Key Points
Adolescents with low VWF–associated HMB had significant bleeding phenotype (BAT score >2; 94%), and severe HMB (BAT HMB domain score ≥2; 90%).
Bleeding complications found included anemia (21%), iron deficiency (60%), hospitalization (10%), and transfusion requirement (12%).
Abstract
Low von Willebrand factor (VWF) in adults is associated with significant bleeding, most notably heavy menstrual bleeding (HMB) and postpartum hemorrhage, although this has not been characterized in adolescents. The objectives of this analysis of a multicenter single arm observational cohort study in adolescents with low VWF–associated HMB were to describe the bleeding phenotype, HMB severity, and related complications. Eligibility criteria included postmenarchal females <21 years of age with HMB (Pictorial Blood Assessment Chart [PBAC] score >100) and low VWF (2 values of VWF activity ≥30 and ≤50 IU/dL). Patients diagnosed with other bleeding disorders were ineligible. Clinical phenotype data, including PBAC and Bleeding Assessment Tool (BAT) scores, laboratory data, and HMB management/outcome details, were extracted. Patient demographics and clinical characteristics were summarized as medians with minimum/maximum values or frequencies with percentages. Groups were compared using a Wilcoxon rank-sum test or Fisher’s exact test. A total of 113 patients met inclusion criteria, and 2 were excluded. Ninety four percent had a significant bleeding phenotype (BAT score >2), with predominantly mucocutaneous bleeding (32%-44%), postprocedural/surgical bleeding (15%), and severe HMB (BAT HMB domain score ≥2; 90%). Bleeding complications included iron deficiency (60%), anemia (21%), transfusion (12%), and hospitalization (10%). Desmopressin challenge response in subjects tested was good and sustained. Several (48%) required combined therapy for HMB (hormonal/hemostatic), and one third did not show improvement despite therapy. Our results suggest that adolescent females with low VWF have a significant bleeding phenotype and resultant complications warranting a focus on prompt diagnosis, appropriate therapy, and prevention of complications.
Introduction
The prevalence of bleeding disorders in adolescents presenting with heavy menstrual bleeding (HMB) has been well reported.1,2 The recently published opinion of the American College of Obstetrics and Gynecology Committee on Adolescent Health Care3 on screening and management of bleeding disorders in adolescents with HMB states that the latter may be an important sentinel sign for bleeding disorders and recommends routine evaluation for underlying bleeding disorders in such patients. According to data on females from the Centers for Disease Control and Prevention’s Universal Data Collection Program,4 von Willebrand disease (VWD) is the most common bleeding disorder in adolescents and adult women with bleeding disorders causing HMB. On the other hand, low von Willebrand factor (VWF) activity (≥30 and ≤50 IU/dL) has been debated to be a risk factor vs a disease.5,6 As a result, there has not been a consistent approach to the diagnosis and management of these patients. Recent studies in adults have shown that low VWF can be associated with significant bleeding,7 especially in women, causing HMB and postpartum hemorrhage.8 However, the impact of low VWF in adolescent females has not been described. We undertook a multicenter study to better define this subset of adolescent females with low VWF–associated HMB, to better comprehend their risk of bleeding, and to tailor their therapy to prevent complications.
Materials and methods
A multicenter observational cohort study to delineate the phenotype and genotype of adolescent females with low VWF–associated HMB was undertaken from February 2017 to June 2019. Tertiary care centers in North America, with expertise in hemostasis, that provided care for adolescents with HMB, and were members of the Foundation for Women and Girls with Blood Disorders, participated in the study. Institutional Review Board approval was obtained by all participating centers, and parental and/or patient consent/assent was obtained from all patients prior to study participation. The objectives of this analysis of the multicenter study, focusing primarily on the bleeding phenotype, were to describe (1) the spectrum of bleeding phenotype and related complications in this patient population, and (2) the severity of HMB, as assessed by Pictorial Blood Assessment Chart (PBAC) score, and the severity of the bleeding phenotype, as assessed by the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (BAT) score.
