Key Points
South Korean MPN patients had a significantly higher risk of developing second primary solid tumors than that of the general population.
Patients with SMF had an overall survival comparable to those with PMF with less risk of developing SAML.
Abstract
This study aimed to elucidate patterns of disease transformation to secondary myelofibrosis (SMF) or secondary acute myeloid leukemia (SAML) and the development of second primary malignancies in South Korean patients with BCR-ABL1–negative myeloproliferative neoplasms (MPNs). By using nationwide public health care insurance claims data, we identified and analyzed 7454 patients with MPNs who were newly diagnosed with essential thrombocythemia (ET), polycythemia vera (PV), or primary myelofibrosis (PMF) from 2008 to 2016 and used the data to appropriately trace the disease course. Transformation to SMF or SAML was rare in patients with ET and PV, but patients with PMF had an 8-year cumulative incidence of SAML of 21.4%. Patients with PV or ET had an 8-year cumulative incidence of second primary solid tumors of ∼14%. Patients with MPNs had a 2 times higher risk of developing second primary solid tumors than that of the general South Korean population. Compared with patients with PMF, patients with SMF had a similar overall survival with a lower risk of developing SAML. The use of ruxolitinib did not increase the risk of developing B-cell lymphoma over a median follow-up period of 16.2 months. Disease transformation to SMF or SAML was rare in patients with ET or PV, but SAML was common in patients with PMF. South Korean patients with MPNs had a significantly higher risk of developing second primary solid tumors than that of the general population, particularly for kidney, prostate, brain, liver, and lung cancers.
Introduction
BCR-ABL1–negative myeloproliferative neoplasms (MPNs) are clonal stem cell disorders of hematopoietic cells of myeloid lineage, comprising polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1 Patients with PV or ET present with increased blood cell counts and have an indolent disease course. However, arterial or venous thrombosis attributed to high blood viscosity can be a serious complication.2 In patients with PMF, excessive secretion of inflammatory cytokines results in fibrotic changes in bone marrow and diminishes the quality of life of the affected patients due to systemic symptoms.3
In addition to vascular complications and constitutional symptoms, disease transformation is an important factor limiting the survival of patients with MPNs. PV and ET can transform into secondary myelofibrosis (SMF) or secondary acute myeloid leukemia (SAML). PMF and SMF can also transform into SAML. In addition, recent studies have suggested that patients with MPNs are at a significantly higher risk of developing second primary solid tumors.4,5
Although there have been significant advances in the field of MPNs since the discovery of the JAK2 V617F mutation in 2005,6 and a therapeutic implication has been established by the JAK inhibitor for myelofibrosis (MF)7 and PV,8 data regarding the epidemiology and disease course of MPNs in Asian populations are still scarce. Two recent South Korean epidemiological studies showed that the annual incidence of MPNs is on the rise.9,10 However, it is not clear whether there is a genuine increase in the population affected by MPNs or if the perceived increase is a result of improved detection rates.
To elucidate patterns of disease transformation to SMF and SAML and the development of second primary malignancies in South Korean BCR-ABL1–negative patients with MPNs, we conducted an epidemiological study using nationwide insurance claims data.
Methods
Data source
The National Health Insurance Service (NHIS) is a South Korean government–operated public health insurance service. All South Korean nationals residing in South Korea, and all health care service providers, both hospitals and community-based clinics, are required to belong to the NHIS, except for ∼3% of the population comprising socioeconomically vulnerable people who are covered by the Medical Aid program. All claims data requested from health care providers for the reimbursement of medical costs of subjects who belong to the NHIS or the Medical Aid program are reviewed by a single public institution, the Health Insurance Review & Assessment Service (HIRA). Access to and use of the HIRA data are regulated by the Rules for Data Exploration and Utilization of the HIRA. Accordingly, we used the data after approval from the data access committee of the HIRA (Big Data Division, Healthcare Data Convergence Department, HIRA, Wonju-si, South Korea). All delivered data were anonymized, and any potentially identifying personal information was not disclosed.
