Key Points
Subcutaneous cladribine shows excellent long-term survival in patients with hairy cell leukemia; progression of disease at 24 or 36 months is not prognostic.
Secondary primary malignancies are the main cause of death but are not significantly increased compared with the normal population.
Abstract
Hairy cell leukemia (HCL) remains an incurable disease. However, first-line treatment with either intravenous or subcutaneous cladribine generally leads to long-lasting remissions. Although there are excellent long-term data for intravenous application, similar data regarding subcutaneous administration are lacking. We therefore analyzed the long-term outcome of 3 prospective multicenter clinical trials on subcutaneous cladribine performed by the Swiss Group for Clinical Cancer Research (SAKK), which recruited 221 patients with classical HCL between 1993 and 2005. Median overall survival from start of treatment was not reached. Pretreatment anemia, higher Eastern Cooperative Oncology Group score, and higher age were associated with poorer overall survival in multivariable analysis, whereas early progression at 24 and 36 months had no significant impact on overall survival. Second-line treatment was necessary in 53 (23.7%) patients after a median of 5 (range, 0.2-20.4) years, and first retreatment was mainly monotherapy with cladribine (66%) or rituximab (15.1%) or a combination of these drugs (15.1%). A total of 44 (19.9%) patients developed second primary malignancies with a median time to occurrence of 5.7 (range, 0.01-17.5) years. Second primary malignancies were the main cause for death (14; 27.5%). Compared with a matched normal Swiss population, the incidence of second primary malignancies was not increased. However, survival of patients with HCL was slightly inferior by comparison (P = .036). In conclusion, the outcome of HCL patients treated with subcutaneous cladribine is excellent, and in most patients, 1 cycle of subcutaneous cladribine is sufficient for long-term disease control.
Introduction
Hairy cell leukemia (HCL) is a rare indolent mature B-lymphoid neoplasm with an incidence between 2.9 and 4.7 per million people per year.1,2 It is characterized by various degrees of pancytopenia, particularly monocytopenia, with typical hairy cells in the peripheral blood or bone marrow in combination with splenomegaly, hepatomegaly, and rarely lymphadenopathy. Over the last decades, the excellent responsiveness to interferon-α and the 2 purine analogs pentostatin and cladribine has substantially improved survival rates, reaching almost normal survival.3-7 Despite the success of purine analog treatment, significant side effects such as infections and secondary malignancies caused by profound and long-lasting immunosuppression are still a matter of concern.8
Continuous intravenous application of cladribine has been applied in most clinical trials and is still used in many centers as first-line treatment of patients with HCL. However, although the plasma half-life of cladribine is short, the drug accumulates within leukemic cells and has a longer intracellular half-life, making the subcutaneous application feasible.9,10 This is an attractive option facilitating outpatient treatment of patients in good health. In the 1990s, the Swiss Group for Clinical Cancer Research (SAKK) developed several trials looking at the outcome of subcutaneous cladribine, showing feasibility using a 5-day schedule. However, long-term follow-up data are mostly based on patients after intravenous cladribine administration in single-center cohorts.7,11-13 Only 1 population-based study with 85 patients analyzed the long-term effect of mainly subcutaneous applied cladribine, suggesting equal long-term efficacy.14 We therefore analyzed the long-term outcome in a large population of patients with classical HCL based on 3 prospective, multicenter SAKK trials with subcutaneous cladribine, and we compared the results with a matched normal Swiss population.
Materials and methods
Patients and trial design
Between 1993 and 2005, the SAKK performed 3 subsequent prospective studies analyzing the feasibility of subcutaneous cladribine treatment (Litak 10, solution for subcutaneous injection or intravenous infusion; Lipomed AG) in patients with HCL (SAKK 32/93, SAKK 32/95, SAKK 32/98). Rationale, regimens, and detailed short-term results are published elsewhere.15-17 The current long-term follow-up analysis is based on the patients included in these 3 studies. Within the clinical trials, all patients were either subject to lifelong follow-up (SAKK 32/93) or until progression, relapse, or death (SAKK 32/95 and SAKK 32/98). All trials were approved by the ethical committees of the Swiss centers. Response was assessed by peripheral blood and bone marrow at 10 weeks. The consensus criteria, published in 1987, applied for remission definition.18 A total of 237 patients were accrued in the 3 trials. Four patients were in 2 of these trials, and 12 patients needed to be excluded because of nonclassical HCL phenotype. Therefore, 221 patients were analyzed. Ninety-two (41.2%) patients had their last follow-up information before 31 May 2013 within the initial study protocol, which is more than 5 years before the data cutoff of 31 May 2018. However, using an additional questionnaire, follow-up data were obtained from 87% of the patients still alive with a cutoff data of 31 January 2018.