The study eligibility criteria included postmenarchal females <21 years of age diagnosed with HMB, defined as PBAC score >100, and low VWF, defined as having ≥2 values of VWF activity (VWF:Act) ≥30 and ≤50 IU/dL (as measured by VWF ristocetin cofactor assay [VWF:RCo] and/or VWF glycoprotein 1bM assay [VWF:Gp1bM]). Patients who did not meet these criteria or who were diagnosed with other bleeding disorders were ineligible for the study. Adolescent females, seen in hematology clinics in participating centers managing HMB patients, were screened for study eligibility, eligible patients were approached for study participation, and patients who consented to participate were enrolled in the study. Clinical phenotype data were extracted prospectively from the patients and retrospectively by reviewing the patient’s electronic medical record. Data collected included the types and severity of bleeding; PBAC score at diagnosis; BAT score (BAT administered by the research team) at study entry; laboratory values, including hemoglobin and hematocrit, ferritin, VWF antigen (VWF:Ag) and VWF:Act levels and factor VIII (FVIII) levels at diagnosis; response to intranasal (IN) or IV desmopressin challenge; HMB management details; and clinical outcomes of HMB. Deidentified patient data from each participating center were entered into the coordinating center’s electronic database; the data were maintained as confidential with access restricted to study investigators by means of password protection.
Statistical analysis
Patient demographics and clinical characteristics were summarized by descriptive statistics, including medians with minimum and maximum values or frequencies with percentage. Groups were compared using the Wilcoxon rank-sum test or Fisher’s exact test. Simple linear regression was used to estimate associations between bleeding outcome scores and hemostasis measures.
Results
Ten centers participated in this multicenter study. One hundred and thirteen adolescent females with a diagnosis of HMB and low VWF, who met the study inclusion and exclusion criteria, were enrolled. Up to one third of the adolescents with HMB seen in these subspecialty clinics have been previously reported to have low VWF.9 One patient each was withdrawn because of the subsequent detection of type 1 VWD and factor XI deficiency. The remaining 111 adolescent females formed the study population for this analysis.
Patient demographics and bleeding characteristics
The median age of the patients was 16.2 years (range, 11.5-20.2). The racial distribution of the patient population was as follows: 87 (78%) white, 8 (7%) African American, 6 (5%) American Indian or Alaska Native, 2 (2%) Asian, 2 (2%) belonging to >1 race, 1 (1%) other, and 5 (5%) declining to disclose their racial origin.
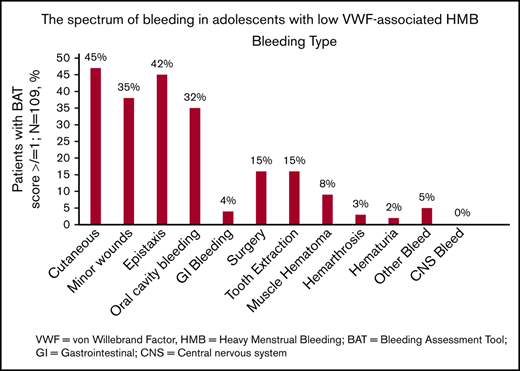
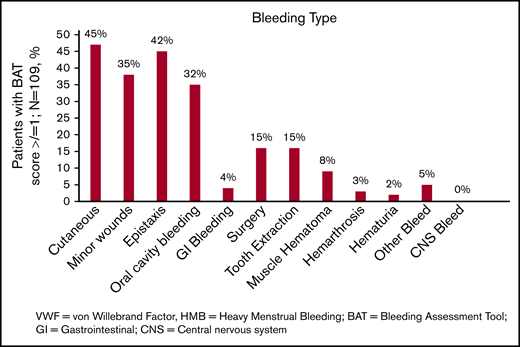
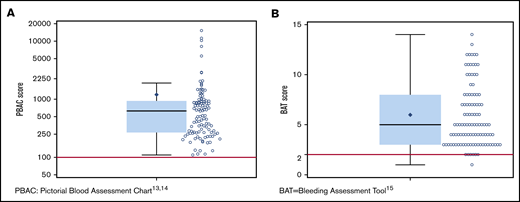
The spectrum of bleeding, in addition to HMB, seen in the study population is illustrated in Figure 1. Mucocutaneous bleeding symptoms were the most common (epistaxis, n = 45, 42%; cutaneous bleeding, n = 48, 45%; bleeding from minor wounds, n = 38, 35%; oral cavity bleeding, n = 35, 32%), followed by postsurgical and dental procedure–related bleeding (n = 16, 15% for each). The median BAT score was 5.0 (N = 109; range, 1-19) (Figure 2B). The median BAT score for patients aged 11 to 14 years, 15 to 18 years, and 19 to 21 years of age was 5.0, 4.5, and 5.0 respectively (P = .72); 4 of 6 patients in the 19- to 21-year-old age group had BAT scores ≤5. Of 109 patients with a BAT score available, 102 (94%) had a BAT score >2, 38 (35%) had a BAT score >6, and 15 (14%) had a BAT score >10. Frequencies of individual domain scores are summarized in Table 1. Seventy-seven patients (of 109; 71%) had >2 bleeding events. The median