Key Points
Iron deficiency anemia requiring intravenous iron is a delayed consequence of bariatric surgery.
Young age, anemia, and low ferritin before surgery are associated with an increased risk of iron deficiency anemia during follow-up.
Abstract
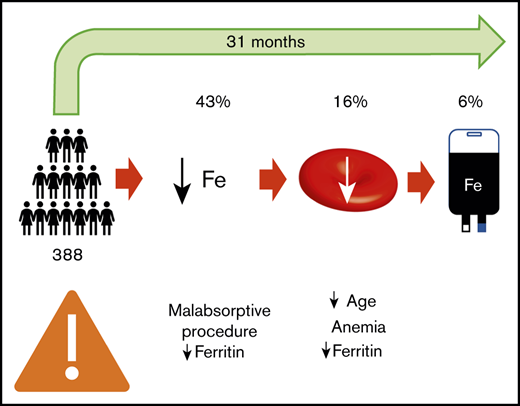
Iron deficiency is a common consequence of bariatric surgery and frequently leads to anemia. Our study reports the incidence and predictors of iron deficiency, iron deficiency anemia (IDA), and IV iron use after bariatric surgery. We conducted a retrospective study of all adult patients who underwent bariatric surgery from January to December 2012 at the regional bariatric surgery center in Hamilton, Ontario, Canada, and were followed for at least 6 months. Time-to-event data were presented as Kaplan-Meier curves. Cox regression analysis was used to identify outcome predictors. A total of 388 patients met the inclusion criteria. Iron deficiency, IDA, and the use of IV iron were reported in 43%, 16%, and 6% of patients, respectively, with a mean follow-up of 31 months. The cumulative incidence of iron deficiency and IDA increased with longer follow-up, and there was a significant increase in IV iron use starting 3 years after surgery. Malabsorptive procedures (hazard ratio [HR], 1.92; 95% confidence interval [CI], 1.20-3.06; P = .006) and low baseline ferritin (HR, 0.96; 95% CI, 0.95-0.97; P < .001) were associated with an increased risk of iron deficiency. Young age (HR, 0.90; 95% CI, 0.82-0.99; P = .028), baseline anemia (HR, 19.6; 95% CI, 7.85-48.9; P < .001), and low baseline ferritin (HR, 0.96; 95% CI, 0.95-0.98; P < .001) were associated with an increased risk of IDA. Our results suggest that IDA is a delayed consequence of bariatric surgery and that preoperative assessment of patient risk may be possible.
Introduction
Obesity is associated with increased incidence of type 2 diabetes mellitus, dyslipidemia, and hypertension and is associated with an increased risk of atherosclerotic disease and mortality.1 Bariatric surgery refers to procedures that achieve weight loss by restricting the capacity of the stomach or causing malabsorption of nutrients, in addition to other identified mechanisms such as effects on incretin response, insulin sensitivity, bile acid metabolism, and gut microbiota.2 In a meta-analysis3 of randomized controlled trials comparing bariatric surgery to medical weight loss strategies, bariatric surgery was associated with improvements in weight, diabetes control, metabolic syndrome, and patient-reported general health. However, bariatric surgery can result in iron deficiency secondary to anatomical exclusion of the duodenum from the digestive tract, decreased gastric acid secretion, decreased tolerance of red meat,4 and potentially increased menstrual regularity,5 among other mechanisms.
Existing literature regarding the burden of iron deficiency after bariatric surgery is limited, because it is prone to restrictive enrollment, inconsistent definitions of iron deficiency, and variability in iron supplementation strategies. Iron deficiency can occur later after surgery, as demonstrated in 2 retrospective studies with a median interval of 42.5 months6 and a mean interval of 51 months7 between bariatric surgery and the administration of IV iron, yet many studies fail to use follow-up periods of sufficient duration.
The primary objective of this study is to report the incidence of iron deficiency and iron deficiency anemia (IDA) and to describe the use of oral and IV iron supplementation in a consecutive population of adult patients who underwent bariatric surgery at our regional bariatric surgery center. We also evaluate the clinical risk factors that increase a patient’s risk of iron deficiency, IDA, and the need for IV iron following bariatric surgery.
Methods
Study design and patient population
We conducted a retrospective study of all consecutive adult patients who underwent any type of bariatric surgical procedure (including sleeve gastrectomy, Roux-en-Y gastric bypass, and duodenal switch) at St. Joseph’s Healthcare Hamilton, a regional bariatric surgery center in Hamilton, Ontario, Canada, from 1 January 2012 to 31 December 2012. All eligible patients were identified by screening operating room listings. We excluded patients who previously underwent bariatric surgery and patients with less than 6 months of postoperative follow-up at our center (because external medical records were not available to the investigators). The study was approved by the Hamilton Integrated Research Ethics Board at St. Joseph’s Healthcare Hamilton prior to data collection.
