Key Points
The 2-year PFS and OS of patients with MYC-R limited-stage DLBCL/HGBL were 78% and 86%, respectively.
There was no benefit of using IIC of R-CHOP in patients with MYC-R limited-stage DLBCL/HGBL.
Abstract
There is a paucity of data regarding outcomes and response to standard therapy in patients with limited-stage (LS) agressive B-cell lymphoma (LS-ABCL) who harbor MYC rearrangement (MYC-R) with or without BCL2 and/or BCL6 rearrangements. We conducted a multicenter retrospective study of MYC-R LS-ABCL patients who received either rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), or more intensive immunochemotherapy (IIC) plus or minus consolidative involved-field radiation therapy (IFRT). One hundred four patients from 15 academic centers were included. Forty four patients (42%) received R-CHOP, of whom 52% had IFRT. Sixty patients (58%) received IIC, of whom 40% had IFRT. Overall response rate was 91% (84% complete response [CR]; 7% partial response). Patients with double-hit lymphoma (DHL; n = 40) had a lower CR rate compared with patients with MYC-R only (75% vs 98%; P = .003). CR rate was higher in the IFRT vs no-IFRT group (98% vs 72%; P < .001). Median follow-up was 3.2 years; 2-year progression-free survival (PFS) and overal survival (OS) were 78% and 86% for the entire cohort, and 74% and 81% for the DHL patients, respectively. PFS and OS were similar across treatment groups (IFRT vs no IFRT, R-CHOP vs IIC) in the entire cohort and in DHL patients. Our data provide a historical benchmark for MYC-R LS-ABCL and LS-DHL patients and show that outcomes for this population may be better than previously recognized. There was no benefit of using IIC over R-CHOP in patients with MYC-R LS-ABCL and LS-DHL.
Introduction
Limited-stage (LS) diffuse large B-cell lymphoma (LS-DLBCL; defined as Ann Arbor stage I to II, confined to a single radiation field in this study) comprises approximately one-third of patients with de novo DLBCL.1 Standard treatment of patients with LS-DLBCL involves extended immunochemotherapy (4-8 cycles2-5 ) vs abbreviated immunochemotherapy (3 cycles) plus involved-field radiotherapy (IFRT).2,4-6 Long-term follow-up of the SWOG 8736 study, the largest prospective phase 3 study in LS-DLBCL, showed similar progression-free survival (PFS) and overall survival (OS) between patients treated with 8 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) vs 3 cycles of CHOP followed by IFRT.7 The phase 2 SWOG S0014 study showed that addition of rituximab increased survival by 10% to 15% in LS-DLBCL patients with at least 1 adverse feature on the stage-modified International Prognostic Index (sm-IPI), with a 4-year PFS and OS of 88% and 92%, respectively. Long-term follow-up (median 12 years) of these patients yielded 5- and 10-year OS of 82% and 67%, respectively.7 In the current era, most clinicians apply an individualized approach to therapy in patients with LS-DLBCL. Although the National Cancer Center Network (NCCN) guidelines recommend either 3 cycles of rituximab with CHOP (R-CHOP) plus IFRT (category 1) or 6 cycles of R-CHOP in this setting, the use of IFRT is preferable in patients for whom poor tolerance to chemotherapy is anticipated or when toxicity of IFRT may be minimal due to the location of disease, as this would allow the use of more abbreviated chemotherapy.
One of the other factors that guide treatment in advanced-stage DLBCL is the rearrangement status of MYC. Patients with MYC rearrangment (MYC-R) DLBCL demonstrate poor outcomes when treated with R-CHOP.8,9 Although there are no specific guidelines regarding the management of these patients and only retrospective data to guide treatment decisions, more intensive therapy approaches are often used compared with DLBCL patients without MYC-R. The presence of concomitant BCL-2 or BCL-6 gene rearrangement (BCL2-R or BCL6-R) confers an even more dismal outcome for patients with MYC-R DLBCL.10-14 Although data are limited, simultaneous rearrangements of BCL-6 and MYC and also BCL-2, BCL-6, and MYC (“triple hit”) appear to adversely impact outcomes.15 Due to the distinct differences in outcomes compared with DLBCL, double-hit lymphoma (DHL)/triple-hit lymphoma have been incorporated as a distinct entity in the 2016 World Health Organization (WHO) Classification of Lymphoid Malignancies as “high-grade B-cell lymphoma (HGBL) with rearrangements of MYC and BCL2 and/or BCL6.”16 Although there are no randomized prospective trials in DHL, R-CHOP is not considered an optimal frontline therapy due to unacceptable rates of progression. Several studies have suggested that escalating upfront therapy from R-CHOP to intensive regimens such as rituximab with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-DA-EPOCH) or fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with high-dose methotrexate and cytarabine (R-HyperCVAD/MA) may improve outcomes in patients with DHL.15,17-19
Historically, most patients with DHL have had a high IPI score and advanced-stage disease at diagnosis; however, with increasing adoption of routine fluorescence in situ hybridization (FISH) testing, an increasing number of cases are being diagnosed in the setting of LS disease. There is a paucity of data regarding prognosis and appropriate management of patients with LS MYC-R/DHLs/triple-hit lymphomas. Herein, we evaluate the practice patterns of management and outcomes of LS MYC-R DLBCL/HGBL (including DHL) and compare the clinical outcomes of patients treated with R-CHOP vs more intensive immunochemotherapy (IIC).