Identification of patients with MPNs
Any patient with a record of diagnostic codes for MPNs from January 2007 to December 2017 were collected (N = 24 431) to select patients: (1) who were newly diagnosed with 1 of 3 types of MPNs (PV, ET, and PMF) between January 2008 and December 2016; and (2) for whom their disease course could be tracked. We then excluded patients by using several steps to maximize data accuracy. First, patients whose MPN diagnostic code was initiated in 2007 or 2017 for 1 year washes before and after the target period were excluded, respectively (n = 6862). For the second step, patients with only a single recording of the diagnostic codes without any continual claims data with the code thereafter were excluded (n = 7989). Third, patients who had a possibility of diagnosis of myeloid diseases other than MPNs were excluded (n = 582). For the fourth step, patients with a diagnostic code for chronic myeloproliferative diseases (D.47.1) were removed; this code was newly introduced to the Korean Standard Classification of Diseases in 2011 and includes chronic neutrophilic leukemia and other unspecified MPNs (n = 256). Finally, we excluded patients with a diagnostic code for other cancers within −1 year to +30 days of MPN diagnosis (n = 1288) to exclude preceding or simultaneous coprimary cancers. Detailed explanations of these exclusions are summarized in supplemental Figure 1.
Analysis of disease transformation and second malignancies in patients with MPNs
Patients with SMF were identified among patients with PV or ET if their diagnostic code was changed into and then continued as MF. Patients with SAML were identified among the patients with PV, ET, PMF, or SMF if their diagnostic code was changed into and maintained as acute myeloid leukemia. Likewise, occurrences of second primary solid tumors were determined in the same manner.
Patients with MF were classified according to ruxolitinib use. Occurrences of lymphoma were counted in the 2 groups from 1 month after initiation of ruxolitinib (in the ruxolitinib-exposed group) or after diagnosis of MF (in the ruxolitinib-nonexposed group) to the last follow-up. The occurrence of tuberculosis and herpes zoster was estimated in the 2 groups.
Statistical analysis
Descriptive statistics were calculated for the crude incidences and transformation rates. Cumulative incidences were estimated for each event of interest, including SMF, SAML, lymphoma, and second primary solid tumors, with death being set as a competing risk according to the Fine and Gray method. Overall survival (OS) was defined as the time from MPN diagnosis to death from any cause, calculated by using the Kaplan-Meier method and compared by using the log-rank test. The data on the number of solid tumors at each site in the South Korean population from 2008 to 2016 were obtained from the National Cancer Registration Statistics of Korea, and the hazard ratios (HRs) of cancer incidence in the selected MPN patients vs the general South Korean population were calculated after adjusting for age and sex. P values were 2-sided, and the level of significance was chosen as .05. Analyses were conducted by using SAS Enterprise Guide version 6.1 and R statistical software (www.r-project.org).
Results
Initial disease status and transformation in the selected patients with MPNs
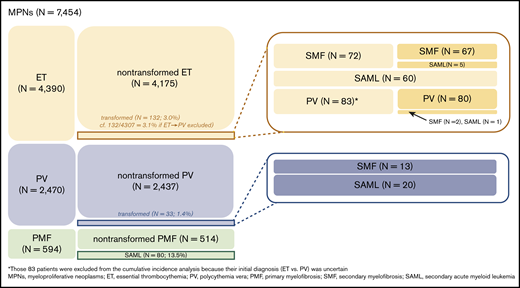
A total of 7454 patients were selected as patients with BCR-ABL1–negative MPNs and were suitable for tracing the disease course (Table 1; supplemental Figure 1). ET (n = 4390; 58.9%) was the most common MPN, followed by PV (n = 2470; 33.1%) and PMF (n = 594; 8.0%). Eighty-three patients initially diagnosed with ET had their diagnosis changed to PV during follow-up; among them, 2 patients had their diagnosis changed to SMF and 1 to SAML. Because the initial diagnosis of 83 patients was ambiguous (ET vs PV), they were excluded from the cumulative incidence analyses (corrected number of ET patients, 4307). Overall, 132 (3.0%) of 4390 ET patients, 33 (1.3%) of 2470 PV patients, and 80 (13.5%) of 594 PMF patients underwent disease transformation to SMF or SAML (Figure 1; supplemental Table 1).