Treatment schedules
Treatment algorithms in the studies were as follows: (1) 5 days of subcutaneous cladribine 0.14 mg/kg followed by a maximum of 2 cycles of 7 days of intravenous cladribine 0.1 mg/kg in case of minor response or no response (SAKK 32/93); (2) single shot of subcutaneous cladribine 0.25 mg/kg followed by a maximum of 2 cycles of subcutaneous cladribine 0.14 mg/kg for 5 days in case of minor response, no response, or relapse (SAKK 32/95); and (3) 5 consecutive days of subcutaneous cladribine 0.14 mg/kg vs the same dose in 5 weekly applications (SAKK 32/98). SAKK 32/93 included 63, SAKK 32/95 included 74, and SAKK 32/98 included 100 patients. In all studies, assessment of early response was at 10 weeks after treatment initiation. In the few non–complete response/partial response (CR/PR) patients, additional treatment was administered as outlined in earlier publications.15-17 Response criteria were similar to those published in the consensus guidelines by Grever in 2017, and the same criteria were applied in all 3 studies.8 Of note, response in the bone marrow biopsy was assessed conventionally and by tartrate resistant acid phosphatase (TRAP) staining. All protocols recommended long-term follow-up every 6 months.
Statistics
Baseline characteristics are shown as median with ranges and absolute numbers with percentages as appropriate. Overall survival (OS) is defined from the start of treatment until death, whereas follow-up time is defined from registration until death or last date known alive. In case that the start of OS is different, this will be specified. OS and follow-up time were assessed by Kaplan-Meier and reverse Kaplan-Meier method, respectively. Prognostic factors were identified by univariable and multivariable Cox regression analysis and are displayed as hazard ratios (HRs), Wald 95% confidence interval (CI), and P values. To identify the optimal cutoff of numerical prognostic factors, P values were adjusted according to Lausen et al.19 Progression of disease at 24 months (POD24) and 36 months (POD36) was defined as experiencing HCL progression within 24 or 36 months from treatment start, respectively. Survival analysis according to POD24 and POD36 was calculated for patients with events (progression or death) within 24 or 36 months or for those with at least 24 or 36 months of follow-up in the case where no POD24- or POD36-defining event was reported. OS was compared with age-adjusted survival of the normal Swiss population. Absolute survival of the study cohort was compared with expected survival of age-, sex-, and period-matched groups of the general population using the life-table approach. Expected survival was estimated using the Ederer II method.20 The relative risk of second primary malignancies (except nonmelanotic skin cancer, which was not reported to the registry) was quantified by comparing the observed number of cancers in the study cohort with the expected number of cancers, based on age-, sex-, and period-specific rates of the Swiss population provided by the National Institute for Cancer Epidemiology and Registration (indirect standardization). The time to next treatment was calculated from the beginning of the first-line treatment to the beginning of subsequent treatment. R 3.5.3 (R Core Team; www.r-project.org) and SAS 9.4 (SAS Institute; www.sas.com) were used to perform the analyses.
Results
Baseline characteristics
Baseline characteristics are shown in Table 1. The median age of patients was 54 years (range, 21-96 years) at the time of diagnosis and 55 years (range, 21-96 years) at study entry. The median time from diagnosis to study entry was short (0.09 years; range, 0-19.0 years). At the time of data analysis, the median follow-up time from study registration was 12.6 years (95% CI, 10.1-14.6) in surviving patients. Most patients were male (170 of 221, 76.9%), were previously untreated (162 of 221, 73.3%), and had a good Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1: 206 of 221, 93.2%). Two thirds of the patients had splenomegaly at study entry (147, 66.5%), whereas only a minority had hepatomegaly (28, 12.7%) or lymphadenopathy (31, 14.0%). Median peripheral blood values showed modest anemia (median hemoglobin, 11.4 g/dL), thrombocytopenia (median, 78 × 109/L), leukopenia (median, 2.7 × 109/L), and monocytopenia (median, 0.1 × 109/L). Treatment of HCL before study inclusion consisted of interferon (51, 23.1%) or splenectomy (22, 10%). All patients were purine analog naïve at inclusion. Twenty-one patients had received more than 1 previous therapy before study entry, explaining why the number of treatments exceeds the number of pretreated patients. At the time of study entry, all patients had treatment indications caused by peripheral cytopenias and/or organomegaly. Treatment of 147 patients in the 32/93 and 32/98 studies was equal except of a weekly administration of the same dose of cladribine in 50 patients in the 32/98 study. The major difference was the schedule of the 32/95 study. However, 58 of those patients were in non-CR/PR at week 10 and had to be treated with a standard cycle of 0.14 mg/kg (subcutaneous) on 5 consecutive days per protocol.16 Therefore, a total of 205 patients or 93% were treated in quite homogenous way.