Methods
Patients
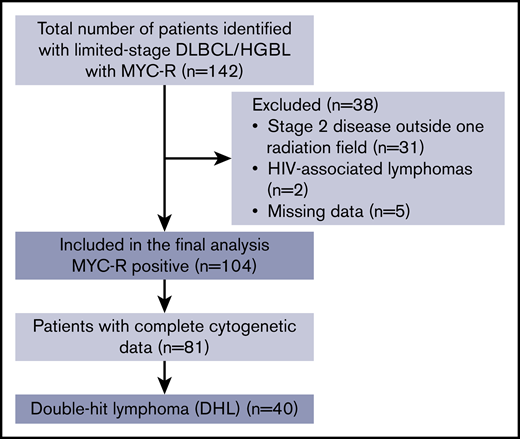
Inclusion criteria included patient age of 18 to 90 years, confirmed histologic diagnosis of DLBCL or HGBL, LS disease on imaging (Ann Arbor stage I to II, confined to a single radiation field), evidence of MYC-R by FISH, and initial treatment with curative intent immunochemotherapy with or without consolidative IFRT. Patients diagnosed between January 2005 and March 2017 were included in the study. Patients were excluded for a diagnosis of Burkitt lymphoma, HIV-associated lymphoma, or posttransplant lymphoproliferative disorder; disease outside 1 irradiation field; and or inadequate clinicopathologic or survival outcomes data (Figure 1, CONSORT diagram). Cell-of-origin classification was performed using the Hans algorithm as previously described.20 Cases were reviewed by hematopathologists at each academic medical center as per routine clinical practice. Criteria and methods for performance of FISH were per the policy of each center. The sm-IPI score incorporated the following pretreatment variables: stage II [vs I], age >60 years, elevated serum lactate dehydrogenase (LDH) level, and Eastern Cooperative Oncology Group (ECOG) performance status ≥2. The choice of frontline immunochemotherapy as well as the mode and regimen for central nervous system (CNS) prophylaxis were determined by the treating physician. Responses were adjudicated by individual investigators using institutional standard-imaging modalities at the time of treatment.
Objectives and end points
The primary objective of this study was to compare PFS and OS in patients with LS-DLBCL/HGBL who received R-CHOP vs IIC. Secondary objectives were to compare overall response rate (ORR) and complete response (CR) rate; determine the impact of baseline clinical or pathologic features on CR, PFS, and OS; and assess the impact of consolidative IFRT on CR, PFS, and OS. A subset analysis of the primary and secondary objectives in LS-DHL patients (defined as MYC-R with BCL2-R and/or BCL6-R) was also planned. Data were collected by retrospective chart review. Outcomes data were censored in October 2017. PFS was defined as time from diagnosis to disease progression, change in therapy, death or last follow-up in remission. OS was defined as time from diagnosis to death from any cause or last follow-up. This protocol was approved by the institutional review board of each participating center.
Statistical methods
Patient characteristics were reported as means, medians, and standard deviations for continuous variables, and as frequencies and relative frequencies for categorical variables. Comparisons were made between groups using the Mann-Whitney U and Fisher's exact tests for continuous and categorical variables, respectively. Associations between treatment selection/outcome and patient demographic, clinical, and treatment characteristics were evaluated using logistic regression models, from which odds ratios and corresponding 95% confidence intervals (CIs) were obtained.
Survival outcomes (PFS and OS) were summarized using standard Kaplan-Meier methods, where estimates of the median and 2-year rates were obtained with 95% CIs. Comparisons were made using the log-rank test. Associations between survival outcomes and patient demographic, clinical, and treatment characteristics were evaluated using Cox regression models, from which hazard ratios and corresponding 95% CIs were obtained.
All analyses were conducted in SAS v9.4 (Cary, NC) at a significance level of 0.05. No adjustments were made for multiple testing and no imputation methods were applied to missing data.