Initial diagnosis of MPNs in the analyzed patients
| . | All MPNs . | ET . | ET, corrected* . | PV . | PMF . |
|---|---|---|---|---|---|
| No. of patients, n (%) | 7454 | 4390 (58.9) | 4307 (57.9) | 2470 (33.1) | 594 (8.0) |
| Age, y | |||||
| Median (range) | 60 (11-106) | 61 (13-106) | 61(13-106) | 58 (11-100) | 63 (21-89) |
| Category, n | |||||
| 0-9 | 0 | 0 | 0 | 0 | 0 |
| 10-19 | 98 | 75 | 75 | 23 | 0 |
| 20-29 | 254 | 167 | 165 | 81 | 6 |
| 30-39 | 573 | 355 | 352 | 189 | 29 |
| 40-49 | 1087 | 615 | 607 | 400 | 72 |
| 50-59 | 1641 | 888 | 867 | 631 | 122 |
| 60-69 | 1664 | 907 | 888 | 590 | 167 |
| 70-79 | 1627 | 1013 | 996 | 447 | 167 |
| ≥80 | 510 | 370 | 357 | 109 | 31 |
| Sex, n | |||||
| Male | 3942 | 1944 | 1912 | 1633 | 365 |
| Female | 3512 | 2446 | 2395 | 837 | 229 |
| Year, n | |||||
| 2008 | 750 | 462 | 448 | 212 | 76 |
| 2009 | 780 | 459 | 443 | 232 | 89 |
| 2010 | 705 | 409 | 397 | 243 | 53 |
| 2011 | 751 | 453 | 444 | 259 | 39 |
| 2012 | 813 | 499 | 491 | 267 | 47 |
| 2013 | 858 | 503 | 496 | 297 | 58 |
| 2014 | 896 | 508 | 498 | 319 | 69 |
| 2015 | 967 | 575 | 569 | 307 | 85 |
| 2016 | 934 | 522 | 521 | 334 | 78 |
| Follow-up period, median (range), mo | 45.63 (6.02-114.61) | 46.74 (6.02-114.61) | 46.38 (6.02-114.61) | 47.40 (6.02-113.68) | 31.78 (6.02-113.78) |
| . | All MPNs . | ET . | ET, corrected* . | PV . | PMF . |
|---|---|---|---|---|---|
| No. of patients, n (%) | 7454 | 4390 (58.9) | 4307 (57.9) | 2470 (33.1) | 594 (8.0) |
| Age, y | |||||
| Median (range) | 60 (11-106) | 61 (13-106) | 61(13-106) | 58 (11-100) | 63 (21-89) |
| Category, n | |||||
| 0-9 | 0 | 0 | 0 | 0 | 0 |
| 10-19 | 98 | 75 | 75 | 23 | 0 |
| 20-29 | 254 | 167 | 165 | 81 | 6 |
| 30-39 | 573 | 355 | 352 | 189 | 29 |
| 40-49 | 1087 | 615 | 607 | 400 | 72 |
| 50-59 | 1641 | 888 | 867 | 631 | 122 |
| 60-69 | 1664 | 907 | 888 | 590 | 167 |
| 70-79 | 1627 | 1013 | 996 | 447 | 167 |
| ≥80 | 510 | 370 | 357 | 109 | 31 |
| Sex, n | |||||
| Male | 3942 | 1944 | 1912 | 1633 | 365 |
| Female | 3512 | 2446 | 2395 | 837 | 229 |
| Year, n | |||||
| 2008 | 750 | 462 | 448 | 212 | 76 |
| 2009 | 780 | 459 | 443 | 232 | 89 |
| 2010 | 705 | 409 | 397 | 243 | 53 |
| 2011 | 751 | 453 | 444 | 259 | 39 |
| 2012 | 813 | 499 | 491 | 267 | 47 |
| 2013 | 858 | 503 | 496 | 297 | 58 |
| 2014 | 896 | 508 | 498 | 319 | 69 |
| 2015 | 967 | 575 | 569 | 307 | 85 |
| 2016 | 934 | 522 | 521 | 334 | 78 |
| Follow-up period, median (range), mo | 45.63 (6.02-114.61) | 46.74 (6.02-114.61) | 46.38 (6.02-114.61) | 47.40 (6.02-113.68) | 31.78 (6.02-113.78) |
CI, confidence interval.
After excluding 83 patients whose initial diagnosis was ET, but was changed to PV during follow-up.