Baseline characteristics
| . | n* . | Percentage . |
|---|---|---|
| Patients | 221 | |
| Age, y† | ||
| At diagnosis | 54.2 | (20.7, 96.1) |
| At start of treatment | 55.5 | (20.7, 96.3) |
| Sex | ||
| Male | 170 | (76.9) |
| Female | 51 | (23.1) |
| ECOG at study entry | ||
| 0 | 148 | (67.0) |
| 1 | 58 | (26.2) |
| 2 | 13 | (5.9) |
| Missing | 2 | (0.9) |
| Stage at study entry | ||
| Untreated | 162 | (73.3) |
| Relapsed | 39 | (17.8) |
| Progressive disease | 20 | (9.0) |
| Previous therapies | ||
| Chemotherapy | 9 | (4.1) |
| Interferon | 51 | (23.1) |
| Radiotherapy | 1 | (0.5) |
| Splenectomy | 22 | (10.0) |
| Others | 5 | (2.3) |
| Organ involvement | ||
| Hepatomegaly | 28 | (12.7) |
| Lymphadenopathy | 31 | (14.0) |
| Splenomegaly | 147 | (66.5) |
| Blood values† | ||
| Hemoglobin, g/dL | 11.4 | (4.5, 16.3) |
| Platelets, ×109/L | 78 | (4.3, 318.0) |
| Leukocytes, ×109/L | 2.7 | (0.2-41.1) |
| Monocytes, ×109/L | 0.1 | (0.0, 12.4) |
| LDH, U/L | 323.5 | (81.0, 792.0) |
| . | n* . | Percentage . |
|---|---|---|
| Patients | 221 | |
| Age, y† | ||
| At diagnosis | 54.2 | (20.7, 96.1) |
| At start of treatment | 55.5 | (20.7, 96.3) |
| Sex | ||
| Male | 170 | (76.9) |
| Female | 51 | (23.1) |
| ECOG at study entry | ||
| 0 | 148 | (67.0) |
| 1 | 58 | (26.2) |
| 2 | 13 | (5.9) |
| Missing | 2 | (0.9) |
| Stage at study entry | ||
| Untreated | 162 | (73.3) |
| Relapsed | 39 | (17.8) |
| Progressive disease | 20 | (9.0) |
| Previous therapies | ||
| Chemotherapy | 9 | (4.1) |
| Interferon | 51 | (23.1) |
| Radiotherapy | 1 | (0.5) |
| Splenectomy | 22 | (10.0) |
| Others | 5 | (2.3) |
| Organ involvement | ||
| Hepatomegaly | 28 | (12.7) |
| Lymphadenopathy | 31 | (14.0) |
| Splenomegaly | 147 | (66.5) |
| Blood values† | ||
| Hemoglobin, g/dL | 11.4 | (4.5, 16.3) |
| Platelets, ×109/L | 78 | (4.3, 318.0) |
| Leukocytes, ×109/L | 2.7 | (0.2-41.1) |
| Monocytes, ×109/L | 0.1 | (0.0, 12.4) |
| LDH, U/L | 323.5 | (81.0, 792.0) |
LDH, lactate dehydrogenase.
Number (%), unless otherwise indicated.
Median (min, max).