Results
Patients
A total of 142 patients with MYC-R LS-DLBCL/HGBL were identified from 15 US academic medical centers; 104 of these patients fulfilled the inclusion criteria. Patient, disease, and treatment characteristics are listed in Table 1. Median age at time of diagnosis was 65 years (range, 21-85 years). Twenty-one percent (n = 22) of patients had stage I disease, 32% (n = 33) stage IE disease, 21% (n = 22) stage II disease, and 26% (n = 27) had stage IIE disease. The majority of patients (69%; n = 70) had a germinal center B-cell (GCB) phenotype.
Baseline clinicopathologic and treatment characteristics of the entire cohort and comparison between patients treated with R-CHOP vs IIC
| Characteristic . | All patients* . | R-CHOP* . | IIC* . | P . |
|---|---|---|---|---|
| Total | 104 (100) | 44 (42) | 60 (58) | |
| Clinical characteristics | ||||
| Median age (range), y | 65 (21-85) | 66.5 (21-85) | 62.5 (21-84) | .38 |
| Sex | .84 | |||
| Male | 68 (65) | 29 (64) | 40 (67) | |
| Female | 36 (35) | 16 (36) | 20 (33) | |
| Stage | .32 | |||
| 1 | 55 (53) | 26 (59) | 29 (48) | |
| 2 | 49 (47) | 18 (41) | 31 (52) | |
| sm-IPI | .15 | |||
| 0-1 | 50 (52) | 24 (62) | 26 (45) | |
| 2-3 | 47 (48) | 15 (38) | 32 (55) | |
| Extranodal disease | .84 | |||
| Yes | 60 (58) | 26 (59) | 34 (57) | |
| No | 44 (42) | 18 (41) | 26 (43) | |
| LDH | .09 | |||
| Normal | 56 (58) | 27 (69) | 29 (50) | |
| Elevated | 29 (42) | 12 (31) | 29 (50) | |
| Disease characteristics | ||||
| Morphology | .44 | |||
| DLBCL | 86 (83) | 38 (86) | 48 (80) | |
| HGBL | 18 (17) | 6 (14) | 12 (20) | |
| Cell of origin | .20 | |||
| GCB | 70 (69) | 26 (62) | 44 (75) | |
| Non-GCB | 31 (31) | 16 (38) | 15 (25) | |
| De novo | 88 (85) | 40 (91) | 48 (80) | .17 |
| Transformed | 16 (15) | 4 (9) | 12 (20) | |
| Mean Ki67 (range) | 80.6 (10-100); n = 94 | 82.2 (10-100); n = 38 | 79.6 (15-100); n = 56 | .66 |
| DHL | 1.00 | |||
| Yes | 40 (49) | 16 (50) | 24 (49) | |
| No | 41 (51) | 16 (50) | 25 (51) | |
| MYC expression | .2 | |||
| <40% | 14 (34) | 9 (45) | 5 (24) | |
| ≥40% | 27 (66) | 11 (55) | 16 (76) | |
| BCL2 expression | .23 | |||
| <50% | 28 (39) | 10 (31) | 18 (46) | |
| ≥50% | 43 (61) | 22 (69) | 21 (54) | |
| Treatment characteristics | ||||
| IIC regimen | NA | NA | R-DA-EPOCH; n = 51 (85%) | NA |
| R-HyperCVAD/MA; n = 7 (12%) | ||||
| R-CODOX-M/IVAC; n = 2 (3%) | ||||
| Median no. of cycles (range) | 6 (1-8) | 6 (1-8) | 6 (2-7) | .5 |
| IFRT | .24 | |||
| Yes | 47 (45) | 23 (52) | 24 (40) | |
| No | 57 (55) | 20 (48) | 36 (60) | |
| CNS prophylaxis | <.001 | |||
| Yes | 43 (54) | 10 (29) | 33 (75) | |
| No | 36 (46) | 25 (71) | 11 (25) |
| Characteristic . | All patients* . | R-CHOP* . | IIC* . | P . |
|---|---|---|---|---|
| Total | 104 (100) | 44 (42) | 60 (58) | |
| Clinical characteristics | ||||
| Median age (range), y | 65 (21-85) | 66.5 (21-85) | 62.5 (21-84) | .38 |
| Sex | .84 | |||
| Male | 68 (65) | 29 (64) | 40 (67) | |
| Female | 36 (35) | 16 (36) | 20 (33) | |
| Stage | .32 | |||
| 1 | 55 (53) | 26 (59) | 29 (48) | |
| 2 | 49 (47) | 18 (41) | 31 (52) | |
| sm-IPI | .15 | |||
| 0-1 | 50 (52) | 24 (62) | 26 (45) | |
| 2-3 | 47 (48) | 15 (38) | 32 (55) | |
| Extranodal disease | .84 | |||
| Yes | 60 (58) | 26 (59) | 34 (57) | |