Cumulative incidence of disease transformation and second malignancy
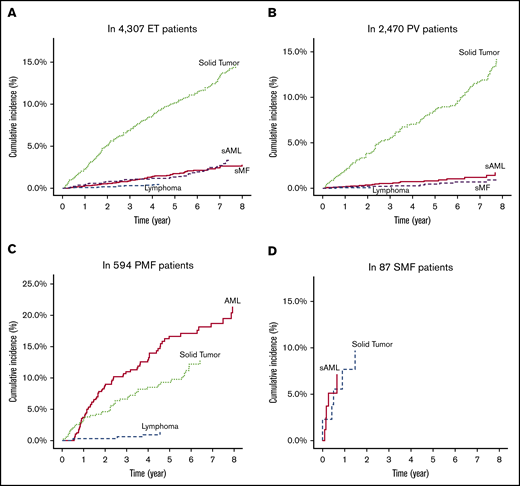
Among the corrected number of 4307 patients with ET (median follow-up period, 46.4 months), the 4- and 8-year cumulative incidence rates were 1.2% and 2.8% for SMF, 1.4% and 3.6% for SAML, 0.4% and 0.5% for lymphoma, and 8.6% and 14.7% for solid tumors, respectively (Figure 2A). Among the 2470 patients with PV (median follow-up period, 47.4 months), the 4- and 8-year cumulative incidence rates were 0.3% and 1.2% for SMF, 0.7% and 1.7% for SAML, 0.1% and 0.1% for lymphoma, and 7.0% and 14.2% for solid tumors (Figure 2B). Among the 594 patients with PMF (median follow-up period, 31.8 months), the 4- and 8-year cumulative incidence rates were 12.9% and 21.4% for SAML, 0.9% and 1.3% for lymphoma, and 8.5% and 12.8% for solid tumors (Figure 2C). Among the 85 patients with SMF (median follow-up period, 10.3 months), the 4-year cumulative incidence rates were 6.8% for SAML, 0% for lymphoma, and 10.3% for solid tumors (Figure 2D).
Cumulative incidences of disease transformation to SMF or acute myeloid leukemia, and development of lymphoid malignancies or solid tumors in the analyzed patients. Patients with essential thrombocythemia (A), polycythemia vera (B), primary myelofibrosis (C), and secondary myelofibrosis (D).
Cumulative incidences of disease transformation to SMF or acute myeloid leukemia, and development of lymphoid malignancies or solid tumors in the analyzed patients. Patients with essential thrombocythemia (A), polycythemia vera (B), primary myelofibrosis (C), and secondary myelofibrosis (D).
Incidence and risk of second primary solid tumors
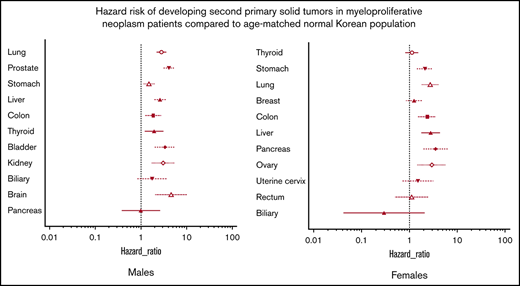
Among the 7454 selected patients with MPNs, 626 were diagnosed with solid tumors during follow-up (supplemental Tables 2-4). Among the male patients with MPNs, the lungs, prostate, and stomach were the most common sites for developing solid tumors, whereas the thyroid, stomach, and lungs were the most common locations in female patients. Patients with MPNs had a significantly increased risk of developing second primary solid tumors compared with the age- and sex-adjusted subjects from the general South Korean population (Table 2). In male patients with MPNs, brain, prostate, bladder, kidney, lung, liver, and stomach cancers had HRs >2.0. Among female patients with MPNs, pancreas, ovary, liver, lung, colon, and stomach cancers had HRs >2.0 (supplemental Figures 2 and 3).
