Key Points
Etranacogene dezaparvovec resulted in clinically relevant increases in FIX activity in severe/moderately severe hemophilia B over 6 months.
Etranacogene dezaparvovec eliminated bleeding and need for FIX replacement in patients with preexisting anti-AAV5 neutralizing antibodies.
Etranacogene dezaparvovec (AMT-061) is a recombinant AAV5 vector including a gene cassette containing the factor IX (FIX) Padua variant under the control of a liver-specific promoter. A phase 2b study was conducted to confirm that a single dose of 2 × 1013 genome copies per kilogram of etranacogene dezaparvovec will result in FIX activity ≥5% 6 weeks after dosing. Secondary end points included FIX activity at other time points, bleed frequency, FIX replacement, and safety. Etranacogene dezaparvovec was administered as a single IV infusion to 3 adults with severe to moderately severe hemophilia B. Before treatment, participants had low levels of preexisting neutralizing antibodies to AAV5. This article reports a planned 26-week interim assessment. At week 6, mean FIX activity was 31% (23.9%-37.8%), increasing to 47% (33.2%-57.0%) at 26 weeks, with 2 subjects exhibiting sustained activity >40%. Consistent with the FIX activity, etranacogene dezaparvovec was associated with a complete bleed cessation with no need for FIX replacement therapy up to 26 weeks. Etranacogene dezaparvovec was generally well tolerated. No clinically significant elevations in levels of liver enzymes or inflammatory markers were observed, and no use of corticosteroids related to treatment was required. In individuals with severe to moderately severe hemophilia B, etranacogene dezaparvovec resulted in clinically relevant increases in FIX activity, cessation of bleeds, and abrogation of the need for FIX replacement, despite the presence of preexisting anti-AAV5 neutralizing antibodies detected by using a highly sensitive luciferase assay. Consistency of results in the 3 participants supported an expanded evaluation of the safety/efficacy of etranacogene dezaparvovec in the HOPE-B (Health Outcomes With Padua Gene; Evaluation in Hemophilia-B) phase 3 trial. The current trial was registered at www.clinicaltrials.gov as #NCT03489291.
Introduction
The current standard of care for people with hemophilia B (PWH), namely exogenous clotting factor replacement, has undergone improvements over recent decades but remains suboptimal in terms of progressive joint damage, the potential for inhibitor development, poor compliance with therapy, and reduced quality of life due to the need for lifelong injections.1 Gene therapy is an attractive therapeutic strategy for PWH because it addresses these limitations by offering the potential for stable long-term expression of endogenous clotting factor activity with a single treatment. Studies have indicated that gene transfer for severe (factor IX [FIX] activity <1% of normal) or moderately severe (FIX <2%) hemophilia B offers the potential to shift the disease to a milder phenotype and reduce or abrogate the bleed risk and FIX concentrate consumption.2-5 Higher levels of FIX activity are likely to have a profound impact on bleed prevention, health outcomes, and quality of life of affected individuals. Experience with clotting factor prophylaxis suggests that even a small rise in circulating clotting factor activity (eg, targeting trough activity values >1%) can substantially ameliorate spontaneous bleeding.6,7 Current prophylactic factor replacement strategies aim to minimize time spent <1% FIX plasma activity and can thereby improve, although not completely avert, the subsequent long-term joint arthropathy outcomes.8-10 Toward that end, relatively higher steady-state levels of FIX activity, now achievable with gene transfer, are likely to have a profound impact on bleeding frequency. Epidemiological data indicate that higher levels of endogenous clotting factor activity are associated with substantial reduction or elimination of joint bleeds and factor usage.11,12 Data from a cohort study of individuals with mild and moderate hemophilia B suggest that a level of 20% is associated with near elimination of predicted bleeds (0.6 bleed/year vs 2.8 bleeds/year at 5% FIX activity).12
Recently, efficacy and safety data have been reported with AMT-060 (adeno-associated virus 5 [AAV5]-wild-type [wt] FIX) in a phase 1/2 trial (CT-AMT-060-01) comprising 2 dose cohorts in 10 subjects with hemophilia B.2,13 Notably, endogenous FIX activity was established in all 10 patients without stimulating a cytotoxic capsid–directed T-cell response, and 9 of the 10 patients receiving FIX at study entry stopped prophylaxis. Mean FIX activity over 3 years was 7.5% in the higher dose cohort.13 Annualized FIX use was reduced by ≥78% each year in this cohort, and the mean annualized spontaneous bleeding rate decreased to 0.5 in the second year of follow-up. A post hoc analysis of samples from the participating subjects, using a highly sensitive luciferase assay,14 indicated that titers of preexisting antibodies against AAV5 had no inhibitory effect on the ability of the gene transfer to establish FIX activity.15 This finding suggests that it may be possible to achieve clinically meaningful outcomes in PWH with neutralizing antibody (NAb) titers at levels generally found in the population. Enabling the inclusion of PWH positive for anti-AAV5 NAbs based on the highly sensitive luciferase assay may therefore open new opportunities for individuals previously ineligible to undergo gene transfer.