time from age at HMB diagnosis and age at BAT score assessment at study entry was 2.2 years (range, 0.1-10.3). The median age at diagnosis of HMB was 13.3 years (N = 105; range, 8-17.4). The median PBAC score was 630 (N = 111; range, 110-15 330) (Figure 2A). The median PBAC score for patients aged 11 to 14 years, 15 to 18 years, and 19 to 21 years was 481, 660, and 338 respectively (P = .61). Ten patients with PBAC scores ≥2000 had an HMB cycle duration ranging from 11 to 62 days, with 4 (40%) using pads and tampons simultaneously for protection, 7 (70%) reporting passing several large clots, and all (100%) reporting overflow on ≥1 day. One hundred (of 110, 91%) had HMB BAT score ≥2, which indicates HMB present since menarche >12 months, requiring therapy/hospitalization/transfusion and/or time off from work or school >2 days per year. Ninety-one (of 107, 85%) reported regular and repetitive menstrual bleeding, and 16 of 107 (15%) reported irregular and prolonged bleeding. Eighty-one (of 109, 74%) reported passing clots, with 73 (67%) passing clots larger than the size of a quarter, and 69 (of 109, 63%) reported having overflow of menstrual bleeding.
The spectrum of bleeding in adolescents with low VWF–associated HMB. Bar graph showing the spectrum of bleeding complications seen in the study population and their percentages (N = 109). CNS, central nervous system; GI, gastrointestinal.
The spectrum of bleeding in adolescents with low VWF–associated HMB. Bar graph showing the spectrum of bleeding complications seen in the study population and their percentages (N = 109). CNS, central nervous system; GI, gastrointestinal.
PBAC and BAT score distribution. (A) Scatter dot plot showing the distribution of PBAC scores13,14 in the study population. The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed value that is within 1.5 times the interquartile range (IQR). The solid black diamond represents the mean value. The median (630) is indicated by the horizontal line within the box, and the cutoff for heavy menstrual bleeding (PBAC score >100) is indicated by the red line. (B) Scatter dot plot showing the distribution of BAT scores15 in the study population. The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed values which are within 1.5 times the IQR. The solid black diamond represents the mean value. The median (5) indicated by the horizontal line within the box, and the cutoff for abnormal BAT score (>2) is indicated by the red line.
PBAC and BAT score distribution. (A) Scatter dot plot showing the distribution of PBAC scores13,14 in the study population. The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed value that is within 1.5 times the interquartile range (IQR). The solid black diamond represents the mean value. The median (630) is indicated by the horizontal line within the box, and the cutoff for heavy menstrual bleeding (PBAC score >100) is indicated by the red line. (B) Scatter dot plot showing the distribution of BAT scores15 in the study population. The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed values which are within 1.5 times the IQR. The solid black diamond represents the mean value. The median (5) indicated by the horizontal line within the box, and the cutoff for abnormal BAT score (>2) is indicated by the red line.
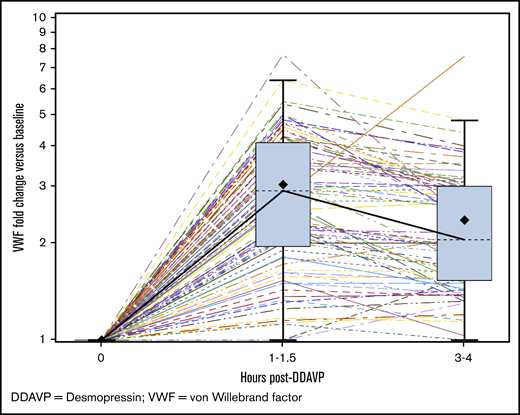
DDAVP challenge test response. Graph showing the fold change in VWF:Act vs baseline at 1 to 1.5 hours and 3 to 4 hours post-DDAVP in the study population. The boxes show the median values (dashed lines) at those time points (2.91 and 2.05, respectively). The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed values which are within 1.5 times the IQR. The solid black diamond represents the mean value. The colored lines with dashed and solid patterns represent results from various patients.