HRs of developing solid tumors as secondary malignancies in 7454 patients with MPNs compared with age- and sex-adjusted subjects from the general South Korean population
| Cancer types and sex . | HR . | Low 95% CI . | High 95% CI . | P . |
|---|---|---|---|---|
| Any type of solid tumors | ||||
| Both sexes | 2.02 | 1.87 | 2.19 | <.0001 |
| Male subjects | 2.19 | 1.98 | 2.43 | <.0001 |
| Female subjects | 1.81 | 1.60 | 2.05 | <.0001 |
| Cancer types and sex . | HR . | Low 95% CI . | High 95% CI . | P . |
|---|---|---|---|---|
| Any type of solid tumors | ||||
| Both sexes | 2.02 | 1.87 | 2.19 | <.0001 |
| Male subjects | 2.19 | 1.98 | 2.43 | <.0001 |
| Female subjects | 1.81 | 1.60 | 2.05 | <.0001 |
Association of exposure to hydroxyurea or anagrelide with disease transformation
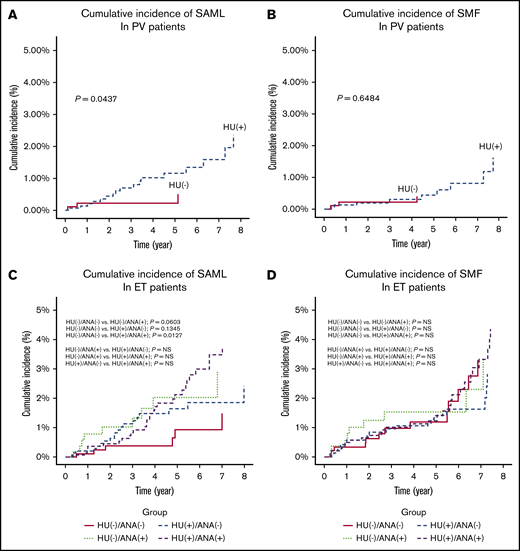
Among the 2470 patients with PV, patients who were exposed to hydroxyurea (HU; n = 1562) reported a higher cumulative incidence of SAML (P = .0437) (Figure 3A) but not SMF (P = .6484) (Figure 3B) than that of patients who were not exposed to HU. Among the 4307 patients with ET, patients exposed to both HU and anagrelide (n = 1433) had a higher cumulative incidence of SAML (n = 899; P = .0127) (Figure 3C) but not SMF (Figure 3D) than that of patients who were exposed to neither HU nor anagrelide.
Cumulative incidences of disease transformation to SMF or acute myeloid leukemia according to exposure to a cytoreductive agent. Cumulative incidence of SAML in PV patients (A), cumulative incidence of SMF in PV patients (B), cumulative incidence of SAML in ET patients (C), and cumulative indicence of SMF in ET patients (D).
Cumulative incidences of disease transformation to SMF or acute myeloid leukemia according to exposure to a cytoreductive agent. Cumulative incidence of SAML in PV patients (A), cumulative incidence of SMF in PV patients (B), cumulative incidence of SAML in ET patients (C), and cumulative indicence of SMF in ET patients (D).
Survival analysis in patients with MF and SAML
There was no difference in the OS between patients with PMF vs patients with SMF (supplemental Figure 4A). Patients with MF who never received a red blood cell (RBC) transfusion exhibited excellent OS compared with those who received any RBC transfusion (P < .001) (supplemental Figure 4B). Among the patients who were exposed to an RBC transfusion, allogeneic hematopoietic stem transplantation improved patient outcomes (P = .052) (supplemental Figure 4C). The median OS of patients with SAML was 7.0 months. Allogeneic hematopoietic stem transplantation led to long-term survival in some patients, but the difference was not statistically significant, probably due to the small number of patients (supplemental Figure 5). Detailed data are summarized in supplemental Table 5.
Impact of ruxolitinib in patients with MF
Among the 681 patients with MF (594 with PMF and 87 with SMF), 192 were administered ruxolitinib. The median time from MF diagnosis to initiation of ruxolitinib was 16.2 months (range, 1-28.8 months). In the ruxolitinib group, only 1 female patient (0.5%) was diagnosed with B-cell non-Hodgkin lymphoma at 6.2 months after the administration of ruxolitinib. In the ruxolitinib-nonexposed group, 4 patients (0.8%) were diagnosed with lymphoma (Table 3). The occurrence rate of tuberculosis was 2.6% (5 of 192) vs 2.3% (11 of 489) in the ruxolitinib exposed group vs the nonexposed group, respectively. The occurrence rate for herpes zoster was 11.5% (22 of 192) vs 8.2% (40 of 489) in the ruxolitinib exposed group vs the nonexposed group.