To retain the favorable characteristics of AMT-060 but provide FIX activity in a range predicted to completely eliminate bleeding and preserve joint function, the AMT-060 FIX transgene cassette was modified with a single amino acid change (R338L) previously shown to result in a highly active FIX protein (etranacogene dezaparvovec [AMT-061]).5,16,17 This naturally occurring variant, termed FIX Padua, has been reported to have a FIX activity-to-protein ratio of ∼6 in animal models and between 5 and 10 in humans.5,16,18 The change to human FIX (hFIX) Padua was expected to result in higher levels of FIX activity with the same dose of vector and thereby further alleviate the bleed risk and need for exogenous therapy for etranacogene dezaparvovec compared with AMT-060. The switch of transgene was not expected to influence other previously reported safety characteristics of AAV5-wt FIX at the established dose of 2 × 1013 genome copies (gc) per kilogram.2 This phase 2b study was proposed by uniQure and supported by the US Food and Drug Administration and the European Medicines Agency to address the change of transgene construct and will inform the dose choice in phase 3. Therefore, the primary aim of the current study was to confirm that a single dose of 2 × 1013 gc/kg of etranacogene dezaparvovec would result in FIX activity levels ≥5% by 6 weeks follow-up. If confirmed, this will represent an advance in the development of gene therapies as it will show that a prospective design modification to a clinically characterized AAV vector platform is able to generate the desired change in humans. Secondary aims include assessing the impact of this change in vector transgene on the efficacy and safety of etranacogene dezaparvovec. This report represents data from a planned 26-week interim analysis.
Methods
Study design and participants
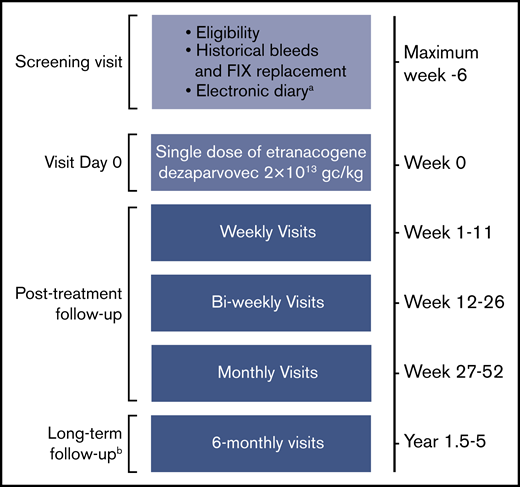
This open-label, single-dose, multicenter phase 2b study included 3 adults with moderate to severe hemophilia B (FIX activity ≤2% of normal) receiving either prophylactic FIX, or on-demand FIX with ≥4 bleeds/year or chronic hemophilic arthropathy (defined as pain, joint destruction, and loss of range of motion in ≥1 joint as assessed by individual investigators) (clinicaltrials.gov #NCT03489291). The primary efficacy assessment in this planned interim analysis is at 6 weeks (Figure 1). The overall study duration will be 52 weeks, with additional long-term follow-up assessments over 4 years. The current article reports a planned interim assessment at 26 weeks.