Overall survival
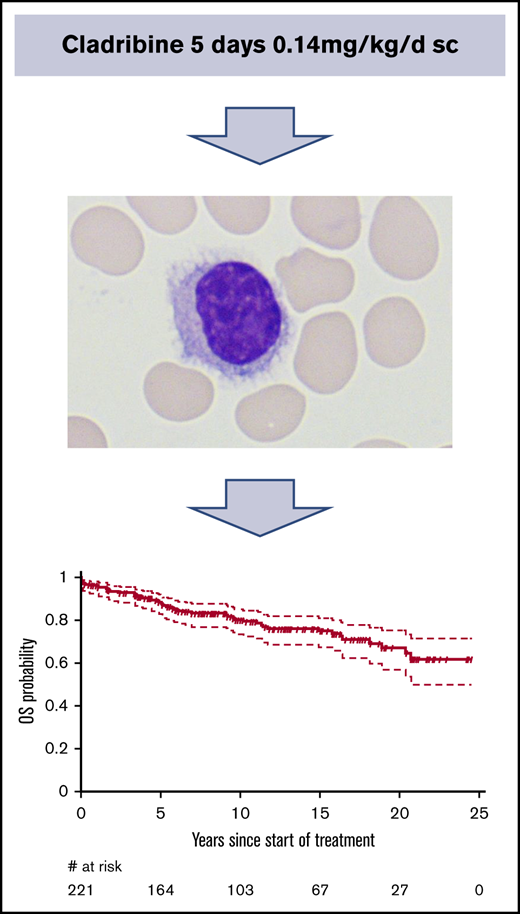
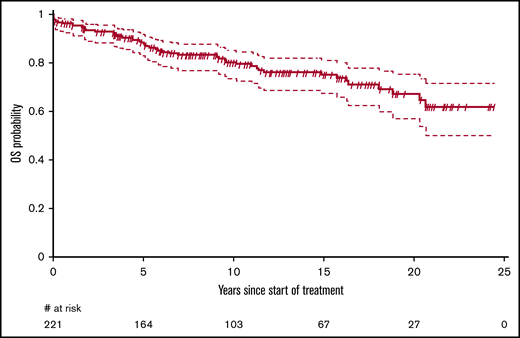
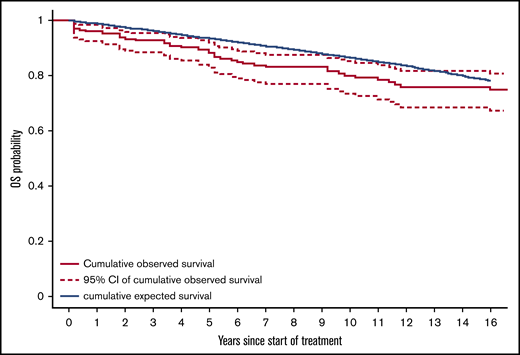
Fifty-one patients have died. The median OS was not reached, but the median OS from diagnosis was 31.6 (95% CI, 31.6-37.8) years. The OS probability at 10 and 20 years was 80.0% (95% CI, 74.3%-86.0%) and 66.9% (95% CI, 58.4%-76.6%), respectively (Figure 1). The principal causes of death were second tumors (14, 27.5%), cardiovascular causes (10, 19.6%), HCL-related causes (7, 13.7%), infections (6, 11.8%), and various other causes (4, 7.8%). In 10 patients, the cause of death was not known. Relative survival of the HCL patients was compared with the age- and sex-adjusted Swiss population (Figure 2). With an observation time of 15 years, patients with HCL showed a slightly inferior OS compared with the age-matched population (P = .036).
OS of patients with HCL treated with subcutaneous cladribine. The median OS was not reached. The survival probability at 10 and 20 years was 80.0% (95% CI, 74.3%-86.0%) and 66.9% (95% CI, 58.4%-76.6%), respectively.
OS of patients with HCL treated with subcutaneous cladribine. The median OS was not reached. The survival probability at 10 and 20 years was 80.0% (95% CI, 74.3%-86.0%) and 66.9% (95% CI, 58.4%-76.6%), respectively.
Relative survival of patients with HCL compared with a matched Swiss population. Absolute survival was calculated from the start of treatment and was compared with expected survival of an age-, sex- and period-matched Swiss general population using the life-table approach. The blue line represents the expected cumulative survival of the matched normal population, the black line represents the observed cumulative survival in patients with HCL, and the hashed lines represent the 95% CI of the cumulative observed survival. Patient with HCL showed a slight statistically significant inferior OS compared with the normal population (P = .036).
Relative survival of patients with HCL compared with a matched Swiss population. Absolute survival was calculated from the start of treatment and was compared with expected survival of an age-, sex- and period-matched Swiss general population using the life-table approach. The blue line represents the expected cumulative survival of the matched normal population, the black line represents the observed cumulative survival in patients with HCL, and the hashed lines represent the 95% CI of the cumulative observed survival. Patient with HCL showed a slight statistically significant inferior OS compared with the normal population (P = .036).
Initial response, prognostic factors, and retreatment
The response to the purine analog treatment was assessed in 218 patients after the first treatment at day 71. The overall response rate was 88.0%, with 48.6% complete and 39.4% partial remissions. Early mortality within 6 months after treatment with subcutaneous cladribine was observed in 8 of 221 patients (3.6%) and was directly linked to the treatment in only 3 cases, whereas 3 deaths were related to cancer and the remaining 2 to cardiovascular causes. None of the patients with treatment-related death were splenectomized.