Data collection
Data were obtained from paper charts and the electronic medical record. Height, weight, comorbidities, and baseline laboratory parameters were collected at the time of the complete preoperative bariatric assessment. The recorded age reflected the age of the patient at the time of the surgery. In accordance with Ontario Bariatric Network Perioperative Task Force Recommendations,8 data were recorded at each routine follow-up appointment, which were scheduled at 1 week, 3 months, 6 months, and 12 months postoperatively and every 12 months thereafter until discharge from the bariatric clinic. Patients were routinely seen by a dietitian, who reviewed the patients’ complete blood count, iron stores, and iron supplementation strategies. At each follow-up interval, the patient’s weight, hemoglobin, mean corpuscular volume, and ferritin were collected, in addition to the oral iron supplementation type, dose, and frequency. We identified all instances where patients received IV iron supplementation, recording the date, type, and dose of IV iron. We recorded all measurements of hemoglobin, mean corpuscular volume, and ferritin after the administration of IV iron, which were regularly monitored by the prescriber of IV iron.
Anemia was defined based on the World Health Organization criteria for anemia (<130 g/L in men and <120 g/L in non-pregnant women).9 Iron deficiency was defined as a ferritin level less than 30 ng/mL. Ferritin has been identified as the best available test for iron deficiency because of its superior area under the receiver operating curve compared with other tests.10 Ferritin is an acute phase reactant that can be normal despite low iron stores in inflammatory states, and there is no generally accepted ferritin cutoff to diagnose iron deficiency. A cutoff of 30 ng/mL was shown to have good sensitivity and specificity in a population of anemic patients who underwent bone marrow biopsy.11 Our data were also reanalyzed using a ferritin cutoff of 40 ng/mL based on the results from a systematic review10 that demonstrated that the positive likelihood ratio for iron deficiency does not start to decrease until 40 ng/mL in an unselected population of patients. Because the population of our study had a low incidence of inflammatory conditions, a ferritin cutoff higher than 40 ng/mL was not appropriate. Perioperative guidelines such as those by the British Committee for Standards in Hematology12 also consider patients to be iron deficient if the transferrin saturation is less than 20% and ferritin level is less than 100 ng/mL, but given that the serum iron and total iron binding capacity was not measured in all patients, we chose to define iron deficiency based on the ferritin level only. IDA was defined as meeting the criteria for both iron deficiency and anemia.
Comorbidity was assessed using the Charlson Comorbidity Index as modified by Quan et al.13 The Chronic Kidney Disease Epidemiology Collaboration14 formula was used to estimate the glomerular filtration rate in each patient because it has been shown to more accurately classify future risk of end-stage renal disease and mortality compared with Modification of Diet in Renal Disease.15 A premenopausal woman was defined as a female younger than age 50 years as of the date of bariatric surgery because a documented menstrual history was not available to the data collectors.
Iron supplementation
The protocol at our bariatric center was to correct iron deficiency before surgery and initiate an iron-containing multivitamin postoperatively, in addition to oral iron supplementation for menstruating individuals. In the case of iron deficiency, patients were started on a ferrous salt, most frequently ferrous sulfate. There was significant variability because of iron preparation tolerance and patient preference. IV iron was administered either by a consulting hematologist or general internist or by a physician at the bariatric clinic in cases where no consultation was requested. The decision to administer IV iron was left to the discretion of the ordering physician. A course of IV iron was defined as consecutive treatments with no more than a 3-month interval between doses.
Statistical analysis
Descriptive statistics were used to report baseline clinical and laboratory characteristics, as well as data for each follow-up interval. We generated Kaplan-Meier curves to display the time-to-event data, where the reported events were as follows: (1) the development of iron deficiency, (2) the development of IDA, and (3) the administration of IV iron. We calculated hazard ratios (HRs) based on Cox regression analysis to identify risk factors for each of the 3 reported events. Age, sex, procedure type (restrictive vs malabsorptive), baseline anemia, baseline ferritin, and weight loss at the 6-month follow-up appointment (as a percentage of total body weight [TBW]) were included in both the univariate and multivariate analyses. The Cox regression model also included an interaction term for age and sex to explore the role of premenstrual bleeding on iron deficiency. For each model, we tested the proportional hazards assumption by checking the P value of each time–covariate interaction term using a time-dependent covariate analysis.
P < .05 was considered statistically significant. Statistical analyses were completed using SPSS Statistics version 25 (IBM Corp., Armonk, NY).
Results
Patient population
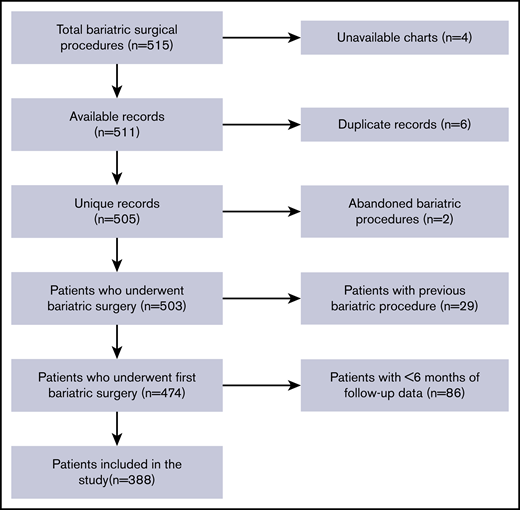
From 1 January 2012 to 31 December 2012, there were 515 total bariatric procedures at our center. We excluded 127 instances (4 unavailable charts, 6 duplicate records, 2 abandoned bariatric procedures, 29 patients with previous bariatric surgery, and 86 patients with <6 months of follow-up data). The final analysis included 388 patients. A flowchart of cohort construction is shown in Figure 1.