DDAVP challenge test response. Graph showing the fold change in VWF:Act vs baseline at 1 to 1.5 hours and 3 to 4 hours post-DDAVP in the study population. The boxes show the median values (dashed lines) at those time points (2.91 and 2.05, respectively). The box extends from the 25th percentile to the 75th percentile. The whiskers extend from the minimum and maximum observed values which are within 1.5 times the IQR. The solid black diamond represents the mean value. The colored lines with dashed and solid patterns represent results from various patients.
Bleeding severity in BAT domains (N = 109)
| BAT domain . | Score 0 . | Score 1 . | Score 2 . | Score 3 . | Score 4 . |
|---|---|---|---|---|---|
| Epistaxis | 64 (59) | 25 (23) | 6 (6) | 10 (9) | 4 (4) |
| Cutaneous | 61 (56) | 41 (38) | 2 (2) | 5 (5) | 0 |
| Minor wound bleeding | 71 (65) | 33 (30) | 4 (4) | 0 | 1 (1) |
| Oral cavity | 74 (68) | 31 (28) | 4 (4) | 0 | 0 |
| Gastrointestinal bleeding | 105 (96) | 1 (1) | 2 (2) | 0 | 1 (1) |
| Hematuria | 107 (98) | 2 (2) | 0 | 0 | 0 |
| Tooth extraction | 93 (85) | 6 (6) | 5 (5) | 4 (4) | 1 (1) |
| Surgery | 93 (85) | 4 (4) | 0 | 7 (6) | 5 (5) |
| HMB | 1 (1) | 8 (7) | 20 (18) | 66 (61) | 14 (13) |
| Muscle hematoma | 100 (92) | 6 (6) | 1 (1) | 2 (2) | 0 |
| Hemarthrosis | 106 (97) | 2 (2) | 0 | 0 | 1 (1) |
| Central nervous system bleeding | 109 (100) | 0 | 0 | 0 | 0 |
| Other bleeding | 104 (95) | 0 | 2 (2) | 2 (2) | 1 (1) |
| BAT domain . | Score 0 . | Score 1 . | Score 2 . | Score 3 . | Score 4 . |
|---|---|---|---|---|---|
| Epistaxis | 64 (59) | 25 (23) | 6 (6) | 10 (9) | 4 (4) |
| Cutaneous | 61 (56) | 41 (38) | 2 (2) | 5 (5) | 0 |
| Minor wound bleeding | 71 (65) | 33 (30) | 4 (4) | 0 | 1 (1) |
| Oral cavity | 74 (68) | 31 (28) | 4 (4) | 0 | 0 |
| Gastrointestinal bleeding | 105 (96) | 1 (1) | 2 (2) | 0 | 1 (1) |
| Hematuria | 107 (98) | 2 (2) | 0 | 0 | 0 |
| Tooth extraction | 93 (85) | 6 (6) | 5 (5) | 4 (4) | 1 (1) |
| Surgery | 93 (85) | 4 (4) | 0 | 7 (6) | 5 (5) |
| HMB | 1 (1) | 8 (7) | 20 (18) | 66 (61) | 14 (13) |
| Muscle hematoma | 100 (92) | 6 (6) | 1 (1) | 2 (2) | 0 |
| Hemarthrosis | 106 (97) | 2 (2) | 0 | 0 | 1 (1) |
| Central nervous system bleeding | 109 (100) | 0 | 0 | 0 | 0 |
| Other bleeding | 104 (95) | 0 | 2 (2) | 2 (2) | 1 (1) |
All data are n (%).