In a univariable Cox regression analysis regarding OS, ECOG performance status (1 vs 0: HR, 2.79; 95% CI, 1.56-5.01; P < .001; 2 vs 0: HR, 3.53; 95% CI, 1.43-8.67; P < .001), age at treatment start (HR, 1.12; 95% CI, 1.09-1.15; P < .001), and lower hemoglobin level as a continuous variable (HR, 0.87; 95% CI, 0.78-0.97; P = .013) were associated with impaired OS (Table 2). The optimal hemoglobin cutoff level was established as 10.2 g/dL (HR, 0.4; 95% CI, 0.2-0.7; Pcorrected, .031), whereas the optimal cutoff for age at start of treatment was 60.3 (HR, 11.8; 95% CI, 5.5-25.1; Pcorrected, <.001). In the multivariable analysis, ECOG performance status, hemoglobin and age remained significant.
Prognostic factors for overall survival
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Log2 (platelets) | 0.91 | 0.89-1.20 | .506 | 0.88 | 0.63-1.23 | .462 |
| Hemoglobin | 0.87 | 0.78-0.97 | .013 | 0.86 | 0.75-1.00 | .048 |
| Log2 (leukocytes) | 1.18 | 0.91-1.53 | .202 | 1.07 | 0.86-1.32 | .564 |
| Log2 (LDH) | 1.39 | 0.83-2.32 | .207 | 1.03 | 0.62-1.69 | .915 |
| Age at treatment start | 1.12 | 1.09-1.15 | <.001 | 1.12 | 1.09-1.15 | <.001 |
| ECOG | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 2.79 | 1.56-5.01 | <.001 | 2.11 | 1.12-4.00 | .022 |
| 2 | 3.53 | 1.43-8.67 | .006 | 1.29 | 0.46-3.60 | .625 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Log2 (platelets) | 0.91 | 0.89-1.20 | .506 | 0.88 | 0.63-1.23 | .462 |
| Hemoglobin | 0.87 | 0.78-0.97 | .013 | 0.86 | 0.75-1.00 | .048 |
| Log2 (leukocytes) | 1.18 | 0.91-1.53 | .202 | 1.07 | 0.86-1.32 | .564 |
| Log2 (LDH) | 1.39 | 0.83-2.32 | .207 | 1.03 | 0.62-1.69 | .915 |
| Age at treatment start | 1.12 | 1.09-1.15 | <.001 | 1.12 | 1.09-1.15 | <.001 |
| ECOG | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 2.79 | 1.56-5.01 | <.001 | 2.11 | 1.12-4.00 | .022 |
| 2 | 3.53 | 1.43-8.67 | .006 | 1.29 | 0.46-3.60 | .625 |
LDH, lactate dehydrogenase.
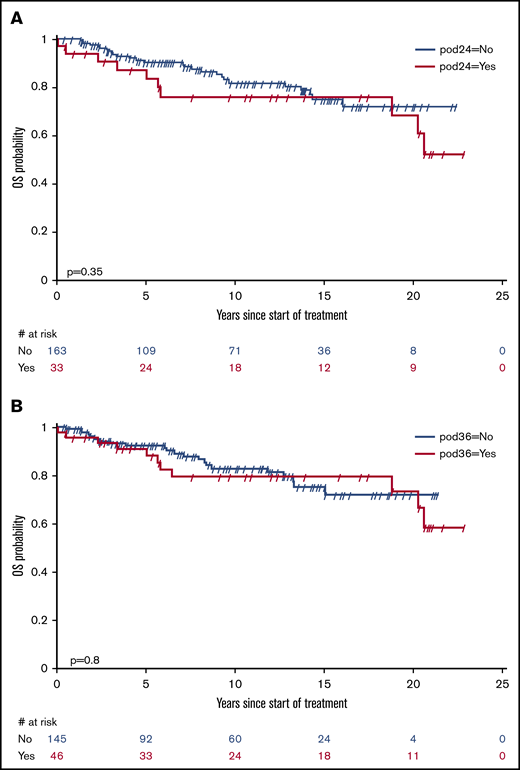
Remission status (PR vs CR) within 71 days after treatment was not significantly predictive for OS (HR, 1.46; 95% CI, 0.73-2.94; P = .284). There was a trend to earlier retreatment in patients in PR. Early progression of disease after 24 months (POD24) occurred in 33 patients and after 36 months (POD36) in 46 patients. As displayed in Figure 3, neither POD24 (HR, 1.43; 95% CI, 0.68-3.02; P = .351) nor POD36 (HR, 1.10; 95% CI, 0.52-2.31; P = .803) was discriminative for OS.