Flowchart of cohort integration. There was a total of 515 bariatric surgical procedures, of which 388 patients met the criteria for inclusion in the study.
Flowchart of cohort integration. There was a total of 515 bariatric surgical procedures, of which 388 patients met the criteria for inclusion in the study.
Frequency and time-to-event analysis of iron deficiency after bariatric surgery
Of the 388 patients meeting the study inclusion criteria, 284 (73%) patients underwent Roux-en-Y gastric bypass, 98 (35%) patients underwent sleeve gastrectomy, and 6 (1.5%) patients underwent a duodenal switch. The mean (standard deviation [SD]) age was 46 (10) years, and 17% were male. The mean (SD) body mass index was 50 (8) kg/m2. The median Charlson comorbidity index was 0, and the most common comorbidities were hypertension (53%), obstructive sleep apnea (46%), gastroesophageal reflux disease (42%), and diabetes mellitus (35%). The mean (SD) baseline hemoglobin was 133 (12) g/L, and median baseline ferritin level was 70 μg/L. The 5 quintiles for baseline ferritin level were <30 μg/L, 30 to 52 μg/L, 53 to 90 μg/L, 91 to 155 μg/L, and 156 μg/L or greater. Baseline anemia, iron deficiency, and IDA were identified in 47 (12%), 72 (19%), and 22 (6%) patients, respectively. Baseline clinical and laboratory data are shown in Tables 1 and 2.
Baseline clinical characteristics
| Variable . | Statistic (N = 388) . |
|---|---|
| Age at time of surgery, mean (SD), y | 46 (10) |
| Sex/menopausal status, n (%) | |
| Male | 65 (17) |
| Premenopausal female | 199 (51) |
| Postmenopausal female | 124 (32) |
| Height, mean (SD), cm | 165 (8) |
| Weight, mean (SD), kg | 137 (28) |
| Body mass index, mean (SD), kg/m2 | 50.2 (8.4) |
| Procedure type, n (%) | |
| Roux-en-Y gastric bypass | 284 (73.0) |
| Sleeve gastrectomy | 98 (25.3) |
| Duodenal switch | 6 (1.5) |
| Comorbidities, n (%) | |
| GERD | 161 (41.5) |
| Hypertension | 207 (53.4) |
| Obstructive sleep apnea | 177 (45.6) |
| Diabetes | 134 (34.5) |
| Chronic complications secondary to diabetes | 18 (4.6) |
| Renal disease | 4 (1.0) |
| Congestive heart failure | 6 (1.5) |
| Chronic pulmonary disease | 5 (1.3) |
| Rheumatologic disease | 15 (3.9) |
| Liver disease | 17 (4.4) |
| Active malignancy | 0 (0.0) |
| Charlson comorbidity index, mean (SD) | 0.2 (0.6) |
| Total months of follow-up, mean (SD) | 31 (21) |
| Variable . | Statistic (N = 388) . |
|---|---|
| Age at time of surgery, mean (SD), y | 46 (10) |
| Sex/menopausal status, n (%) | |
| Male | 65 (17) |
| Premenopausal female | 199 (51) |
| Postmenopausal female | 124 (32) |
| Height, mean (SD), cm | 165 (8) |
| Weight, mean (SD), kg | 137 (28) |
| Body mass index, mean (SD), kg/m2 | 50.2 (8.4) |
| Procedure type, n (%) | |
| Roux-en-Y gastric bypass | 284 (73.0) |
| Sleeve gastrectomy | 98 (25.3) |
| Duodenal switch | 6 (1.5) |
| Comorbidities, n (%) | |
| GERD | 161 (41.5) |
| Hypertension | 207 (53.4) |
| Obstructive sleep apnea | 177 (45.6) |
| Diabetes | 134 (34.5) |
| Chronic complications secondary to diabetes | 18 (4.6) |
| Renal disease | 4 (1.0) |
| Congestive heart failure | 6 (1.5) |
| Chronic pulmonary disease | 5 (1.3) |
| Rheumatologic disease | 15 (3.9) |
| Liver disease | 17 (4.4) |
| Active malignancy | 0 (0.0) |
| Charlson comorbidity index, mean (SD) | 0.2 (0.6) |
| Total months of follow-up, mean (SD) | 31 (21) |
GERD, gastroesophageal reflux disease.