Study design.aRecording of bleeds and FIX replacement before, and after, etranacogene dezaparvovec treatment. Assessment of bleeds and FIX replacement before screening were based on medical records. bNo e-diary recording during long-term follow-up.
Study design.aRecording of bleeds and FIX replacement before, and after, etranacogene dezaparvovec treatment. Assessment of bleeds and FIX replacement before screening were based on medical records. bNo e-diary recording during long-term follow-up.
Full inclusion/exclusion criteria are listed in supplemental Table 1. Individuals who had detectable anti-AAV5 antibody titers, measured by using a sensitive luciferase-based NAb assay,14,15 were included. The study was approved by the institutional review board/institutional ethics committee at each center, and all participants provided written informed consent. The trial was performed according to the Declaration of Helsinki and the principles of Good Clinical Practice.
Etranacogene dezaparvovec
Etranacogene dezaparvovec is a recombinant AAV5 vector including a gene cassette containing the FIX Padua variant16 of a codon-optimized human FIX complementary DNA under the control of a liver-specific promoter. Except for the inclusion of the FIX Padua variant, etranacogene dezaparvovec is similar to AMT-060 (AAV5-wt FIX) in that it uses the same AAV5 vector and liver-specific promoter. AMT-060 has been extensively tested in a phase 1/2 clinical trial.2,13 Etranacogene dezaparvovec was manufactured in accordance with Good Manufacturing Practices by using insect cell cultures and a baculovirus expression system.19 Vector titer was measured by using a conventional quantitative polymerase chain reaction–based approach with plasmid DNA as the primary reference. This approach is comparable with those reported for the characterization of internationally accepted AAV reference standards.20,21
Anti-AAV5 antibody measurements
Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) was performed at a central laboratory, as recently published by Majowicz et al.15 Briefly, diluted serum samples were incubated on AAV5-coated immunosorbent plates for 1 hour at room temperature. After washing, anti-human immunoglobulin M (IgM) or IgG-conjugated horseradish peroxidase was added, which binds to the Fc region of any bound IgM or IgG. After a further wash, a horseradish peroxidase substrate was added, enabling antibody levels to be deduced by colorimetry. Specificity for AAV5 was confirmed by preincubating the test sera with excess AAV5 before the ELISA.
Luciferase assay
The luciferase assay was performed at a central laboratory, as recently published by Majowicz et al.15 Briefly, serial dilutions of test sera were incubated with an AAV5-based reporter vector carrying the luciferase gene. The test sera mixtures were then incubated for 2 hours with HEK293T cells in 96-well plates to allow the AAV5-reporter vector to transduce cells. The supernatants of each well were then replaced by cell culture medium and incubated overnight. Wells were analyzed for luciferase expression. The anti-AAV5 NAb titer was determined by using LabKey software analysis (https://www.labkey.com).
Procedures
Etranacogene dezaparvovec was administered as a single, 500-mL IV infusion of 2 × 1013 gc/kg infused over 1 hour to the 3 participants. Participants were monitored for 24 hours after administration and later weekly until week 12, every other week from week 12 to 26, and monthly thereafter to week 52. Individuals were dosed 14 calendar days apart to allow for appropriate safety monitoring. At baseline, participants received a single dose of short-acting FIX (40 IU/kg) that provided sufficient FIX coverage for 2 to 3 days posttreatment. At day 3, an additional dose of short-acting FIX could be administered at the investigator’s discretion. FIX prophylaxis could be restarted if FIX activity was <2% in ≥2 consecutive central laboratory measurements. FIX prophylaxis could also be re-initiated on the basis of clinical judgment and participant preference if endogenous FIX activity was between 2% and 5%. Treatment with prednisone was not recommended routinely but could be administered if alanine aminotransferase (ALT) elevations ≥twofold over baseline levels were observed.