Laboratory values, management, and outcome
Seventy-two patients (65%) had VWF:Act (lowest value) between 30-40 IU/dL, with a median VWF:Act of 35 IU/dL (range, 30-39 IU/dL), and 39 (35%) had VWF:Act (lowest value) between 40 and 50 IU/dL, with a median VWF:Act of 44 IU/dL (range, 40-49 IU/dL). The median PBAC scores for patients with VWF:Act 30-40 IU/dL vs 40-50 IU/dL were 578 (range, 110-15 330) and 639 (range, 114-11 305), respectively, and the difference was not statistically significant (P = .87). The median HMB domain scores in BAT for patients with VWF:Act 30-40 IU/dL vs 40-50 IU/dL were 3 (range, 1-4) and 3 (range, 0-4), respectively, and the difference was not statistically significant (P = .31). The median BAT scores for patients with VWF:Act 30-40 IU/dL vs 40-50 IU/dL were 5.0 (range, 2-18) and 5.0 (range, 1-19) respectively, and the difference was not statistically significant (P = .96). The median FVIII level was 75 IU/dL (range, 25-243 IU/dL). The median FVIII/VWF:Ag ratio was 1.92 (range, 0.69-6.94). One hundred one (91%) patients had a FVIII/VWF:Ag ratio of >1, and 10 (9%) patients had a ratio of <1. Ninety-seven (87%) patients underwent desmopressin challenge testing; 75 (77%) underwent IN, and 22 (23%) underwent IV challenge, respectively. The median fold rise in VWF:Act at 1 to 1.5 hours postdesmopressin was 2.91 (N = 96; range, 1-7.7), and the median fold rise at 3 to 4 hours was 2.05 (N = 78; range, 1-7.6) (Figure 3). When the responses to desmopressin (DDAVP) at 1 and 4 hours among patients with an FVIII/VWF:Ag ratio of >1 and those with an FVIII/VWF:Ag ratio of <1 were compared, there was no statistically significant difference between the 2 groups (P = .154 and .638, respectively). BAT scores were higher among patients with a greater-than-twofold rise in DDAVP response at 1 hour than among patients with a less-than-twofold rise (median score of 5 vs 4, P = .029); there was no significant difference in PBAC scores between the 2 DDAVP response groups (P = .228). In regression plots, the BAT score was higher for increasing VWF:Act at 1 hour (slope = 0.01; P = .86) and showed no change for VWF:Act at 3 to 4 hours (slope = 0; P = .79); it was higher for increasing FVIII at 1 hour (slope = 0.01; P = .6) and higher for increasing FVIII at 3 to 4 hours (slope = 0.02; P = .02). In regression plots, the PBAC score was higher for increasing VWF:Act at 1 hour (slope = 50; P = .23) and higher for VWF:Act at 3 to 4 hours (slope = 46; P = .26); it was higher for increasing FVIII at 1 hour (slope = 14; P = .09) and higher for increasing FVIII at 3 to 4 hours (slope = 8.9; P = .2). Bleeding complications are summarized in Table 2. Twenty-three (21%) patients had anemia, with hemoglobin <12 g/dL, and 62 (60%) had iron deficiency, with ferritin <20 ng/mL, at the time of HMB diagnosis. Eleven (10%) patients were hospitalized for HMB, and 13 (12%) received a red blood cell transfusion for HMB. When PBAC and BAT scores were compared with hemoglobin ≥12 g/dL vs <12 g/dL, there was no statistically significant correlation between the groups (median PBAC of 650 vs 462, P = .57 and median BAT of 5 vs 5, P = .24, respectively). When PBAC and BAT scores were compared with ferritin ≥20 ng/mL vs <20 ng/mL, there was no statistically significant correlation between the groups (median PBAC of 746 vs 578, P = .28 and median BAT of 5 vs 5, P = .62, respectively). Hormonal therapy received by the patients for HMB management included (1) estrogen-only therapy, including IV estrogen (n = 3, 3%) and estrogen-only patches (n = 4, 4%), (2) progesterone-only therapy, including progestin-only pills (n = 28, 25%), levonorgestrel intrauterine device (n = 12, 11%), etonogestrel implant (n = 3, 3%), and intramuscular depot medroxyprogesterone injection (n = 15, 14%), or (3) combined estrogen and progesterone therapy, including combined oral contraceptives (n = 59, 53%) or combined patches (n = 10, 9%). Overall, 41 (37%) patients used hormonal therapy only, with resolution, improvement, no change, or worsening seen in 3 (7%), 25 (61%), 10 (24%), and 2 (5%) patients, respectively; the outcome was not known in 1 patient. Hemostatic therapy included oral epsilon aminocaproic acid (n = 11, 10%), oral/IV tranexamic acid (n = 44, 40%), IN/IV desmopressin (n = 45, 41%), or IV plasma-derived VWF (n = 2, 2%). Overall, 15 (14%) patients used hemostatic therapy only, with improvement or no change seen in 5 (33%) and 10 (67%) patients, respectively. Fifty (45%) patients received both hormonal and hemostatic therapy for HMB control; 48 patients used both therapies simultaneously, and 3 patients used them sequentially. Resolution, improvement, and no change with combined hormonal and hemostatic therapy was seen in 9 (18%), 29 (58%), and 11 (22%) patients, respectively; the outcome was unknown in 1 patient. One patient (2%) underwent balloon tamponade; this patient suffered a straddle injury while horseback riding and was admitted to the hospital with severe HMB. Examination under anesthesia did not reveal any laceration of her genital tract or uterine cervix, with bleeding noted from the cervical opening, which stopped in 2 days with balloon tamponade, hormonal therapy, and tranexamic acid. No patient reported having a hysterectomy. Among 103 patients with recorded outcomes, HMB was improved in 58 (56%) subjects, resolved in 12 (12%) subjects, the same in 31 (30%) subjects, and worse in 2 (2%) subjects.