POD24 and POD36 after treatment of HCL with subcutaneous cladribine. (A) POD24. (B) POD36. Early progression of disease after 24 months (POD24) occurred in 33 patients and after 36 months (POD36) in 46 patients. Neither POD24 (HR, 1.43; 95% CI, 0.68-3.0221; P = .351) nor POD36 (HR, 1.10; 95% CI, 0.52-2.31; P = .803) was discriminative for OS.
POD24 and POD36 after treatment of HCL with subcutaneous cladribine. (A) POD24. (B) POD36. Early progression of disease after 24 months (POD24) occurred in 33 patients and after 36 months (POD36) in 46 patients. Neither POD24 (HR, 1.43; 95% CI, 0.68-3.0221; P = .351) nor POD36 (HR, 1.10; 95% CI, 0.52-2.31; P = .803) was discriminative for OS.
Type and criteria for late retreatment were up to the discretion of the treating physician. Retreatment was initiated in 53 (24.0%) patients after a median of 5 (range, 0.2-20.4) years. First retreatment was mainly cladribine (35, 66.0%), rituximab (8, 15.1%), or cladribine and rituximab (8, 15.1%). Twenty-two patients (9.8%) required more than 1 retreatment. Patients achieving a PR after initial treatment were more likely to receive a rituximab-containing regimen compared with patients relapsed after CR (14% vs 8%).
Second primary malignancies
A total of 44 (19.9%) patients developed second primary malignancies from the start of treatment with a mean time to occurrence of 5.7 (range, 0.01-17.5) years. Mortality from second cancers was 14 of 44 (31.8%) with a broad variety of tumor types. There was no significant difference of the incidence of second primary malignancies compared with a matched normal population (standardized incidence ratio, 1.24; 95% CI, 0.89-1.74; P = .206). Most of the second primary malignancies were of nonhematologic origin (36, 85.7%), and most were skin cancer (13, 30.9%), followed by prostate cancer (8, 19.0%) and colorectal cancer (19.0%; Table 3). Eight patients (19%) developed hematologic second primary malignancies with a predominance of B-lymphoid neoplasms. Only 1 case of acute myeloid leukemia was observed.
Secondary primary malignancies
| . | n* . | Percentage . |
|---|---|---|
| Nonhematologic malignancies | 36 | (81.8) |
| Skin tumors | ||
| Melanoma | 2 | (4.5) |
| Basal cell carcinoma | 4 | (9.1) |
| Squamous cell carcinoma | 5 | (11.4) |
| Merkel cell carcinoma | 1 | (2.3) |
| Nonskin tumors | ||
| Gastrointestinal cancer | 8 | (18.2) |
| Pancreas cancer | 1 | (2.3) |
| Prostate cancer | 8 | (18.2) |
| Breast cancer | 1 | (2.3) |
| Lung cancer | 1 | (2.3) |
| Glioblastoma | 1 | (2.3) |
| Renal cell cancer | 2 | (4.5) |
| Adenocarcinoma with unknown primary | 1 | (2.3) |
| Soft tissue sarcoma | 1 | (2.3) |
| Hematologic malignancies | 8 | (18.2) |
| Acute myeloid leukemia | 1 | (2.3) |
| Diffuse large B-cell lymphoma | 1 | (2.3) |
| Follicular lymphoma | 2 | (4.5) |
| Marginal zone lymphoma | 1 | (2.3) |
| Lymphoplasmocytic lymphoma | 1 | (2.3) |
| T-cell lymphoma | 1 | (2.3) |
| Multiple myeloma | 1 | (2.3) |
| . | n* . | Percentage . |
|---|---|---|
| Nonhematologic malignancies | 36 | (81.8) |
| Skin tumors | ||
| Melanoma | 2 | (4.5) |
| Basal cell carcinoma | 4 | (9.1) |
| Squamous cell carcinoma | 5 | (11.4) |
| Merkel cell carcinoma | 1 | (2.3) |
| Nonskin tumors | ||
| Gastrointestinal cancer | 8 | (18.2) |
| Pancreas cancer | 1 | (2.3) |
| Prostate cancer | 8 | (18.2) |
| Breast cancer | 1 | (2.3) |
| Lung cancer | 1 | (2.3) |
| Glioblastoma | 1 | (2.3) |
| Renal cell cancer | 2 | (4.5) |
| Adenocarcinoma with unknown primary | 1 | (2.3) |
| Soft tissue sarcoma | 1 | (2.3) |
| Hematologic malignancies | 8 | (18.2) |
| Acute myeloid leukemia | 1 | (2.3) |
| Diffuse large B-cell lymphoma | 1 | (2.3) |
| Follicular lymphoma | 2 | (4.5) |
| Marginal zone lymphoma | 1 | (2.3) |
| Lymphoplasmocytic lymphoma | 1 | (2.3) |
| T-cell lymphoma | 1 | (2.3) |
| Multiple myeloma | 1 | (2.3) |
Number (%).