Outcome measures
The primary outcome was to confirm whether a single dose of 2 × 1013 gc/kg of etranacogene dezaparvovec would result in FIX activity levels ≥5% at 6 weeks after dosing. Secondary efficacy end points comprised FIX activity at other time points, bleeding rates, and the use of FIX replacement therapy. FIX activity was assessed at the central laboratory by using the one-stage activated partial thromboplastin time assay (HemosIL SynthASIL, Instrumentation Laboratory, Bedford, MA). From screening onward, subjects recorded their FIX replacement therapy utilization and bleeding episodes in a dedicated e-diary, which was regularly reviewed by investigators. Before the screening period, bleeds and FIX replacement in the year before etranacogene dezaparvovec treatment were assessed by reviewing medical records. Secondary safety end points included adverse events (AEs), hematology and serum chemistry parameters, ALT and aspartate aminotransferase (AST) levels, corticosteroid use for ALT and/or AST elevations, parameters on antibody formation to AAV5 and hFIX, enzyme-linked immunospot for AAV5 capsid specific T-cell response, inflammatory markers (ELISA, interferon-γ, interleukin [IL]-1β, IL-2, IL-6, and monocyte chemoattractant protein-1 [MCP-1]), and vector DNA in semen and blood. Preexisting antibodies to AAV5 were analyzed by using a sensitive in vitro reporter system as previously described.14,15
Data analysis
This report represents a planned 26-week interim analysis with data current to March 21, 2019; further analyses of this same population are planned to provide a total of 5 years of follow-up. It should be noted that this is a confirmatory trial to support the innovative transition from the phase 1/2 AMT-060 trial to the phase 3 etranacogene dezaparvovec trial based on the prospective design change in the AMT-060 vector construct to use the FIX Padua variant. This trial was therefore performed to confirm the safety and efficacy of the 2 × 1013 gc/kg dose of etranacogene dezaparvovec for subsequent assessment in the HOPE-B (Health Outcomes With Padua Gene; Evaluation in Hemophilia-B) phase 3 clinical trial that is currently enrolling.22 From a clinical perspective, 3 subjects was considered sufficient to provide a reliable assessment of FIX activity at 6 weeks after dosing. No formal sample size calculation was performed. Considering the small sample size, no formal statistical analyses were performed, no analysis populations were defined, and all results are reported by using descriptive statistics.
Results
Demographic and baseline characteristics
Three individuals were screened and underwent etranacogene dezaparvovec treatment. At the time of this interim analysis, all participants remained in the study and were undergoing continuing assessment. All participants had severe to moderately severe hemophilia B, required regular FIX prophylaxis before etranacogene dezaparvovec treatment, and had preexisting NAbs to AAV5 (Table 1). None of the participants had target joints.
Baseline characteristics
| Characteristic . | Participant . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Age, y | 43 | 50 | 47 |
| Weight, kg | 89 | 81 | 82 |
| HIV status | Negative | Positive, controlled | Positive, controlled |
| Hep B/Hep C | Hep B negative | Hep B negative | Hep B negative |
| Hep C; resolved | Hep C; resolved | Hep C; resolved | |
| Hemophilia B status | FIX = 1% | FIX <1% | FIX <1% |
| Prescreening FIX regimen | Prophylaxis (EHL FIX) | Prophylaxis (EHL FIX) | Prophylaxis (EHL FIX) |
| Annualized bleed rate 1 y before screening* | 3 | 1 | 5 |
| NAb titer (AAV5) (luciferase assay)† | Positive | Positive | Positive |
| 48 | 44 | 25 | |
| Characteristic . | Participant . | ||
|---|---|---|---|
| 1 . | 2 . | 3 . | |
| Age, y | 43 | 50 | 47 |
| Weight, kg | 89 | 81 | 82 |
| HIV status | Negative | Positive, controlled | Positive, controlled |
| Hep B/Hep C | Hep B negative | Hep B negative | Hep B negative |
| Hep C; resolved | Hep C; resolved | Hep C; resolved | |
| Hemophilia B status | FIX = 1% | FIX <1% | FIX <1% |
| Prescreening FIX regimen | Prophylaxis (EHL FIX) | Prophylaxis (EHL FIX) | Prophylaxis (EHL FIX) |
| Annualized bleed rate 1 y before screening* | 3 | 1 | 5 |
| NAb titer (AAV5) (luciferase assay)† | Positive | Positive | Positive |
| 48 | 44 | 25 | |
EHL, extended half-life; Hep, hepatitis.