Baseline laboratory data
| Variable . | Statistic (N = 388) . |
|---|---|
| Hemoglobin, mean (SD), g/L | 133 (12) |
| Platelets, mean (SD), ×109/L | 266 (65) |
| Leukocytes, mean (SD), ×109/L | 7.7 (1.9) |
| MCV, mean (SD), fL | 87.7 (5.2) |
| Ferritin, median, μg/L | 70 |
| <30, n (%) | 72 (19) |
| 30-52, n (%) | 78 (20) |
| 53-90, n (%) | 79 (21) |
| 91-155, n (%) | 76 (19) |
| 156+, n (%) | 77 (20) |
| Iron, mean (SD), μmol/L | 13 (5) |
| IBC, mean (SD), μmol/L | 62 (8) |
| Transferrin saturation, mean (SD), % | 21.9 (8.5) |
| Vitamin B12, mean (SD), pmol/L | 345 (199) |
| Creatinine, mean (SD), μmol/L | 67 (17) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 98 (18) |
| Hemoglobin A1c, mean (SD), % | 6.5 (1.5) |
| Iron deficiency status, n (%) | |
| Anemia | 47 (12.1) |
| Iron deficiency | 72 (18.6) |
| Iron deficiency anemia | 22 (5.7) |
| Variable . | Statistic (N = 388) . |
|---|---|
| Hemoglobin, mean (SD), g/L | 133 (12) |
| Platelets, mean (SD), ×109/L | 266 (65) |
| Leukocytes, mean (SD), ×109/L | 7.7 (1.9) |
| MCV, mean (SD), fL | 87.7 (5.2) |
| Ferritin, median, μg/L | 70 |
| <30, n (%) | 72 (19) |
| 30-52, n (%) | 78 (20) |
| 53-90, n (%) | 79 (21) |
| 91-155, n (%) | 76 (19) |
| 156+, n (%) | 77 (20) |
| Iron, mean (SD), μmol/L | 13 (5) |
| IBC, mean (SD), μmol/L | 62 (8) |
| Transferrin saturation, mean (SD), % | 21.9 (8.5) |
| Vitamin B12, mean (SD), pmol/L | 345 (199) |
| Creatinine, mean (SD), μmol/L | 67 (17) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 98 (18) |
| Hemoglobin A1c, mean (SD), % | 6.5 (1.5) |
| Iron deficiency status, n (%) | |
| Anemia | 47 (12.1) |
| Iron deficiency | 72 (18.6) |
| Iron deficiency anemia | 22 (5.7) |
eGFR, estimated glomerular filtration rate; IBC, iron-binding capacity; MCV, mean corpuscular volume.
The mean (SD) weight loss was 26% (7%) of TBW at 6-month follow-up, reaching a peak of 33% (11%) of TBW at 2 years. At 6-month follow-up, 41% of patients were receiving oral iron, of which ferrous sulfate was the most common, whereas 49% of patients were receiving an iron-containing prenatal vitamin only. Mean hemoglobin remained relatively stable throughout follow-up, but the median ferritin level decreased throughout follow-up from a peak of 78 μg/L at 3 months. Anemia, iron deficiency, and IDA were identified in 8%, 17%, and 3% of patients at 6-month follow-up, respectively, but all 3 frequencies increased with later follow-up intervals. Patient data for each of the follow-up appointments up to 6 years are presented in supplemental Table 1.
The 388 patients included in the study were followed for a mean (SD) of 31 (21) months. A total of 166 (43%) patients developed iron deficiency (as defined by a ferritin level <30 ng/mL), 63 (16%) patients developed iron deficiency anemia, and 24 (6%) patients received IV iron. Four additional patients received red blood cell transfusions, 3 of whom met criteria for iron deficiency anemia and 1 patient who was diagnosed with acute myeloid leukemia. Using an alternative ferritin level cutoff of 40 ng/mL, 214 (55%) and 77 (20%) patients developed iron deficiency and IDA, respectively.
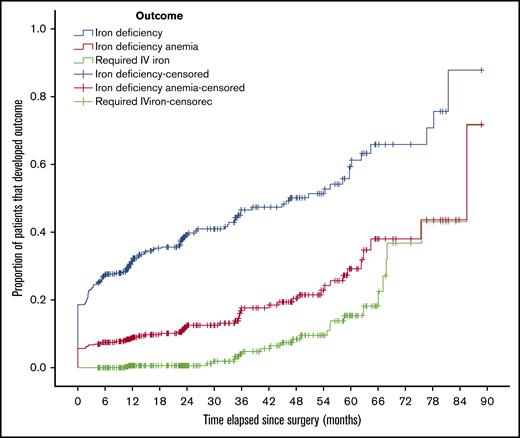
Kaplan-Meier curves showing the time-to-event analysis for the initial diagnosis of iron deficiency, IDA, and the administration of IV iron are shown in Figure 2. The Kaplan-Meier curve for the ferritin threshold of 40 ng/mL was similar and is shown in supplemental Figure 1.
Kaplan-Meier curve of outcomes. Proportion of patients that developed iron deficiency or IDA or required IV iron, plotted against time since bariatric surgery.
Kaplan-Meier curve of outcomes. Proportion of patients that developed iron deficiency or IDA or required IV iron, plotted against time since bariatric surgery.
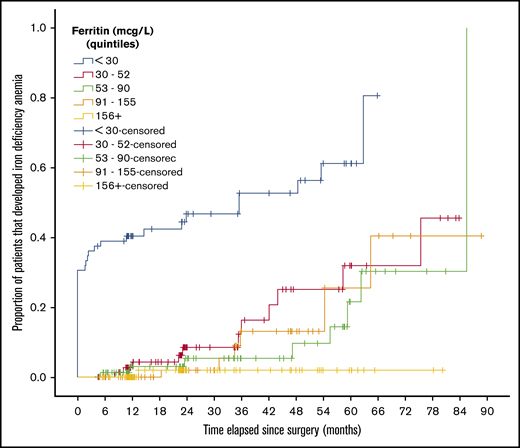
Kaplan-Meier curves stratified by quintile of baseline ferritin level are shown in Figure 3 and supplemental Figures 2 and 3. A difference between ferritin level quintiles was detected in time-to-event models for iron deficiency (P < .001) and IDA (P < .001), but there was no statistically significant difference in IV iron administration between ferritin level quintiles (P = .053), albeit there was a trend toward a higher risk with lower baseline ferritin levels.