| No | 44 (42) | 18 (41) | 26 (43) | |
| LDH | .09 | |||
| Normal | 56 (58) | 27 (69) | 29 (50) | |
| Elevated | 29 (42) | 12 (31) | 29 (50) | |
| Disease characteristics | ||||
| Morphology | .44 | |||
| DLBCL | 86 (83) | 38 (86) | 48 (80) | |
| HGBL | 18 (17) | 6 (14) | 12 (20) | |
| Cell of origin | .20 | |||
| GCB | 70 (69) | 26 (62) | 44 (75) | |
| Non-GCB | 31 (31) | 16 (38) | 15 (25) | |
| De novo | 88 (85) | 40 (91) | 48 (80) | .17 |
| Transformed | 16 (15) | 4 (9) | 12 (20) | |
| Mean Ki67 (range) | 80.6 (10-100); n = 94 | 82.2 (10-100); n = 38 | 79.6 (15-100); n = 56 | .66 |
| DHL | 1.00 | |||
| Yes | 40 (49) | 16 (50) | 24 (49) | |
| No | 41 (51) | 16 (50) | 25 (51) | |
| MYC expression | .2 | |||
| <40% | 14 (34) | 9 (45) | 5 (24) | |
| ≥40% | 27 (66) | 11 (55) | 16 (76) | |
| BCL2 expression | .23 | |||
| <50% | 28 (39) | 10 (31) | 18 (46) | |
| ≥50% | 43 (61) | 22 (69) | 21 (54) | |
| Treatment characteristics | ||||
| IIC regimen | NA | NA | R-DA-EPOCH; n = 51 (85%) | NA |
| R-HyperCVAD/MA; n = 7 (12%) | ||||
| R-CODOX-M/IVAC; n = 2 (3%) | ||||
| Median no. of cycles (range) | 6 (1-8) | 6 (1-8) | 6 (2-7) | .5 |
| IFRT | .24 | |||
| Yes | 47 (45) | 23 (52) | 24 (40) | |
| No | 57 (55) | 20 (48) | 36 (60) | |
| CNS prophylaxis | <.001 | |||
| Yes | 43 (54) | 10 (29) | 33 (75) | |
| No | 36 (46) | 25 (71) | 11 (25) |
GCB, germinal center B-cell; NA, not applicable.
Values are n (%) unless otherwise specified in row headings.
Forty-four patients (42%) received R-CHOP, of whom 23 (52%) received consolidative IFRT. Sixty patients (58%) received IIC, of whom 24 (40%) received consolidative IFRT. Four patients in the IIC group received R-CHOP for 1 cycle and then escalated to IIC following the finding of adverse cytogenetics. R-DA-EPOCH was the most common IIC regimen used (85% of patients), followed by R-HyperCVAD/MA (12%). Forty patients (85%) had achieved a CR and 7 (15%) had achieved a partial response (PR) prior to the administration of consolidative IFRT. Age, stage, presence of extranodal disease, serum LDH, sm-IPI, morphology, and double-hit status were similar between patients receiving R-CHOP and IIC. The median number of cycles and proportion of patients who received IFRT did not differ between the 2 groups (P = .17). The median number of cycles was lower in the IFRT vs no IFRT group (4 vs 6; P = .02). Forty-three patients received CNS prophylaxis (54%); data were not available for 25 patients (24%). The mode of CNS prophylaxis was not available for analysis. Patients receiving IIC were more likely to receive CNS prophylaxis (75%, n = 35) when compared with patients who received R-CHOP (29%; n = 10; P < .001).
Outcomes
Median follow-up was 3.2 years (range, 0.5-12.5 years). In the entire cohort (n = 104), the ORR to frontline therapy was 91% (84% CR; 7% PR) (Table 2). The ORR and CR rates were similar between the IIC and R-CHOP groups (ORR, 93% vs 88% [P = .51]; CR, 82% vs 85% [P = .79]). The CR rate was higher in the IFRT compared with the no-IFRT group (98% vs 72%; P < .001). Of the 7 patients with a PR prior to administration of consolidative IFRT, 6 patients converted to a CR after IFRT. In patients who had relapsed/refractory disease (n = 26), distant relapses were more common in the IFRT vs no-IFRT group (92% vs 43%; P = .014).