Development of tuberculosis, herpes zoster, and lymphoma according to ruxolitinib exposure in patients with MF
| . | N . | PMF: SMF . | Male: female . | Follow-up duration, median (range), mo . | No. of patients with tuberculosis* . | No. of patients with herpes zoster* . | No. of patients with lymphoma* . |
|---|---|---|---|---|---|---|---|
| Patients exposed to ruxolitinib | 192 | 161:31 | 125:67 | 16.2 (1.0-28.8)† | 5 (2.6%) | 22 (11.5%) | 1 (0.5%) |
| 2 male subjects and 3 female subjects | 13 male subjects and 9 female subjects | A 54-year-old female patient | |||||
| Median age, 68 y (range, 44-77 y) | Median age, 66.5 y (range, 40-86 y) | Diagnosed with B-cell non-Hodgkin lymphoma | |||||
| Median time from ruxolitinib to tuberculosis, 12.9 mo (range, 1.34-16.38 mo) | Median time from ruxolitinib to herpes zoster, 6.92 mo (range, 0-23.55 mo) | Time from ruxolitinib to diagnosis of lymphoma, 6.2 mo | |||||
| Patients not exposed to ruxolitinib | 489 | 433:56 | 292:197 | 28.78 (1-113.8)‡ | 11 (2.3%) | 40 (8.2%) | 4 (0.8%) |
| 10 male subjects and 1 female subject | 26 male subjects and 14 female subjects | 3 male subjects and 1 female | |||||
| Median age, 76 y (range, 66-87 y) | Median age, 63 y (range, 27-83) | Median age: 71.5 y (range, 54-75 y) | |||||
| Median time from diagnosis of MF to tuberculosis, 19.9 mo (range, 2.3-88.5 mo) | Median time from diagnosis of MF to herpes zoster: 18.8 mo (range, 1.74-81.6 mo) | Median time from diagnosis of MF to lymphoma, 25.7 mo (range, 6.0-54.6 mo) |
| . | N . | PMF: SMF . | Male: female . | Follow-up duration, median (range), mo . | No. of patients with tuberculosis* . | No. of patients with herpes zoster* . | No. of patients with lymphoma* . |
|---|---|---|---|---|---|---|---|
| Patients exposed to ruxolitinib | 192 | 161:31 | 125:67 | 16.2 (1.0-28.8)† | 5 (2.6%) | 22 (11.5%) | 1 (0.5%) |
| 2 male subjects and 3 female subjects | 13 male subjects and 9 female subjects | A 54-year-old female patient | |||||
| Median age, 68 y (range, 44-77 y) | Median age, 66.5 y (range, 40-86 y) | Diagnosed with B-cell non-Hodgkin lymphoma | |||||
| Median time from ruxolitinib to tuberculosis, 12.9 mo (range, 1.34-16.38 mo) | Median time from ruxolitinib to herpes zoster, 6.92 mo (range, 0-23.55 mo) | Time from ruxolitinib to diagnosis of lymphoma, 6.2 mo | |||||
| Patients not exposed to ruxolitinib | 489 | 433:56 | 292:197 | 28.78 (1-113.8)‡ | 11 (2.3%) | 40 (8.2%) | 4 (0.8%) |
| 10 male subjects and 1 female subject | 26 male subjects and 14 female subjects | 3 male subjects and 1 female | |||||
| Median age, 76 y (range, 66-87 y) | Median age, 63 y (range, 27-83) | Median age: 71.5 y (range, 54-75 y) | |||||
| Median time from diagnosis of MF to tuberculosis, 19.9 mo (range, 2.3-88.5 mo) | Median time from diagnosis of MF to herpes zoster: 18.8 mo (range, 1.74-81.6 mo) | Median time from diagnosis of MF to lymphoma, 25.7 mo (range, 6.0-54.6 mo) |
From 1 month after initiation of ruxolitinib (in ruxolitinib-exposed patients) or diagnosis of MF (in the ruxolitinib-nonexposed patients) to last follow-up or death.
From initiation of ruxolitinib to last follow-up or death.
From diagnosis of PMF or SMF to last follow-up or death.
In patients with MF, those who were treated with ruxolitinib reported significantly superior OS compared with those who were not exposed to ruxolitinib (P < .001), although baseline characteristics, including risk factors from the International Prognostic Scoring System (IPSS) or myelofibrosis secondary to PV and ET-prognostic model (MYSEC-PM), could not be compared (supplemental Figure 6). Among 192 patients with MF treated with ruxolitinib, 122 patients eventually discontinued ruxolitinib. During a median follow-up period of 9.5 months, 1- and 2-year OS after discontinuation of ruxolitinib was 70.7% and 58.0%, respectively (supplemental Figure 7).