Kaplan-Meier curve of the diagnosis of IDA, stratified by ferritin. Proportion of patients that developed IDA for each quintile of baseline ferritin level. The difference between groups was statistically significant (P < .001).
Kaplan-Meier curve of the diagnosis of IDA, stratified by ferritin. Proportion of patients that developed IDA for each quintile of baseline ferritin level. The difference between groups was statistically significant (P < .001).
Characteristics of patients who received IV iron
Of the 24 patients who received IV iron, only 1 (4%) patient was male. Nineteen (79%) and 4 (17%) patients were pre- and postmenopausal women, respectively. The mean (SD) hemoglobin before surgery, before IV iron, and after the first course of IV iron were 130 (10), 100 (21), and 120 (16) g/L, respectively. The median (interquartile range [IQR]) ferritin levels before surgery, before IV iron, and after the first course of IV iron were 45 (28-72), 8 (5-10), and 112 (50-172) μg/L, respectively. Before IV iron, 18 (75%) patients had IDA, and 4 (17%) patients had iron deficiency without anemia. Two of 24 (8%) patients were anemic but did not meet criteria for iron deficiency. This includes 1 male patient who was receiving darbepoetin alfa and IV iron sucrose with hemodialysis (with a pretreatment ferritin of 444 ng/mL), and 1 pregnant patient who met criteria for iron deficiency 2 weeks prior.
Although guidelines and local protocols suggest ferrous salts as the preferred treatment of IDA after bariatric surgery, the use of dedicated ferrous salt supplements before IV iron was documented in only 6 of 24 (25%) of patients. In many cases, other iron formulations were being used because of issues with tolerability or patient preference.
Only 2 (8%) patients were found to have gastrointestinal bleeding. One patient had rectal bleeding, with a tubular adenoma and hyperplastic polyp on colonoscopy. The other patient had an occult gastrointestinal bleed in the context of gastric antral vascular ectasia, which was treated with argon plasma coagulation.
The most common IV iron was iron sucrose. The mean (SD) dose was 214 (52) mg, with a median (IQR) of 6 (4-10) doses per course. Nine of 24 (38%) patients required multiple courses of IV iron, and there was a median (IQR) of 13 (6-27) months between the first and second courses of IV iron. Characteristics of the patients who received IV iron are shown in supplemental Table 2.
Risk factors for iron deficiency after bariatric surgery
In the multivariate Cox regression model, an increased risk of iron deficiency was associated with malabsorptive procedures (HR, 1.92; 95% confidence interval [CI], 1.20-3.06; P = .006) and a low baseline ferritin level (HR, 0.96; 95% CI, 0.95-0.98; P < .001). Young age, female sex, and baseline anemia were risk factors for iron deficiency in the univariate models, but this association was not seen in the multivariate analysis. A multivariate analysis that excluded the potential mediating factors of anemia and ferritin demonstrated that age (HR, 0.98; 95% CI, 0.97-1.00; P = .028), female sex (HR, 2.30; 95% CI, 1.27-4.17; P = .006), and malabsorptive procedure (HR, 2.36; 95% CI, 1.50-3.71; P < .001) were independently predictors of iron deficiency. The interaction between age and female sex (HR, 0.94; 95% CI, 0.87-1.00; P = .077) did not meet statistical significance but suggests that young age might interact with female sex to increase the risk of iron deficiency.
An increased risk of IDA was associated with young age (HR, 0.90; 95% CI, 0.82-0.99; P = .028), baseline anemia (HR, 19.6; 95% CI, 7.85-48.9; P < .001), and a low baseline ferritin level (HR, 0.96; 95% CI, 0.95-0.98; P < .001). A multivariate analysis that excluded the potential mediating factors of anemia and ferritin demonstrated no statistically significant predictors of IDA, but young age (HR, 0.98; 95% CI, 0.96-1.00; P = .088) approached statistical significance. The interaction between age and female sex (HR, 0.96; 95% CI, 0.86-1.06; P = .377) did not meet statistical significance.
Because there were only 24 patients who received IV iron, model overfit was avoided by performing Cox regression using only 2 covariates: baseline anemia and baseline ferritin level. Neither parameter was a statistically significant risk factor for needing IV iron, although low ferritin (HR, 0.99; 95% CI, 0.98-1.00; P = .080) approached significance. The univariate model did not yield any statistically significant risk factors.
The Cox regression models for iron deficiency, IDA, and the administration of IV iron are shown in Table 3.