Total bleeds (treated + untreated).
AAV5 NAb is considered positive if titer is ≥2.15
Endogenous FIX activity after gene transfer
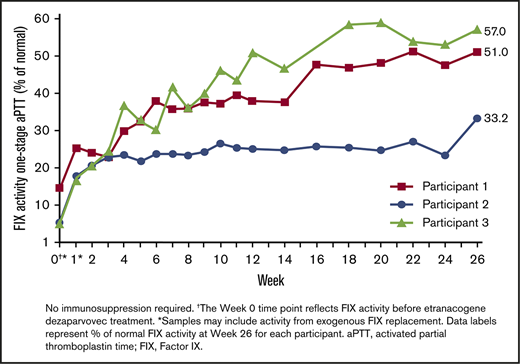
Before etranacogene dezaparvovec treatment, all participants had endogenous FIX activity ≤1% (Table 1). FIX activity in the first week of follow-up was potentially contaminated by exogenous short-acting FIX that was administered at baseline as recommended by the protocol. Increases in FIX activity were observed from the first uncontaminated measurement at week 2 post–gene transfer with a one-stage activated partial thromboplastin time assay. At week 6, mean FIX activity was 31% of normal with individual levels of 37.8%, 23.9%, and 30.0% in participants 1 through 3, respectively, thus fulfilling the primary end point (Figure 2; supplemental Figure 1). At 12 weeks, mean FIX activity was 38.0% with individual levels of 37.9%, 24.9%, and 51.1% in participants 1 through 3. FIX activity continued to increase to the interim follow-up at 26 weeks (mean activity, 47%; Participant 1, 51.0%; Participant 2, 33.2%; and Participant 3, 57.0%).
FIX activity measured by using a one-stage activated partial thromboplastin time (aPTT) assay. Individual FIX activity posttreatment (one-stage aPTT). No immunosuppression required. †The week 0 time point reflects FIX activity before etranacogene dezaparvovec treatment. *Samples may include activity from exogenous FIX replacement. Data labels represent the percentage of normal FIX activity at week 26 for each participant.
FIX activity measured by using a one-stage activated partial thromboplastin time (aPTT) assay. Individual FIX activity posttreatment (one-stage aPTT). No immunosuppression required. †The week 0 time point reflects FIX activity before etranacogene dezaparvovec treatment. *Samples may include activity from exogenous FIX replacement. Data labels represent the percentage of normal FIX activity at week 26 for each participant.
Use of exogenous FIX concentrate and bleeding events
In the year before etranacogene dezaparvovec treatment, all participants received prophylactic FIX replacement as well as additional doses of FIX to treat bleeding events. Medical records from that prior year indicated that Participants 1, 2, and 3 experienced 3, 1, and 5 bleeds requiring FIX treatment, respectively. Participant 3 also reported a suspected bleed during the screening period.
After etranacogene dezaparvovec treatment, there were no reported bleeds and no requirement for FIX replacement up to 26 weeks. As permitted by the protocol, Participant 1 received 2 doses of short-acting FIX (day of dosing and day 3 posttreatment), whereas the remaining participants both received 1 dose of short-acting FIX on the day of dosing. Apart from this, no further FIX infusions were administered.
Safety
Two AEs judged to be possibly related to etranacogene dezaparvovec were reported, both in Participant 1. The first was a transient, self-limiting headache on day of dosing and the second was a mild elevation in C-reactive protein level on day 14 posttreatment (7.4 mg/L; reference range, 0-3 mg/L) that resolved without intervention. There were no serious AEs or deaths during the study.