Discussion
We reported a higher risk of developing SAML in patients with PMF than in those with ET or PV. This finding is consistent with data in a Western population: in the Mayo Clinic cohort, which included 826 patients with MPNs, the median follow-up time for living patients was 17.3 years for ET, 11.8 years for PV, and 7.7 years for PMF. The cumulative incidence rates of SAML were 3.8% for patients with ET (95% cumulative incidence, 1.3-6.2), 6.8% for patients with PV (95% cumulative incidence, 3.3-10.2), and 14.2% for patients with PMF (95% cumulative incidence, 9.5-18.6).11 European studies have reported a 10% to 20% cumulative incidence of SAML in patients with PMF at 10 years, which was also significantly higher than that in patients with PV or ET.12-16 In the current study, patients with ET had a higher cumulative incidence of SAML than that in patients with PV (3.57% vs 1.71% at 8 years). This result is probably because a portion of patients with SAML that evolved from ET would actually be diagnosed with a prefibrotic stage of MF,17 but these patients could not be separated by using diagnostic codes. This hypothesis is supported by findings showing that 5 of 65 patients with SAML that evolved from ET had a fibrotic stage (ie, SMF), but none of the 20 patients with SAML that evolved from PV had a fibrotic stage. An independent diagnostic code for prefibrotic MF is required for future epidemiological and health care research as well as the evidence of its divergent prognosis.17
Although there are significant differences in the incidence of solid tumors between Asian and Western subjects,18 we found that the risk of developing any type of second primary solid tumor in patients with MPNs is significantly higher than that in age- and sex-adjusted subjects from the general population, which is shown here for the first time in an Asian population, to the best of our knowledge. The HR of 2.02 (95% cumulative incidence, 1.87-2.19) found in the current study is even higher than the HRs reported in recent Western studies, which ranged from 1.29 to 1.44.4,5,19,20 The current finding may be supported by the fact that the 8-year cumulative incidence of second primary solid tumors in our patients was ∼14% vs the 12.7%4 and 13.1%20 10-year cumulative incidence rates in the US studies. Additional studies are warranted to define whether Asian populations are more vulnerable to developing second primary solid tumors.
Recently published Swedish studies have reported that cancers of the skin (both non-melanoma skin cancer and melanoma), kidneys, brain, pancreas, lungs, head and neck, and esophagus/stomach had higher standardized incidence ratios among all patients with MPNs compared with the incidence rates of the general population.5 The majority of those sites also had a high HR in our study (supplemental Figures 2 and 3), raising the possibility of an underlying genetic mechanism. Intriguingly, thyroid and breast cancers, 2 of the most common cancers in South Korean female subjects, did not show a significantly higher HR in female patients with MPNs than in the general female population. Breast cancer was also not associated with a higher risk in the Swedish study. Additional confirmation and relevant genetic studies are warranted to determine why cancers of certain sites, such as the kidneys, brain, and lungs, are more likely to occur in patients with MPNs than in the general population.
In the current study, the use of HU was associated with an increased risk of SAML in patients with PV. The use of HU or anagrelide alone was not significantly associated with the incidence of SAML in patients with ET, but patients exposed to both HU and anagrelide had a higher risk of developing SAML than did those who were exposed to neither HU nor anagrelide. Increased blood counts, such as leukocytosis21 or extreme thrombocytosis17 in patients with ET, or white blood cell counts ≥15 × 109/L in patients with PV,22 are known to be risk factors for developing SAML. Therefore, it is not clear whether aggressive disease features are associated with increased blood cell counts, or the potential mutagenic effect of cytoreductive agents is a genuine cause of SAML transformation. The leukemogenic risk related to the use of cytoreductive agents, particularly HU, is currently less advocated but has been controversial for a long time,23 and we cannot make any conclusion from our uncontrolled data. However, it is clear that patients with MPNs who are not exposed to any cytoreductive agents during their disease course have a reduced risk of SAML.