Cox regression of iron deficiency, iron deficiency anemia, and administration of IV iron, using both univariate and multivariate analyses
| Risk factor . | Univariate analysis . | Multivariate analysis . | Multivariate excluding mediators* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Iron deficiency | |||||||||
| Age, y | 0.98 | 0.96-0.99 | .005† | 0.99 | 0.92-1.06 | .735 | 0.98‡ | 0.97-1.00 | .028† |
| Female sex | 2.85 | 1.58-5.13 | <.001† | 0.80§ | 0.01-44.3 | .914 | 2.30§ | 1.27-4.17 | .006† |
| Malabsorptive procedure | 2.46 | 1.59-3.80 | <.001† | 1.92 | 1.20-3.06 | .006† | 2.36 | 1.50-3.71 | <.001† |
| Baseline anemia | 2.94¶ | 1.88-4.60 | <.001† | 1.49¶ | 0.92-2.44 | .107 | — | — | — |
| Baseline ferritin, μg/L | 0.96¶ | 0.95-0.97 | <.001† | 0.96¶ | 0.95-0.97 | <.001† | — | — | — |
| Weight loss at 6 mo, %TBW | 1.01 | 0.99-1.03 | .480 | 0.98 | 0.96-1.00 | .066 | 0.99 | 0.97-1.01 | .492 |
| Iron deficiency anemia | |||||||||
| Age, y | 0.98 | 0.96-1.00 | .072 | 0.90 | 0.82-0.99 | .028† | 0.98 | 0.96-1.00 | .088 |
| Female sex | 1.64 | 0.70-3.81 | .252 | 0.02§ | 0.00-2.62 | .114 | 1.48§ | 0.62-3.52 | .376 |
| Malabsorptive procedure | 1.01 | 0.58-1.77 | .973 | 0.84 | 0.45-1.59 | .602 | 1.01 | 0.55-1.85 | .969 |
| Baseline anemia | 24.6¶ | 11.0-55.1 | <.001† | 19.6¶ | 7.85-48.9 | <.001† | — | — | — |
| Baseline ferritin, μg/L | 0.96¶ | 0.94-0.97 | <.001† | 0.96¶ | 0.95-0.98 | <.001† | — | — | — |
| Weight loss at 6 mo, %TBW | 0.99 | 0.95-1.02 | .399 | 0.98 | 0.94-1.02 | .335 | 0.98 | 0.95-1.02 | .278 |
| Administration of IV iron|| | |||||||||
| Age, y | 0.98 | 0.94-1.01 | .203 | — | — | — | 0.98 | 0.94-1.02 | .262 |
| Female sex | 2.45 | 0.33-18.3 | .382 | — | — | — | 2.12§ | 0.28-16.1 | .469 |
| Malabsorptive procedure | 1.62 | 0.60-4.36 | .342 | — | — | — | — | — | — |
| Baseline anemia | 1.39 | 0.47-4.14 | .550 | 1.21 | 0.40-3.63 | .736 | — | — | — |
| Baseline ferritin, μg/L | 0.99 | 0.98-1.00 | .071 | 0.99 | 0.98-1.00 | .080 | — | — | — |
| Weight loss at 6 mo, %TBW | 0.98 | 0.92-1.04 | .450 | — | — | — | — | — | — |
| Risk factor . | Univariate analysis . | Multivariate analysis . | Multivariate excluding mediators* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Iron deficiency | |||||||||
| Age, y | 0.98 | 0.96-0.99 | .005† | 0.99 | 0.92-1.06 | .735 | 0.98‡ | 0.97-1.00 | .028† |
| Female sex | 2.85 | 1.58-5.13 | <.001† | 0.80§ | 0.01-44.3 | .914 | 2.30§ | 1.27-4.17 | .006† |
| Malabsorptive procedure | 2.46 | 1.59-3.80 | <.001† | 1.92 | 1.20-3.06 | .006† | 2.36 | 1.50-3.71 | <.001† |
| Baseline anemia | 2.94¶ | 1.88-4.60 | <.001† | 1.49¶ | 0.92-2.44 | .107 | — | — | — |
| Baseline ferritin, μg/L | 0.96¶ | 0.95-0.97 | <.001† | 0.96¶ | 0.95-0.97 | <.001† | — | — | — |
| Weight loss at 6 mo, %TBW | 1.01 | 0.99-1.03 | .480 | 0.98 | 0.96-1.00 | .066 | 0.99 | 0.97-1.01 | .492 |
| Iron deficiency anemia | |||||||||
| Age, y | 0.98 | 0.96-1.00 | .072 | 0.90 | 0.82-0.99 | .028† | 0.98 | 0.96-1.00 | .088 |
| Female sex | 1.64 | 0.70-3.81 | .252 | 0.02§ | 0.00-2.62 | .114 | 1.48§ | 0.62-3.52 | .376 |
| Malabsorptive procedure | 1.01 | 0.58-1.77 | .973 | 0.84 | 0.45-1.59 | .602 | 1.01 | 0.55-1.85 | .969 |
| Baseline anemia | 24.6¶ | 11.0-55.1 | <.001† | 19.6¶ | 7.85-48.9 | <.001† | — | — | — |
| Baseline ferritin, μg/L | 0.96¶ | 0.94-0.97 | <.001† | 0.96¶ | 0.95-0.98 | <.001† | — | — | — |
| Weight loss at 6 mo, %TBW | 0.99 | 0.95-1.02 | .399 | 0.98 | 0.94-1.02 | .335 | 0.98 | 0.95-1.02 | .278 |
| Administration of IV iron|| | |||||||||
| Age, y | 0.98 | 0.94-1.01 | .203 | — | — | — | 0.98 | 0.94-1.02 | .262 |
| Female sex | 2.45 | 0.33-18.3 | .382 | — | — | — | 2.12§ | 0.28-16.1 | .469 |
| Malabsorptive procedure | 1.62 | 0.60-4.36 | .342 | — | — | — | — | — | — |
| Baseline anemia | 1.39 | 0.47-4.14 | .550 | 1.21 | 0.40-3.63 | .736 | — | — | — |
| Baseline ferritin, μg/L | 0.99 | 0.98-1.00 | .071 | 0.99 | 0.98-1.00 | .080 | — | — | — |
| Weight loss at 6 mo, %TBW | 0.98 | 0.92-1.04 | .450 | — | — | — | — | — | — |
—, not included in the analysis.