Liver biomarkers
No clinically significant signals in liver transaminase levels were observed after etranacogene dezaparvovec administration. Participant 1 had normal transaminase levels at screening for study eligibility, but later analysis of the baseline samples indicated that ALT (109 U/L; reference range, 6-41 U/L) and AST (113 U/L; reference range, 9-34 U/L) levels were elevated before the gene transfer procedure on the day of etranacogene dezaparvovec dosing. ALT levels remained raised at week 1 (53 U/L; reference range, 6-41 U/L) but was within normal limits by week 2. At week 22, ALT increased to 44 U/L (normal range, 5-41 U/L), but this resolved without intervention, was not associated with an elevated AST level, and was not considered to be clinically significant (supplemental Figure 1). Participant 2 had transient elevated AST levels at weeks 2 and 4 (43 and 48 U/L, respectively), which resolved quickly without additional treatment. These AST elevations in Participant 2 were not accompanied by ALT elevations. ALT and AST levels were within the reference range at all time points in Participant 3. There was no requirement for steroid treatment to address liver enzyme elevations; however, Participant 3 required a short course of prednisone (50 mg daily for 5 days starting at day 94) for bronchitis.
Immune responses and inflammatory markers
All 3 participants had NAbs to AAV5 at screening (titers 48, 44, and 25 in Participants 1, 2, and 3, respectively; titers >2 considered positive based on assay-derived parameters) detected by using a sensitive luciferase-based assay. Based on ELISA data, no participants had anti-AAV5 IgG antibodies at screening or baseline. Participant 1 had anti-AAV5 IgM at screening and baseline. There were no detectable anti-FIX antibodies or FIX inhibitors at screening or during the study.
Participant 1 had a slightly elevated MCP-1 level before treatment at week 0 (816 pg/mL; reference range, 200-722 pg/mL) that returned to normal by week 2. At week 8, there was a further isolated elevation in MCP-1 above normal (748 pg/mL) in Participant 1 that returned to normal levels by week 9 (supplemental Table 2) and did not seem to affect clinical outcomes. There were no increases above the reference range in the other inflammatory markers tested (interferon-γ, IL-1β, and IL-6) in Participant 1. Neither of the other participants had elevations in any of the tested inflammatory markers at any time point. No T-cell–mediated anti-AAV5 capsid responses were detected in viable samples from any of the participants collected during weeks 1 through 26. There was no loss of FIX activity in any of the participants (Figure 2).
Clearance of vector DNA
By week 26, Participants 1 and 2 had levels of vector DNA below the limit of detection in semen samples, although the criteria for clearance were not achieved because negative results from 3 consecutive samples were required for confirmation. For Participant 3, data for vector shedding in semen were not available at week 26, and vector DNA was still detected at week 12. Vector DNA was detectable in the blood of all 3 participants at week 26, although for Participant 2 the level was less than the lower limit of quantification for this assay.
Discussion
In this dose confirmation study, 3 participants with severe to moderately severe hemophilia B (FIX ≤1%) and low titers of preexisting anti-AAV5 NAb, who experienced 1 to 5 bleeds in the year before treatment despite routine FIX prophylaxis, each received a single infusion of 2 × 1013 gc/kg of etranacogene dezaparvovec. This dose met the primary end point of FIX activity >5% at 6 weeks, with a mean activity of 31% (range, 23.9%-37.8%). By the 26-week interim time point, mean FIX activity was 47% (range, 33.2%-57.0%). Because FIX activity was still increasing to the 26-week interim time point, longer follow-up will be needed to confirm steady-state FIX activity. Consistent with the observed FIX activity, etranacogene dezaparvovec treatment was associated with a complete cessation of bleeds up to the 26-week interim end point. In addition, except for protocol-recommended administration at dosing and on day 3 after treatment in 1 participant, there was no requirement for FIX replacement therapy during follow-up.