The OS of patients with SMF in the current study was comparable to that of patients with PMF. However, interpretation needs caution because the number of patients with SMF was relatively small (n = 85), and their follow-up duration was short (median, 10.3 months). There are still few data that directly compare OS between PMF vs SMF patients. For an indirect comparison, the median OS of the IPSS study for PMF was 69 months,24 whereas that of the MYSEC-PM study for SMF was 112 months,25 suggesting that patients with SMF may have better OS compared with those with PMF. Masarova et al26 directly compared OS in 755 patients with PMF, 181 with post-PV MF, and 163 with post-ET MF referred to the MD Anderson Cancer Center from 1984 to 2013. The median OS of PMF vs post-PV MF vs post-ET MF was 45 vs 48 vs 73 months, respectively (P < .001), suggestive of more favorable long-term outcomes with post-ET MF. We did not classify SMF patients into post-ET and post-PV MF because of the limited patient numbers. A notable finding from our study is that patients with SMF had a lower 4-year cumulative incidence of developing SAML than that of patients with PMF (6.8% vs 12.9%). This result is in line with the findings from the MYSEC study on SMF,25 in which the most frequent cause of death was nonclonal disease progression (38%) rather than SAML (32%). In contrast, in the DIPSS study (Dynamic International Prognostic Scoring System) on PMF,27 the most common cause of death was SAML (31%), followed by progression of PMF (18%). Considering this difference, patients with SMF may have the potential for longer OS if follow-up duration is extended, because they are under less risk of disease transformation, and nonclonal disease progression would be delayed by the possible higher efficacy of JAK2 inhibitors.28
In South Korea, ruxolitinib has been linked to a higher IPSS24 risk level for patients with MF since 2013. There was a concern that treatment of MF with JAK inhibitors may increase the risk for developing aggressive B-cell lymphoma.29 However, the result was derived from a small number of patients. In addition, several anecdotal reports suggested the association of ruxolitinib with infectious complications, such as tuberculosis30,31 and herpes zoster.32 We could not find any association between the use of ruxolitinib and the development of lymphoma or increased frequencies of tuberculosis or herpes zoster, although the duration of follow-up after ruxolitinib use was relatively short.
We are aware that the current study has limitations. First, because our case identification of patients with MPNs was not based on histological diagnosis but registration of claims data, diagnostic accuracy is to some extent inherently limited. Patients with any reactive increase of blood cell counts or those with other myeloid diseases, including chronic myelogenous leukemia and myelodysplastic syndrome with overlapping feature of MPNs, could be falsely registered with MPN diagnostic codes in the database. To minimize this misidentification, we initially gathered 24 431 patients but finally included only 7454 patients (supplemental Figure 1). However, there may even be a few who were not genuine MPN patients. Accuracy may also have a direct influence on the reported results. For example, we excluded patients (n = 83) who had an initial diagnosis of ET and changed the diagnosis to PV. However, these cases may represent a natural change of the phenotype of ET, and this category of patients may be associated with higher risk of transformation. Thus, a very low number of exclusions might have a strong influence on the results of the study. Second, as we already discussed, patients with a prefibrotic stage of MF could not be discriminated from those with overt PMF or ET. Although the majority of previous MPN epidemiologic studies did not separate these populations,4,5,9,10,33,34 it should be reflected in future studies. Establishing a separate diagnostic code for the prefibrotic stage of MF could be considered. Finally, from the claims data, we could not acquire information on several factors that are known to be crucial to predict prognosis, including mutation status, complete blood cell count, and presence of constitutional symptom.
In summary, disease transformations to SMF or SAML were rare events in patients with ET or PV, but SAML was common in patients with PMF, with an 8-year cumulative incidence of 21.4%. South Korean patients with MPNs had a 2 times higher risk of developing second primary solid tumors than that of the general population. Further genetic analyses for this phenomenon in both Western and Asian subjects are warranted. Regular medical check-ups and preventive measures for second primary cancers should be emphasized for these patient populations.
Acknowledgment
This study was supported by a research fund from Seoul National University Bundang Hospital (13-2018-018).
Authorship
Contribution: S.-M.B. conceived the original idea; J.H., J.M.B., J.Y.L., Y.K., D.-Y.S., J.-O.L., S.M.H., H.S.C., I.K., S.-S.Y., and S.-M.B. contributed to analysis of data and reviewed the manuscript; J.H.L. contributed to data collection and curation; J.H. and J.H.L. conducted statistical analysis and interpretation; and J.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Soo-Mee Bang, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82, 173 Beon-gil, Gumi-ro Bundang-gu, Seongnam-si 13620, Republic of Korea; e-mail: smbang7@snu.ac.kr.
References
Author notes
All data-sharing requests should be sent to the corresponding author, S.-M.B. (smbang7@snu.ac.kr).
The full-text version of this article contains a data supplement.