This multivariate analysis excludes baseline anemia and baseline ferritin.
P < .05, statistically significant.
The effect of age was not statistically significant with the inclusion of an interaction term for age and sex.
There was no significant interaction between age and sex.
Time-dependent covariates were used because proportional hazards assumption was not satisfied in univariate analysis. Time measured in months.
The multivariate analysis included only 2 covariates to prevent overfit.
The Cox regression that was performed using an alternative ferritin cutoff of 40 ng/mL had similar results, other than 2 pertinent differences, as shown in supplemental Table 3. In the multivariate analysis of iron deficiency that excluded potential mediators, the interaction between age and female sex (HR, 0.93; 95% CI, 0.88-0.99; P = .030) became statistically significant, suggesting that young age confers a greater risk of iron deficiency in women compared with in men. In the univariate analysis of IDA, young age attained statistical significance as a risk factor when a ferritin level of 40 ng/mL was used to define iron deficiency.
Discussion
Our study reports the frequency of iron deficiency and IDA in a general population of patients who underwent bariatric surgery. We found that iron deficiency and IDA are common in this population and that the need for IV iron is a delayed consequence that usually occurs multiple years after the procedure. We demonstrated that malabsorptive procedures and low baseline ferritin level were associated with an increased risk of iron deficiency. Young age, baseline anemia, and low baseline ferritin level were associated with an increased risk of IDA.
We found baseline iron deficiency in 19% of our patients at their preoperative bariatric appointment and iron deficiency anemia in 6%. Twelve percent of our patients were anemic at baseline compared with a range of 10% to 40% described in the literature.4
In our study, we found that 43% of patients developed iron deficiency, and 16% developed IDA. As shown in the Kaplan-Meier curve (Figure 2), the development of iron deficiency and IDA is slow but progressive, with an approximate 8% annual incidence of iron deficiency after bariatric surgery and nearly a 4% annual incidence of IDA. The overall frequency reported by our study likely underestimates the burden of iron deficiency in this patient population, because the mean follow-up duration was only 31 months. At 5 years of follow-up, the cumulative incidence of iron deficiency and IDA were 60% and 38%, respectively. This suggests that iron deficiency is a long-term rather than immediate consequence of bariatric surgery. These results can be contrasted with a large cohort study (959 patients) describing iron deficiency after bariatric surgery,16 which showed 41% of patients who underwent laparoscopic Roux-en-Y procedures had a ferritin level less than 30 μg/L at some point during follow-up. Another retrospective longitudinal study17 of patients followed for 4 years after a Roux-en-Y procedure reported iron deficiency (defined as ferritin level <30 ng/mL or transferrin saturation <20%) in 48% of patients. Our study reports similar incidences of iron deficiency to those published in the literature, extending the results to a more general population that includes various procedure types. By reporting the annual incidence of iron deficiency and IDA, our study provides a clearer description of the timing of iron deficiency than previous studies.
In our study, only 6% of patients received IV iron; however, this underestimates the long-term use of IV iron because of the short mean follow-up duration of 31 months. As shown in the Kaplan-Meier curve (Figure 2), very few patients required IV iron during the first 3 years after surgery, but the proportion of patients requiring IV iron increased significantly starting at the 3-year mark. Fifteen percent of patients had received IV iron by 5 years and 38% by 6 years, although selection bias caused by loss to follow-up may have led to overestimation of these figures. Our finding of IV iron administration to 6% of patients is comparable to multiple studies that reported frequencies from 7% to 9%,7,16,18 although those studies were limited to Roux-en-Y procedures or based on claims data. Two retrospective studies demonstrate a median interval of 42.5 months6 and a mean interval of 51 months7 between bariatric surgery and the administration of IV iron, which is consistent with the late onset of IV iron administration reported in our study.
In our Cox regression model, malabsorptive procedures and low baseline ferritin level were associated with an increased risk of iron deficiency. Young age, baseline anemia, and low baseline ferritin level were associated with an increased risk of IDA. No factor predicted the administration of IV iron with statistical significance, although low baseline ferritin level approached significance (P = .080). These results contrast a study by McCracken et al19 that reported the risk of severe anemia was decreased in men less than 40 years old and increased in patients with baseline anemia, low baseline ferritin level, or a 6-month weight loss of greater than 35% of TBW. Follow-up ferritin levels were not measured in that study, and iron deficiency was not a study outcome.