As predicted, FIX activity levels after etranacogene dezaparvovec administration were several-fold higher than those observed with AAV5-wt FIX.2 It has been reported previously that the Padua hFIX variant has a FIX activity-to-protein ratio of between 5 and 10 in humans,16,18 which agrees with the findings in this study. Achieving higher FIX activity by combining a previously described AAV5 vector platform and promotor with the Padua hFIX variant codon-optimized transgene (etranacogene dezaparvovec), rather than the wt FIX codon-optimized transgene (AMT-060), confirms the ability to obtain prospectively engineered improvements after a logical change in the design of a gene transfer construct. Furthermore, this initial follow-up indicates a safety and immunogenicity profile that seems similar to that previously reported for AMT-060.2
Elevations in liver biomarker levels have been observed generally in the first 2 to 3 months after liver-directed gene transfer in previous trials and, in some cases, these elevations were associated with reductions in FIX activity.3-5 Although this study included a limited number of participants, there was no indication of clinically relevant liver toxicity. There was an isolated and transient ALT elevation in Participant 1 at a time point beyond the usual critical window for events, and Participant 2 had transient elevations in AST but not ALT. Although these isolated findings of transaminitis occurred in 2 patients, both events were considered unrelated to etranacogene dezaparvovec, resolved without intervention, and did not affect FIX activity. It should be noted, however, that liver enzyme elevations were observed in 3 of 10 participants who were treated with AMT-060, which shares the same AAV5-vector as etranacogene dezaparvovec.2 Hence, the liver safety profile of etranacogene dezaparvovec will be explored more fully in the ongoing phase 3 study.
None of the 3 participants in this trial required corticosteroids related to treatment. Immune responses to gene transfer, indicated by liver transaminase elevations, and generally treated with corticosteroids, were observed in ∼20% to 40% of participants in trials in hemophilia B2-5 and 89% in a trial in hemophilia A.23 Corticosteroid treatment is associated with a range of potential adverse effects, including risks of infection, diabetes, fracture, hypertension, venous thromboembolism, avascular necrosis, and osteoporosis24 ; these kinds of AEs have not been reported in gene transfer trials that used corticosteroids, however. Short-term use for <30 days at doses as low as 20 mg/d may elevate the risk of developing sepsis, venous thromboembolism, and/or fractures.24 Many PWH contracted hepatitis and HIV infections through contaminated blood products in the 1980s,25 and corticosteroids may be of particular concern because their use can trigger the reactivation of dormant hepatitis infections.26
All 3 participants had NAbs to AAV5 at screening detected by using a highly sensitive in vitro AAV5-luciferase transgene assay (titers 24 to 48).14,15 Using the same highly sensitive luciferase assay to assess the AMT-060 phase 1/2 trial, 3 of 10 participants had NAbs to AAV5 at titers ranging from 21 to 340 without affecting clinical outcomes.15 The same study then examined NAbs to AAV5 in a cohort of 100 healthy male subjects and found that 53% had titers <2 (indicating no detectable anti-AAV5 NAb) and 92% had titers <340. This scenario suggests that the majority of people in the general population have AAV5 titers below the level that would interfere with AAV5-mediated gene transfer. Data in non-human primates (NHP) from the same study indicated that titers up to 1030 (measured with the highly sensitive assay) did not interfere with gene transfer. If the NHP findings can be extrapolated to humans, then based on the seroprevalence data, 97% of the general population would not be excluded from AAV5-based gene transfer on the basis of NAb titer. This finding is of particular interest as many trials exclude participants on the basis of preexisting NAbs, although there is currently no standardized protocol in place for determining NAbs. Indeed, 2 of the participants in this trial had failed screening for another gene transfer trial due to NAbs to the vector serotype used in that study. For AAV8, a titer of 1:5 blocked liver transduction in NHP.27 For AAV2, a NAb titer of 1:17, but not 1:2, adversely affected titration efficacy in human participants.28 Therefore, although it is difficult to compare studies due to the different NAb assays used, it is possible that low-level titers of AAV2 and AAV8 NAbs are more likely to impair transduction efficacy for vectors based on their respective AAV serotype. The reason for this potential difference between AAV vector serotypes is unclear and is likely to be the subject of further studies. Given that immunity to naturally occurring AAV serotypes is common, the ability to include participants with generally prevalent titers of preexisting NAbs to AAV5 based on the luciferase assay and achieve clinically important outcomes is of particular interest in terms of extending access to gene transfer.
Two AEs were possibly related to etranacogene dezaparvovec, both in Participant 1 (transient, self-limiting headache on the day of dosing, and a mild elevation in C-reactive protein level on day 14). The data are insufficient to speculate on the cause of these AEs.