Comparison between outcomes of patients with MYC-R LS-DLBCL/HGBL treated with R-CHOP vs IIC
| . | All (MYC-R) patients, N = 104 . | R-CHOP, N = 44 . | IIC, N = 60 . | P . |
|---|---|---|---|---|
| ORR, n (%) | 94 (91) | 41 (93) | 53 (88) | .51 |
| CR, n (%) | 87 (84) | 36 (82) | 51 (85) | .79 |
| PR, n (%) | 7 (7) | 5 (11) | 2 (3) | .13 |
| 2-y PFS, % | 78 | 79 | 77 | .79 |
| 2-y OS, % | 86 | 88 | 83 | .28 |
| . | All (MYC-R) patients, N = 104 . | R-CHOP, N = 44 . | IIC, N = 60 . | P . |
|---|---|---|---|---|
| ORR, n (%) | 94 (91) | 41 (93) | 53 (88) | .51 |
| CR, n (%) | 87 (84) | 36 (82) | 51 (85) | .79 |
| PR, n (%) | 7 (7) | 5 (11) | 2 (3) | .13 |
| 2-y PFS, % | 78 | 79 | 77 | .79 |
| 2-y OS, % | 86 | 88 | 83 | .28 |
Thirty-four patients (33%) progressed or died. Of the 23 deaths, 15 were attributable to progressive lymphoma, 1 was due to treatment-related toxicity from R-CHOP, and 7 died of unrelated causes. The 2-year PFS and OS were 78% and 86%, respectively, for the entire cohort and 74% and 81%, respectively, for DHL patients (Figure 2).
Kaplan-Meier plots for the entire cohort and for DHL patients. (A) PFS. (B) OS.
Kaplan-Meier plots for the entire cohort and for DHL patients. (A) PFS. (B) OS.
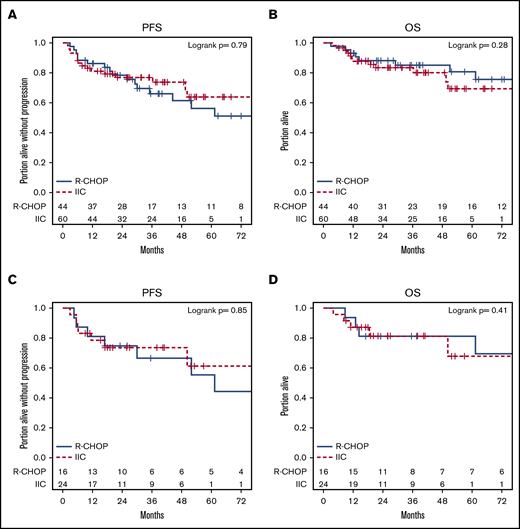
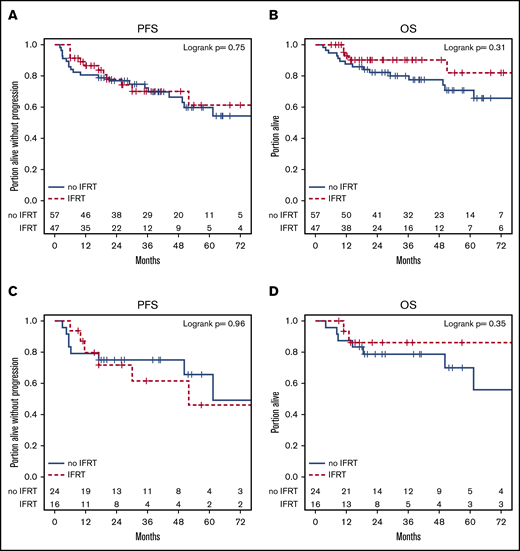
sm-IPI ≥ 2 (hazard ratio [HR], 2.32; P = .026), elevated serum LDH (HR, 2.08; P = .043), and transformed status (HR, 2.42; P = .019) were associated with a lower PFS in univariate analysis (supplemental Table 1), however, only transformed status was prognostic in multivariate models (HR, 2.32; P = .031) (supplemental Table 2). sm-IPI ≥ 2 (HR, 2.80; P = .025) and age ≥70 years (HR, 4.06; P = .001) were associated with inferior OS in univariate, but not multivariate, analysis (supplemental Tables 3 and 4). Presence of B symptoms, stage, extranodal disease, morphology, cell of origin, and double-hit status did not affect survival. PFS and OS were similar across treatment groups (R-CHOP vs IIC, IFRT vs no IFRT) (Figures 3 and 4) in the entire cohort. One patient had a CNS relapse: this patient had not received CNS prophylaxis as part of frontline therapy. Use of CNS prophylaxis was not associated with improved PFS (HR, 0.62 [95% CI, 0.27, 1.40]) or OS (HR, 0.98 [95% CI, 0.34, 2.85]). The findings of univariate analysis were confirmed in a multivariate model controlling for all 3 treatment variables (R-CHOP vs IIC, IFRT vs no IFRT, and CNS prophylaxis vs no CNS prophylaxis), which showed no statistical difference in PFS and OS.
