 days).
Among the group of 86 patients diagnosed with VTE during or after hospital admission, 77 (89%) had a “high-risk” PADUA score (4 or greater); of these 77 patients, 74 (96% of all patients with VTE) experienced VTE during their hospital stay, and 4 (4.6% of all patients with VTE) experienced a postdischarge VTE. D-dimer values were captured on all patients in this study and were found to have a median value of 7.59 (interquartile range of 3.07 to 32.24), which is significantly elevated over those without DVT/PE and which is dramatically elevated compared with normal values in the population.
The frequency of clinical VTE occurring after hospital discharge was low; of 2075 hospitalized patients who did not have an episode of VTE during their inpatient stay and who survived until discharge, only 3 experienced VTE (0.14%). Of the 86 patients diagnosed with new VTE during their hospital stay, 43 (50%) met our definition of prophylaxis failure. Of these 43 patients, 7 received exclusively mechanical prophylaxis, 4 received unfractionated heparin prophylaxis, 5 received exclusively low-molecular-heparin prophylaxis, and 27 received some combination of both mechanical and pharmacologic prophylaxis. Finally, 27.9% (24/86) of patients with a diagnosis of VTE died by 21 May 2020. By comparison, the proportion of patients in the overall hospitalized cohort who had died was 24.4% (671/2748).
Discussion
Within a cohort of 2748 patients hospitalized for COVID-19 between 1 March 2020 and 1 May 2020, 3.1% had developed VTE as of 21 May 2020. As expected, the proportion of mechanically ventilated patients who were diagnosed with VTE was higher (7.2%). The rate of posthospitalization VTE among the 2075 admitted patients who were discharged alive and who did not experience an in-hospital VTE was 0.14%. Although our overall rate of VTE in hospitalized patients was low, half of our cases occurred in patients who were receiving effective VTE prophylaxis prior to their VTE.
Most patients with COVID-19 are asymptomatic or have mild to moderate disease. Despite this, our study confirms that hospitalization, particularly if it requires ICU admission, is associated with a high mortality rate. However, our observation that VTE occurred in <10% of patients requiring mechanical ventilation (the vast majority of whom were probably receiving effective VTE prophylaxis) suggests that the prophylaxis “failure rate” among very sick patients with COVID-19 may not be dramatically different from what has been previously described in other critically ill populations.8,9 Among patients successfully discharged from the hospital, we found a rate of posthospital VTE similar to that seen in other medically ill patients requiring hospitalization.
In this data set, we found a second subgroup of patients (n = 22) that were on renal replacement therapy who experienced frequent clotting of their dialysis circuit requiring heparinization. This appears to be a clinically distinct entity from the DVT/PE population as these patients were significantly more ill (100% on ventilators and with an 81% mortality rate). This study presents overall rates of VTE that are much lower than some previously published results4,10,11 but are consistent with others.6,7,12 The large difference in the rates of VTE is not due to this cohort being a selected low-risk patient group; among those hospitalized, almost 25% died and >7% required renal replacement therapy. An important explanation for some of the differences in published COVID-19–associated thrombosis rates is differences in outcome definitions. We focused only on VTE, whereas other authors have included multiple forms of thrombosis (eg, arterial thrombosis, extracorporeal circuit thrombosis, or exclusively microvascular thrombosis) in their overall reported rates.13
This analysis has significant limitations. First, our EHR search strategy may have not captured VTE cases. Although we used several different strategies to minimize the number of missed VTE cases, the authors recognize that an administrative EHR query can be less comprehensive than a manual record review. It is also possible that clinicians in some centers have a higher or lower threshold to order diagnostic testing, potentially impacting events rates. Cattaneo et al have raised the intriguing possibility that the very high PE-to-DVT ratio reported by some centers might reflect a high frequency of in situ pulmonary artery thrombosis or occlusion, the pathophysiology of which may be different from traditional, clinically apparent VTE.7,12 As more data on COVID-19 are published, it appears that average events rates are lower than initially suspected, possibly due to ascertainment bias in the initial papers. For example, Bilaloglu et al have reported that VTE occurred in 13.6% of 829 patients admitted to ICU and 4.2% of 2505 hospitalized patients who did not require ICU admission11 ; these results, while not identical, are certainly consistent with our findings. Similarly, Roberts and colleagues have reported a postdischarge VTE rate of 0.48% among patients hospitalized for COVID-19,12 an event frequency that is quite similar to what we found. There are many possible explanations for the different VTE rates reported at different centers; 1 possibility is that there is a difference in the biology of the SARS-CoV-2 itself, that is, different genotypes may manifest with different clinical characteristics.
Second, outpatients who sought care outside the Ochsner Health System after hospital discharge would not be identified by our strategy, but the number of such patients is likely small because Ochsner is a multistate system with comprehensive commercial and state payer contracts. Third, our strategy would not have captured postdischarge VTE events that either were fatal or did not lead the patient to seek medical attention. Our study also did not employ any form of adjudication, and we systematically excluded patients with current use of anticoagulation at the time they tested positive for SARS-CoV-2. Many patients included in this analysis remain at risk of VTE; further follow-up would likely reveal additional events. However, given the size of the present cohort, it seems unlikely that these study design characteristics would change our high-level observations. Last, we did not systematically screen for venous thromboembolic events, so it is possible that such events, which were not clinically relevant, were not captured in this cohort.
Our findings, in combination with those previously published, suggest that VTE rates (and the rate of prophylaxis failure) in hospitalized patients with COVID-19 may be somewhat higher than expected. However, more information is needed, not only about the strength of the association between COVID-19 and VTE risk but also about the most effective way to reduce the risk of VTE in this disease. Until additional prospective outcome data are reported, and underlying mechanisms are better understood, our findings support a traditional approach to VTE prophylaxis, both during and after hospitalization, for patients with COVID-19.
Data requests may be made by e-mailing the corresponding author, Jason B. Hill, at jahill@ochsner.org.
Authorship
Contribution: J.B.H. compiled data for paper, composed methods and statistical analyses, and revised and edited background/results and discussion; M.C. and S.D. were the subject matter experts in thrombosis, and assisted in data validation and drafting the background section as well as editing and compiling the results and discussion; D.G. was the subject matter expert in thrombosis, assisted in data validation and drafting the background section as well as editing and compiling results and discussion, and completed the initial draft of Figure 1; S.P. and B.S. worked to complete validation of initial search query with manual chart abstraction; and K.C. verified statistical significance and analysis of data pulls and results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason B. Hill, OMC-NS Hospital Medicine, Associate CMIO, Ochsner Health System, 100 Medical Center Dr, Slidell, LA 70460; e-mail: jahill@ochsner.org.