Bleeding complications in adolescents with low VWF–associated HMB
| Bleeding complication . | n/N (%) . |
|---|---|
| Iron deficiency (ferritin <20 ng/mL) | 62/108 (60) |
| Anemia (hemoglobin <12 g/dL) | 23/110 (21) |
| Red blood cell transfusion | 13/108 (12) |
| Hospitalization for HMB | 11/109 (10) |
| Bleeding complication . | n/N (%) . |
|---|---|
| Iron deficiency (ferritin <20 ng/mL) | 62/108 (60) |
| Anemia (hemoglobin <12 g/dL) | 23/110 (21) |
| Red blood cell transfusion | 13/108 (12) |
| Hospitalization for HMB | 11/109 (10) |
Discussion
Low VWF has been reported to be prevalent in up to 32% of adolescents with HMB managed in a subspecialty clinic compared with other bleeding disorders with lower prevalence rates.9 In adults, low VWF levels can be associated with significant bleeding which, in turn, can lead to serious complications and increased health care utilization.7 The Low von Willebrand in Ireland Cohort (LoVIC) study8 documented HMB in 89% of adult women in their study population: of these, 45.8% developed iron deficiency. Postpartum hemorrhage was self-reported in 63.5% of adult women, with 21.6% requiring transfusion, critical care, or radiological or surgical intervention. In addition, low VWF diagnosis was not expedited in women who presented to physicians with complaints of HMB. Recently, Jacobson et al10 reported on a national claims database of 23 888 girls and adolescents aged from 10 to 17 years with heavy menses, among whom VWD screening was performed in only 8% with HMB and 16% with severe HMB. We hope that our observations of the substantial burden of low VWF will lead to further awareness, early identification of these patients, and development of clinical pathways of care, because such studies are lacking. Interestingly, a recent prospective evaluation of tonsillectomy bleeding in children11 failed to show a correlation with low VWF levels, yet Shui et al have advocated for systematic evaluation of BAT in these patients.12 Pollio and colleagues reported increased perioperative bleeding risk in children with low VWF compared with healthy controls.13 Our study is the first to evaluate the bleeding spectrum and severity in adolescent females with HMB and low VWF utilizing HMB and overall bleeding assessment tools (ie, PBAC and BAT score, respectively).
Bleeding spectrum and severity
In addition to HMB in all subjects, the majority of our study patients had other bleeding symptoms. The bleeding symptoms were predominantly mucosal or cutaneous, with more than one third of the patients reporting epistaxis, oral cavity bleeding, and cutaneous bleeding. In addition, 31% of the patients reported bleeding after trauma, and 15% reported postdental extraction and/or postoperative bleeding. Two thirds of the patients had >2 bleeding complications, which implies a sizable burden of disease and the need to identify and manage these patients systematically. Our findings are similar to those of adults in the LoVIC study,7 which showed that bleeding scores remained significantly elevated in women, even after removing HMB and postpartum hemorrhage–related scores.