Kaplan-Meier plots of PFS and OS by R-CHOP vs IIC. (A-B) Entire cohort. (C-D) DHL patients.
Kaplan-Meier plots of PFS and OS by R-CHOP vs IIC. (A-B) Entire cohort. (C-D) DHL patients.
Kaplan-Meier plots of PFS and OS by IFRT vs no IFRT. (A-B) Entire cohort. (C-D) DHL patients.
Kaplan-Meier plots of PFS and OS by IFRT vs no IFRT. (A-B) Entire cohort. (C-D) DHL patients.
Further analyses revealed that no subset of patients benefitted more with IIC over R-CHOP when stratified by their baseline characteristics such as age, sex, stage, extranodal disease, high serum LDH, sm-IPI score, cell of origin, morphologic classification, transformation status, or double-hit status (supplemental Figure 1a-d).
Subgroup analysis of patients with DHL
Of 104 patients with MYC-R, 81 patients had data on BCL2 and BCL6. Of the 81 patients, 40 (49%) had DHL: 26 patients with MYC-R/BCL2-R, 10 patients with MYC-R/BCL6-R, and 4 patients with triple-hit lymphoma (MYC-R/BCL2-R/BCL6-R). Twenty-three patients without complete cytogenetic data were excluded from this subgroup analysis comparing DHL patients with MYC-R–only patients. The ORR and CR rates in patients with DHL were 85% and 75%, respectively (Table 3). CR rates were lower in patients with DHL compared with those with MYC-R only (75% vs 98%; P = .003). ORR was comparable in DHL patients treated with IIC (n = 24; 60%) and R-CHOP (n = 16; 40%): 88% vs 81% (P = .68). The CR rate was higher in the IIC group compared with the R-CHOP group, however, the difference did not reach statistical significance, perhaps due to the small sample size (83% vs 63%; P = .16). The odds of achieving a CR were higher with IIC than R-CHOP in patients with GCB cell of origin and among those who did not receive IFRT, however, this benefit was not reflected in ORR, PFS, or OS (supplemental Figure 2a-d).
Comparison between outcomes of patients with LS-DHL treated with R-CHOP vs IIC
| . | All DHL patients, N = 40 . | R-CHOP, N = 16 . | IIC, N = 24 . | P . |
|---|---|---|---|---|
| ORR, n (%) | 34 (85) | 13 (81) | 21 (88) | .67 |
| CR, n (%) | 30 (75) | 10 (63) | 20 (83) | .16 |
| PR, n (%) | 4 (10) | 3 (19) | 1 (4) | .28 |
| 2-y PFS, % | 74 | 75 | 74 | .85 |
| 2-y OS, % | 81 | 81 | 81 | 1.00 |
| . | All DHL patients, N = 40 . | R-CHOP, N = 16 . | IIC, N = 24 . | P . |
|---|---|---|---|---|
| ORR, n (%) | 34 (85) | 13 (81) | 21 (88) | .67 |
| CR, n (%) | 30 (75) | 10 (63) | 20 (83) | .16 |
| PR, n (%) | 4 (10) | 3 (19) | 1 (4) | .28 |
| 2-y PFS, % | 74 | 75 | 74 | .85 |
| 2-y OS, % | 81 | 81 | 81 | 1.00 |
The 2-year PFS and OS of the DHL cohort was 74% and 81%, respectively. PFS and OS were similar across treatment groups (IFRT vs no IFRT, R-CHOP vs IIC) in the DHL patients (Figures 3 and 4). Rates of CNS prophylaxis were similar in DHL (64%; n = 21) vs MYC-R–only patients (49%; n = 20; P = .23). DHL patients receiving IIC (81%; n = 17) were more likely to receive CNS prophylaxis than those receiving R-CHOP (33%; n = 4; P = .01). Administration of CNS prophylaxis did not impact ORR, CR rate, PFS, or OS in LS-DHL patients.
Discussion
In our study, intensification of chemotherapy did not improve survival in LS-DLBCL/HGBL patients who harbor a MYC rearrangement. A subset analysis of patients with DHL yielded similar findings, although responses tended to be better in patients treated with IIC. Additionally, the use of IFRT or CNS prophylaxis was not associated with differences in survival outcomes in our cohort. Although the number of DHL patients in this cohort is modest (n = 40), our study addresses a knowledge gap regarding the management and outcomes of patients with LS-DHL, an area in which little is known.