Premenopausal status did not independently predict iron deficiency in our multivariate model; however, both young age and female sex were predictors of iron deficiency in the univariate model. In the multivariate model that did not include baseline ferritin level or baseline anemia, we demonstrated that young age and female sex independently predicted iron deficiency. This suggests that young age and female sex are true risk factors, but their effects may be mediated by low baseline ferritin levels, which are more strongly associated with iron deficiency. The interaction between age and sex was not statistically significant at a ferritin cutoff of 30 μg/L, but the interaction was statistically significant at a ferritin cutoff of 40 μg/L. Our study supports existing research, such as the cohort study by Obinwanne et al,16 that identifies premenopausal status as a risk factor for iron deficiency and IV iron. However, their study did not assess the mediating effect of baseline ferritin on outcomes.
Our multivariate model also demonstrated that young age was an independent predictor of IDA, but the effect of sex was not statistically significant, even with removal of potential mediators from the model. The small proportion of male patients may have limited our study’s power to detect a significant sex effect.
We demonstrated an increased risk of iron deficiency in patients who underwent malabsorptive procedures such as Roux-en-Y gastric bypass and duodenal switch, but no effect on the risk of IDA. This contrasts with a meta-analysis20 that found no significant difference in iron deficiency or anemia between Roux-en-Y gastric bypass and sleeve gastrectomy and another study21 showing no significant difference in the absorption of iron isotopes between the procedure types.
Ferritin was a strong predictor of iron deficiency and IDA in our study. Baseline ferritin level less than 30 μg/L was associated with the highest risk of IDA, whereas ferritin level of 156 μg/L or greater carried a minimal risk of IDA even after 6 years of follow-up, as shown in Figure 3.
Only 24 patients required IV iron, which limited the power of our study to show statistically significant effects from risk factors such as baseline ferritin and premenopausal status. Notably, the only male patient to receive IV iron had anemia that was ascribed to chronic kidney disease rather than iron deficiency. Only 2 of the patients had gastrointestinal bleeding, suggesting that gastrointestinal bleeding is not a major factor in most cases of IDA after bariatric surgery. Although guidelines22 and local protocols suggest ferrous salts as the initial treatment of iron deficiency, our data show that as many as three quarters of patients were receiving alternative iron formulations because of issues with tolerability and compliance. This observation raises the possibility that poor tolerance of oral iron may be a dominant factor that leads to IV iron supplementation.
American,22 European,23 and Ontario8 bariatric surgery guidelines suggest monitoring iron studies every 3 to 6 months in the first postoperative year and annually thereafter. Our results support their recommendations for long-term follow-up of iron studies. We question the lower frequency of testing that starts at 1 year after bariatric surgery, because the risks of IDA and requiring IV iron infusions increase with time. The guidelines22 also suggest a “preoperative aggressive case-finding approach” to detect iron deficiency, but our results suggest that even patients with borderline low ferritin levels are at increased risk of iron deficiency and may benefit from preoperative supplementation.
The strengths of our study are the inclusion of a diverse population of bariatric surgery patients that was not restricted to patients with diabetes or Roux-en-Y procedures and a Cox regression analysis that accounted for covariance between risk factors. Our study is limited by its single-center and retrospective design, the use of age as a surrogate marker for menopausal status, and the variability in prescribed iron supplements. Our definition of iron deficiency is limited by the use of ferritin, which is an acute phase reactant that increases in inflammatory states. However, ferritin is the preferred noninvasive test for iron deficiency. We found similar results using a ferritin level cutoff of 40 μg/L as with 30 μg/L, suggesting that false negatives secondary to inflammation did not significantly impact our results. Many patients were not followed beyond 2 years, and the mean follow-up of 31 months was shorter than desired. There is a potential for selection bias in which patients with iron deficiency were followed for longer than the rest of the cohort, but this could not be avoided because of the retrospective nature of the study.
In conclusion, our results support previous reports that iron deficiency and IDA are common after bariatric surgery and suggest that they are associated with low baseline ferritin level, among other factors. Premenopausal status predicts iron deficiency but is mediated by its effects on baseline ferritin level. The increased use of IV iron after the first 3 years of surgery adds to the growing body of evidence that IDA is a delayed consequence of bariatric surgery. Prospective studies are needed to explore oral iron supplementation strategies to reduce the risk of IDA and to define the role of IV iron in this patient population.
Data may be requested from the corresponding author at siegald@mcmaster.ca.
Authorship
Contribution: Z.G., M. Crowther, and D.M.S. conceived and designed the study; Z.G., A.L., and M. Conroy collected the data; Z.G. and L.M. performed statistical analysis; Z.G., M.T., M. Crowther, L.M., and D.M.S. drafted the manuscript; all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deborah M. Siegal, Department of Medicine, McMaster University, 711 Concession St, Hamilton, ON L8V 1C3, Canada; e-mail: siegald@mcmaster.ca.
References
Author notes
The full-text version of this article contains a data supplement.