Discussion
Our study shows excellent long-term outcome of patients with HCL treated with subcutaneous cladribine. Median OS from diagnosis was more than 31 years. Compared with a matched healthy Swiss population, survival was only slightly inferior. For most patients, 1 cycle of subcutaneous cladribine was sufficient for long-term disease control. Interestingly, early disease progression was not associated with an impaired OS. Hemoglobin levels, age, and performance status were associated with poorer survival and may help to predict outcome in patients receiving subcutaneous cladribine treatment. Second primary malignancies occurred in 19.9% of the patients; however, they were not more frequent compared with the age-matched normal population. Nevertheless, physicians should pay attention to second primary malignancies and patients should undergo lifelong follow-up.
Several published studies have shown excellent long-term survival after initial treatment with different types and regimens of purine analogs. A large long-term follow-up analysis of 233 patients mostly treated with pentostatin showed a 15-year survival of 78%.21 In a single-center study, 44 patients received intravenous cladribine as continuous infusion over 7 days with a 12-year OS of 79%.11 Chadha and colleagues published their experience in 86 HCL patients treated with intravenous cladribine, showing a median 12-year OS of 86%.22 Eighty-eight younger patients (<40 years of age) with a follow-up of >19 years treated with intravenous cladribine had a median OS of 251 months.12 The only publication looking at subcutaneous administration of cladribine was based on Swedish registry data and found a 15-year OS of >80% in 85 younger patients.14 However, most publications are based on retrospective single-center analyses using a variety of different drugs and regimens.23 Thus, our study is the largest analysis of HCL patients treated with subcutaneous cladribine based on 3 multicenter prospective studies using a uniform treatment approach for all patients. The 10-year OS of 80% is comparable with other studies using intravenous treatment, although our population is slightly older than other study populations.24
We compared OS to an age- and sex-matched Swiss population. Survival was slightly inferior to the normal population. However, even with longer follow-up, the curves do not diverge. This suggests that HCL-related mortality and the mortality from second primary malignancies in patients with HCL treated with subcutaneous cladribine do not impact survival more than in a normal population. Moreover, HCL-related mortality will likely be further reduced because of availability of treatment options, such as regular use of rituximab, BRAF inhibitors, or CD22 antagonists.25
The complete remission rate in our cohort (CR, 48.6%; PR, 39.4%) was lower compared with other studies reporting a range between 70% and 100%. This might be explained by the early time point of the bone marrow assessment in the 3 trials (mainly day 71). In the literature, CR rates increase with time from 80%22 if assessed after 3 to 4 months to almost 100%11,26 after 6 months. Indeed, response improvement can be observed up to 9 months.27 Unfortunately, in many publications, the time point and method of response assessment are not clearly stated, making comparison of CR rates among different studies difficult; therefore, differences should be interpreted with caution. Current guidelines recommend response assessment not earlier than 4 to 6 months after treatment with purine analogs.8 Second, although many studies determined CR based on conventional hematoxylin and eosin staining, in the 3 SAKK studies, remission assessment was based on staining for TRAP. Histochemical positivity of remaining hairy cells facilitates their detection and increases the sensitivity of the method. Hence, some complete remissions by HE staining might have been assessed as partial remissions using the TRAP staining potentially lowering the rate of CR in our study.11,28 Third, comparison of the CR rates between studies using cladribine or pentostatin might be misleading because pentostatin is generally given until best response, whereas cladribine is given as a fixed dose potentially resulting in lower CR rates when assessed early.29 Last, rituximab was used more frequently in PR than in CR patients. Rituximab is known to improve response rate30 and might have counteracted some of the inferior prognosis of patients in PR. It is therefore recommended to take the addition of rituximab into consideration in patient needing retreatment to delay further relapses.31 Reassuringly, long-term OS of our patient population was excellent and comparable to studies using intravenous cladribine, making it unlikely that the lower CR rates reflect real differences in the response rates.