A series of 129 patients with DHL treated at the MD Anderson Cancer Center with frontline R-CHOP were reported to have a CR rate of 40%, which was significantly lower than 68% for R-DA-EPOCH and R-HyperCVAD/MA.15 Patients treated with R-DA-EPOCH had a significantly improved event-free survival rate compared with those who received R-CHOP (HR, 0.38; P = .008). In this report, 4% of patients had stage 1 disease and 12% had stage 2 disease. Of the total 20 patients with stage 1 or 2 disease, 11 received R-CHOP, 8 received R-DA-EPOCH, and 1 received R-HyperCVAD/MA. None of the 20 patients with early-stage disease planned at diagnosis to have a short course (<6 cycles) of immunochemotherapy. Treatment was changed in 6 patients (30%) to different regimens after <6 cycles because of disease progression after 2, 3, and 4 cycles (n = 2 for each). Of the patients who received at least 6 cycles of primary immunochemotherapy, 5 received consolidative radiation therapy. Patients with early-stage disease had a CR rate of 60%, not different from advanced-stage patients (54%; P = .628).
A US multicenter study of 311 patients with DHL reported a significantly lower CR rate for patients treated with R-CHOP compared with R-DA-EPOCH, and a shorter median PFS for patients receiving R-CHOP compared with intensive frontline therapy (7.8 v 21.6 months).17 In this series, 18% of patients had stage 1 or 2 disease. Although treatment regimens and responses in patients with LS disease were not specifically delineated, the authors did find leukocytosis (white blood cell count 10 × 109/L), elevated serum LDH, advanced-stage disease, and CNS involvement to be adverse prognostic markers on multivariate analysis. Another multicenter retrospective study of 149 patients with DHL who achieved first complete remission following completion of frontline therapy included 38 patients with stage 1/2 disease and showed that consolidation with high-dose chemotherapy followed by autologous stem cell transplantation did not improve outcomes.18 In a phase 2, prospective, single-arm study, in which 10 of 53 patients had stage 1-2 disease and over one-half (24 of 53) had DHL, R-DA-EPOCH produces a 4-year event-free survival of 71% and 4-year OS of 76.7% for patients with MYC-R aggressive B-cell lymphoma.19 Although the phase 3 Alliance/CALGB 50303 study did not show any outcome differences between R-CHOP and R-DA-EPOCH in DLBCL, only a limited number of patients with MYC-R (13 of 491) and DHL (3 of 491) were included, which precludes us from drawing any conclusions in this patient population.21 In a recently published retrospective study of 171 LS-DLBCL patients treated with R-CHOP, with or without radiation therapy, patients with DHL (n = 7) did not have an inferior PFS or OS. Definitive conclusions are difficult to be drawn given the low number of DHL patients.22
The current study is the first multicenter analysis to focus specifically on LS-DLBCL/HGBL with MYC-R and/or BCL2-R and/or BCL6-R within 1 radiation field. Although the 2-year PFS and OS in our cohort (78% and 86%, respectively) were lower than historical cohorts of LS-DLBCL,23,24 they are significantly better than previous reports that included patients with advanced-stage MYC-R DLBCL/HGBL and DHL.17,19 We excluded LS patients with MYC-R with disease outside of 1 radiation field to limit heterogeneity of disease, minimize bias that could be involved in frontline therapy selection (ie, treating physician may tend to use IIC in stage 2 disease outside of 1 radiation field with adverse cytogenetics), and allow direct comparison of patients treated with and without radiation therapy. However, this factor needs to be considered while comparing our data with previously published data on patients with LS disease in which such patients may not have been excluded. We had a balanced distribution of patients between the R-CHOP and IIC arm and did not find any difference in PFS and OS in patients treated with R-CHOP vs IIC. It is important to note that in the IIC cohort, 85% of patients were treated with R-DA-EPOCH. Hence, any conclusions from the comparison were mainly driven by this regimen.
Next, we addressed the role of consolidative IFRT in LS MYC-R DLBCL/HGBL. Whereas IFRT led to a higher CR rate, the receipt of IFRT did not affect PFS and OS in this cohort. Of the patients who had relapsed/refractory disease and had received IFRT (n = 12), only 1 had local relapse whereas the remaining patients had distant relapses (n = 11). Previous studies have shown that relapse rates after IFRT vary from 0% to 34% and, although IFRT induces better local tumor control, it does not prevent systemic relapse in LS-DLBCL patients.25 Our study corroborates these findings in MYC-R LS-DLBCL patients.