In conclusion, etranacogene dezaparvovec gene transfer resulted in sustained, clinically relevant increases in endogenous FIX activity without requirement for immunosuppression and was associated with cessation of both clinically apparent bleeds and the need for FIX replacement over the follow-up period. These results were observed in participants with preexisting AAV5 NAbs, suggesting that commonly encountered levels of NAbs detected by the luciferase assay may not be a barrier to AAV5-mediated gene transfer. Moreover, the safety and tolerability of etranacogene dezaparvovec seemed to be in line with other liver-directed hemophilia gene therapies and, unlike comparators,3-5 no elevations in liver enzymes that required corticosteroids were observed in this small cohort. If these results are confirmed in the phase 3 trial, physicians and PWH will be provided with increased confidence that reliable clinical outcomes can be achieved after gene transfer. Although these observations are limited to 3 participants, the consistency of results among participants supports the ongoing phase 3 HOPE B trial, which will further assess the safety and efficacy of etranacogene dezaparvovec at the 2 × 1013 gc/kg dose in ∼50 individuals with severe or moderately severe hemophilia B.22
Acknowledgments
The authors thank the individuals who participated in the study, the study site staff, and the uniQure clinical operations team (Stephanie Verweij, Emily Skelton, Lindsay Toto, and Ilse Tuinhof).
This study was supported by research funding from uniQure B.V. Michael Lappin, a medical writer supported by funding from uniQure, provided drafts and editorial assistance to the authors during preparation of the manuscript.
Authorship
Contribution: All authors were involved with the trial, provided critical revisions at all stages of the manuscript preparation, provided final approval before submission, and agreed to be accountable for the work.
Conflict-of-interest disclosure: A.V.D. has received honoraria for participating in scientific advisory board panels, consulting, and speaking engagements from uniQure, Biomarin, Novo Nordisk, Bayer, Bioverativ/Sanofi, Spark Therapeutics, HEMA Biologics, Pfizer, CSL Behring, Sanofi, Shire, and Takeda; receives research funding from Bioverativ/Sanofi, and Pfizer; and is a cofounder and a member of the Board of Directors of Hematherix LLC., a biotech company that is developing superFVa therapy for bleeding complications. A.G. received honoraria for advisory board meetings from uniQure, Bioverativ, Genentech/Roche, Biomarin, and Novo Nordisk; and speaker’s fees from Bioverativ, Genentech/Roche, and Alexion. G.C. participated in the Advisory Board of uniQure; received fees as a speaker or to participate in Advisory Boards from Bayer, Shire/Takeda, CSL Behring, Sobi, Novo Nordisk, Roche, Werfen, Ablynx, and Kedrion; and received research grants directly to his institution from Pfizer, Sobi, and CSL Behring. N.S.K. has received consultant fees from uniQure for participation on the Steering Committee for this study. S.L. reports consultant fees from uniQure during the conduct of the study. F.W.G.L. has received unrestricted research grants from CSL Behring and Shire/Takeda; is a consultant for UniQure, Shire, and Novo Nordisk, of which the fees go to the university; and is a Data and Safety Monitoring Board member for a study by Roche. W.M. has received consultant fees from UniQure. B.V. during the conduct of the study; grants and personal fees from Novo Nordisk; and personal fees from Bayer, Shire, Biotest, Pfizer, Octapharma, LFB, CSL Behring, SOBI, Biogen, and BPL outside the submitted work. M.R. has received research support for his institution from Bioverativ, Genentech, Novo Nordisk, and Shire; reports consultant fees from Bioverativ, CSL Behring, Genentech, Kedrion, Novo Nordisk, Pfizer, Shire, and uniQure; and is the immediate past chair, Board of Directors, American Thrombosis and Hemostasis Network. A.L., R.G., and E.K.S. are uniQure employees. S.W.P. has served as a consultant to ApcinteX, Bayer, Biomarin, Bioverativ, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, Roche/Genentech, Sanofi, Shire, Spark Therapeutics, and uniQure.
Correspondence: Steven W. Pipe, Special Coagulation Laboratory, University of Michigan, 1500 E Medical Center Dr, Ann Arbor, MI 48109-5718; e-mail: ummdswp@med.umich.edu.
References
Author notes
The full-text version of this article contains a data supplement.