PBAC and BAT scores in patients with low VWF
Overall, bleeding was significant in most subjects with elevated PBAC and/or BAT scores. PBAC score has been used in adolescent14 and adult15 females with HMB as a clinical and research tool, and BAT score was shown by Lavin and colleagues8 to be a more sensitive tool for assessing HMB. Ninety-four percent of the patients had BAT scores >2, which is a significant score for the pediatric population younger than 18 years of age.16 Although a BAT score >5 is considered an abnormal score for adult females, this has not been defined for an exclusively adolescent population. Only 4 patients in the 19- to 21-year-old age group had a BAT score ≤5. Nevertheless, it is important to note that, in the menorrhagia domain of the BAT score, having no menorrhagia gets a score of 0; hence, normal menstruation seen in postmenarchal adolescents should not increase the BAT score in these patients, whereas experiencing HMB will increase the score. The majority (90%) had more severe HMB, with a BAT HMB domain score ≥2. In addition, three fourths of the patients reported passing clots and having overflow of menstrual bleeding. Lavin et al reported similar results in adult women, with a majority of the women with low VWF experiencing significant HMB.8 The high prevalence of severe HMB in adolescents with low VWF calls for prompt evaluation for underlying bleeding disorders in all adolescents at the onset of HMB to avoid a delay in diagnosis. Patients were treated with hemostatic and/or hormonal therapy for HMB control, with approximately half of the patients requiring both types of treatment. Despite therapy, one third of the patients did not have improvement in HMB, illustrating the severity of HMB in low VWF patients. This also emphasizes the need to study combined treatment strategies, such as hormonal and hemostatic therapy, or dual hemostatic therapy, such as tranexamic acid and desmopressin (DDAVP).
Although a minority require procedures to control HMB, including balloon tamponade in our study and dilatation and curettage in 19%, endometrial ablation in 4%, and hysterectomy in 7.5% in the Lavin et al study,8 these findings highlight the severity and complexity of HMB management during adulthood, especially if these patients are not promptly diagnosed early during adolescence. Likewise, postpartum hemorrhage noted in 63.5% of adult women with low VWF8 emphasizes the need to diagnose low VWF in adolescent females with bleeding phenotype to prevent subsequent maternal morbidity related to bleeding. The delay in diagnosis of low VWF documented in adult women8 and the severity of HMB and overall bleeding symptomatology in adult and adolescent females shown in the LoVIC study7 and in our studies emphasize the requirement for physician awareness of this entity as a significant cause of bleeding, with a need for prompt medical attention and expedited evaluation.
BAT and PBAC scores were not significantly different in patients with VWF levels of 30 to 40 IU/dL vs 40 to 50 IU/dL, supporting the finding from the LoVIC study7 that, in patients with low VWF, bleeding symptoms and severity do not appear to correlate with VWF levels within the 30 to 50 IU/dL range.
Pathophysiology of low VWF
Studies have shown that increased clearance of VWF by varying mechanisms is a potential cause for low VWF levels.17,18 This may explain the cases of low VWF levels without pathologic VWF gene variants. Although the majority of patients in this study had an FVIII/VWF:Ag ratio >1, which was previously shown to indicate decreased VWF synthesis and/or secretion,19 a small subset of patients had a ratio <1, indicating increased clearance of VWF. VWF propeptide/VWF:Ag ratio,20 another measure to help identify patients with increased clearance of VWF, was not analyzed in our study. The lack of correlation between FVIII/VWF:Ag ratio and DDAVP response, as well as between BAT and PBAC scores and post-DDAVP FVIII and VWF:Act levels, as shown in the study by Atiq et al,20 may be due to our study’s smaller sample size. Future studies specifically addressing the pathogenesis of low VWF activity by looking at the clearance mechanisms and pathogenic gene variants of VWF clearance–associated genes may shed additional light on this topic. Analysis of gene variants affecting hemostasis, thrombosis, and vascular biology is underway in our patient cohort.
Outcome and complications of bleeding
Overall, the good response to DDAVP challenge at 1 hour and the sustained response at 4 hours post-DDAVP, consistent with previous studies,7,21 indicate that DDAVP is a good therapeutic choice for mucosal and menstrual bleeding in these patients. Although a recent study found that DDAVP response predicted bleeding severity,20 our findings did not support this observation, perhaps as a result of our smaller sample size. Two thirds of the patients using hormonal therapy only or combined hemostatic and hormonal therapy exhibited resolution of or improvement in HMB, whereas this was noted in one third of the patients using hemostatic therapy only. Approximately half of the patients required both hormonal and hemostatic agents, most simultaneously, reflecting the severity of HMB. Morbidity was high, with one third lacking improvement despite treatment, similar to the observation by Lavin and colleagues in adult women with low VWF.8 We hope that the efficacy of hormonal therapy, used frequently in combination with hemostatic therapy, noted in our study will help to raise awareness among hematologists that they should collaborate with gynecologists to consider the use of hormonal therapy, especially in patients not responding well to hemostatic therapy alone. We hope that future studies in adolescents will explore understudied therapeutic options, such as the levonorgestrel intrauterine device, alone or in combination with other hemostatic agents.