In this cohort, DHL patients had a lower CR rate than MYC-R–only patients. The 2-year PFS and OS was 74% and 81%, respectively, in the DHL cohort. Although ORR and CR rates tended to be higher in DHL patients if treated with IIC, the survival rates were comparable in the R-CHOP vs IIC groups. Although these data need to be interpreted cautiously due to the limited number of patients in each group, it is reassuring to note that patients with LS-DHL have good outcomes with R-CHOP (2-year OS, 82%), in contrast to advanced-stage DHL. Whether CNS prophylaxis or intensification of therapy should be performed across the board in this patient population cannot be answered definitively by our study; however, the data suggest that there is a role for individualization of therapy, and outcomes are better than previously appreciated in this high-risk patient population. Outcomes with R-CHOP vs IIC and IFRT vs no IFRT were not remarkably different in terms of PFS and OS, and consideration should be given to patient’s performance status and comorbidities in guiding the therapeutic decision. Our study also suggests that perhaps the biology of LS and advanced-stage single-hit lymphoma/DHL is different, thus producing varied outcomes.
Our study has several limitations, mostly inherent to its retrospective nature. Selection bias, lack of central response assessment, and central pathology review and/or missing data may have confounded the analysis. There could have been interinstitutional differences in practice and treatment philosophies guiding the choice of therapy. Because the number of patients contributed by each institution varied significantly, a statistical estimate of this bias is not possible. Although we were able to gather information on 104 patients with MYC-R LS-DLBCL/HGBL through a multi-institutional collaboration of 15 academic centers, the number of LS-DHL patients was only 40, thus curtailing the significance of the subgroup analysis. The majority of patients in our series were detected to have MYC-R using a 8q24 break-apart probe. Hence, we are unable to comment on the impact of the translocation partner for MYC, although there is emerging evidence that the negative prognostic impact of MYC-R in DLBCL is largely observed in patients in whom MYC is translocated to an immunoglobulin partner.26,27 Lastly, immunohistochemistry data for MYC expression and BCL2 expression was missing or inconclusive in 61% and 32% of patients, respectively, hence a reliable analysis of the differences in outcomes between double-expressor and non-double-expressor DLBCL/HGBL could not be performed.
In conclusion, the 2-year PFS and OS of patients with MYC-R LS-DLBCL/HGBL were 78% and 86%, respectively. There was no apparent benefit of using IIC over R-CHOP or adding CNS prophylaxis in this cohort. Although consolidative IFRT led to a higher CR rate, the PFS and OS were similar in comparison with the non-IFRT group. Distant relapses were observed, perhaps explaining the observed PFS and OS. Patients with LS-DHL had lower CR rates than those with MYC-R as the sole cytogenetic abnormality. Although intensification of therapy tended to produce higher response rates, this did not translate into improved survival in LS-DHL. Our data provide a historical benchmark for patients with LS-DLBCL/HGBL with MYC-R and LS-DHL patients who have disease confined to 1 radiation field, and show that outcomes for this population may be better than were previously recognized. Prospective trials incorporating novel/targeted agents targeting Bcl-2 family members or C-MYC function rather than further intensification of chemotherapy regimens may yield better outcomes in patients with MYC-R with or without BCL2-R and/or BCL6-R DLBCL/HGBL.
Presented as an oral abstract at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 2 December 2018.
For original data, please contact pallawi.torka@roswellpark.org or shalin.kothari@yale.edu.
Acknowledgment
This work was supported by a biostatistics shared resource supported by a Roswell Park Comprehensive Cancer Center support grant from the National Institutes of Health, National Cancer Institute (P30CA016056).
Authorship
Contribution: All authors provided study materials or patients, contributed to data collection and assembly, reviewed the data analysis, edited and revised the manuscript, and approved the final version of the manuscript; in addition, P.T. and S.K.K. participated in study design, analyzed and interpreted the data, and wrote and revised the manuscript; and A. Groman and K.A. performed data analysis and interpretation.
Conflict-of-interest disclosure: K.J.M. has received research support from Bristol-Myers Squibb, Merck, Pharmacyclics, and Novartis, and has been an advisory board member for Pharmacyclics, Celgene, Teva, Seattle Genetics, AstraZeneca, and Bayer. R.K. has received research support from Kite/Gilead, Celgene/Juno, Takeda, and Bristol-Myers Squibb; has been an advisory board member for Kite/Gilead and Celgene/Juno; and has been on speakers’ bureaus for Kite/Gilead and AstraZeneca. A.J.O. has received research support from Genentech/Roche, Spectrum Pharmaceuticals, and TG Pharmaceuticals. B.H. has received research support and consulting remuneration from Genentech. D.P. has equity holdings in Bristol-Myers Squibb and Merck, and has received associate research funding from Celgene and ER Squibb and Sons. The remaining authors declare no competing financial interests.
Correspondence: Pallawi Torka, Roswell Park Comprehensive Cancer Center, Elm and Carlton Streets, Buffalo, NY 14263; e-mail: pallawi.torka@roswellpark.org.
References
Author notes
P.T. and S.K.K. contributed equally to the work.
The full-text version of this article contains a data supplement.