Because patients relapsing may remain stable over a long period of time, we analyzed time to retreatment as a clinically relevant parameter. In total, 24% of the study population required retreatment after a median of 5 years, which was in line with earlier studies. We analyzed factors other than treatment that might influence outcome in our population. Earlier studies established a correlation of overall and disease-free survival with anemia and thrombocytopenia,7 and newer data found a correlation with IGHV4-34 molecular variants32 or telomer length.33 In our study, only conventional pretreatment variables such as clinical data and standard blood values were available. There was a correlation between anemia, age, ECOG status, and survival in line with earlier findings correlating anemia and thrombocytopenia with lower disease-free survival.7 Anemia does not seem to impact on early mortality but rather on long-term survival.
Early progression of disease was recently introduced in several low-grade lymphomas as a dynamic marker for the aggressiveness of the disease. POD24 was able to delimit a group with poorer OS in follicular lymphoma and marginal zone lymphoma.34-36 In contrast, in our cohort of patients with classical HCL, neither POD24 nor POD36 was associated with poorer OS. We speculate that this difference might be explained by the different biology of these low-grade lymphomas. Although in follicular and marginal zone lymphoma, early progression most likely selects patients with a disease in transformation, this does not occur in patients with HCL.
Although our study focused on long-term efficacy and toxicity, early side effects are still a matter of concern, and patients with active infections should not be treated with cladribine.8 However, the way of administration does not seem to have a major impact on early toxicity despite missing data based on randomized studies.9,11,15,37,38 In a pooled analysis of all 3 studies, we found an overall infection rate according to World Health Organization grading of 18.5% grade 1, 9.3% grade 2, 6% grade 3, and 5.6% grade 4 (supplemental Data). In addition, weekly vs daily schedules were of no benefit when compared in a randomized fashion.17,39 In summary, no treatment schedule shows clear superiority or inferiority in early toxicity. Evaluation of long-term toxicitiy is therefore of further importance to have the complete basis to decide on the most effective, safe, and convenient initial treatment schedule.
The overall incidence of second primary tumors in patients with HCL who had received subcutaneous cladribine was 19.9% without a clear predominance of a specific tumor entity and without increase of therapy-related myeloid malignancies. This is in line with epidemiologic data that found a 24% incidence of second cancers in patients with HCL.40 Likewise, we did not observe a statistically significant difference of these second primary malignancies (nonmelanotic skin cancers are excluded) compared with the incidence of primary ones in a normal matched Swiss population. In the literature, the association between HCL treatment and second primary malignancies remains controversial. Although several studies showed an increased rate of second primary malignancies,28,40-42 others could not find a difference.7,43 Of note, the largest registry study provides indirect evidence of higher rates of second primary malignancies since the introduction of purine analogs.40 Some studies suggest that a higher cumulative dose of the purine analog is associated with a higher risk of developing second primary malignancies. In the current analysis, retreatment was infrequent; therefore, a correlation of the risk for secondary primary malignancies with the total amount of cladribine could not be determined.
Our study has several limitations. Because of the long-term follow-up and multicenter design, a potential underreporting of second primary malignancies and retreatment cannot be excluded. However, both are in line with other studies, making a significant effect rather unlikely. Second, the different treatment schedules in the 3 clinical studies and the selection of the patients could have had an impact on the outcome of the patients despite 93% having a similar treatment schedule. Third, in some of the analyses, the relatively low number of events might preclude statistically significant differences.
In summary, subcutaneous cladribine at a dose of 0.14 mg/kg per day on 5 consecutive days is a convenient and effective treatment in HCL, with excellent long-term survival similar to the normal population. Even under long-term observation, no differences were observed compared with the intravenous application with regard to early progression, OS, and secondary malignancies, ensuring the possibility of starting treatment in an outpatient facility.
Data may be requested from the corresponding author at rudolf.benz@stgag.ch.
Acknowledgments
This study was supported by a grant from the Cancer League of the Canton Thurgau and research agreements with the following institutions: State Secretary for Education, Research and Innovation, the Swiss Cancer Research Foundation, and the Swiss Cancer League.
Authorship
Contribution: R.B. and G.S. designed the study; R.B., G.S., and D.R. interpreted data and wrote the manuscript; K.A., M.A., T.P., M.B., U.N., F.H. U.H., R.Z., Y.C., U.M., S. Blum, D.R., N.C., M.B., E.B-P., A.O.S., J.P., and A.L. contributed clinical patient data; S. Berardi, Q.L., and A.F. collected data and performed the statistical analysis; and all authors revised and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rudolf Benz, Division of Hematology and Oncology, Kantonsspital Muensterlingen, CH 8596 Muensterlingen, Switzerland; e-mail: rudolf.benz@stgag.ch.
References
Author notes
The full-text version of this article contains a data supplement.