Complications of HMB were frequent, with anemia in 21%, iron deficiency in 60%, blood transfusion requirement in 12%, and hospitalization in 10% of the patients, similar to previous adult studies.8 Interestingly, a recent study by Zia et al found similar rates of hospitalization for packed red blood cell transfusion and/or parenteral iron in a prospective study of 200 adolescents with HMB, with or without an identifiable hemostatic defect, suggesting that hematologic complications in adolescents with HMB are not unique.22 The deleterious effects of anemia and iron deficiency in females, including fatigue, poor cognitive functioning and academic performance, depression, and impaired cardiac stress response,23-27 warrant thorough evaluation and prompt diagnosis of low VWF, because appropriate management can provide a positive impact on patient well-being and significantly improve their overall quality of life.
Study limitations include small sample size, recall bias in the patient and/or parent reporting of bleeding history, selection bias (adolescents with bleeding are more likely to be seen in specialized centers for bleeding disorders), lack of an adolescent-specific BAT score, and lack of centralized laboratory data. Nevertheless, our study is the first to describe the spectrum and severity of the bleeding phenotype in adolescents with low VWF–associated HMB and the resultant complications that may negatively impact their quality of life.
In conclusion, our study confirms for the first time that low VWF in postmenarchal adolescent females is associated with a significant bleeding phenotype, similar to adult women.7,8 Moreover, HMB is among the most common symptoms associated with low VWF, with associated iron deficiency, anemia, transfusion requirement, and hospitalization in some patients, consistent with previously published findings in adults.7,8 Compared with other pediatric studies,11-13 our study is the first to illustrate the frequency and severity of bleeding manifestations in this young population. The prevalence of clinically significant bleeding and resultant complications in adolescents with low VWF is high and supports the need for prompt intervention with hemostatic and/or hormonal therapy. The upcoming VWD clinical practice guidelines28 recommends confirming the diagnosis of type 1 VWD in patients with VWF levels between 30 and 50 IU/dL and abnormal bleeding. Our findings further confirm that adolescents with low VWF have a true bleeding disorder that warrants prompt evaluation by health care providers, including blood counts and iron and coagulation studies. Future studies are needed to elucidate the pathogenesis of low VWF, as well as to address coexisting factors that may modify the hemostatic balance in these patients, to identify optimal treatment strategies for this younger population. Rather than waiting for ≥12 months, screening for a bleeding disorder at the first symptoms of HMB should be the standard of care in adolescent females, because they are already being affected by social and emotional burdens that may worsen if they are overlooked.
Data sharing requests should be sent to Lakshmi Srivaths (e-mail: lvsrivat@txch.org).
Acknowledgment
This work was supported by investigator-initiated research grant (IIR-USA-BXLT-001980-H16-30985) from Shire US Inc., now part of Takeda.
Authorship
Contribution: L.S. conducted literature searches, designed the study, collected and interpreted data, wrote the manuscript, and created figures and tables; S.H.O., A.P.W., E.M., M.S., R.S., S.J., A.Z., M.V.R., R.K., and J.E.D. collected data and edited the manuscript; C.G.M. analyzed data and created figures; and P.A.K. conducted literature searches, designed the study, and edited the manuscript.
Conflict-of-interest disclosure: L.S. has received research funding from Shire US Inc., now part of Takeda. R.S. has an investigator-initiated grant from Takeda Pharmaceuticals for the ATHN 9 study and is a local principal investigator for the PEGylated Recombinant FVIII (BAX 855) previously untreated patients study. M.V.R. has received institutional research funding from Alnylam/Sanofi, ATHN, Biomarin, Bioverativ, Sangamo, and SPARK and consulting fees from Alnylam/Sanofi, Biomarin, Bioverativ, and SPARK. The remaining authors declare no competing financial interests.
Correspondence: Lakshmi Srivaths, Section of Hematology, Department of Pediatrics, Baylor College of Medicine/Texas Children’s Hospital, 6701 Fannin St, Houston, TX 77030; e-mail: lvsrivat@txch.org.