Key Points
Breath analysis is a feasible novel method to detect and potentially monitor graft-versus-host disease.
Abstract
Volatile organic compounds (VOCs) are generated during pathologic processes, and their assessment can be used to diagnose and monitor a variety of diseases. Given the role of the microbiome in graft-versus-host disease (GVHD), we hypothesized that microorganisms producing volatile metabolites may alter VOCs expelled in breath in patients with gastrointestinal (GI) GVHD. In this pilot study, exhaled breath samples were obtained from 19 patients with grade 2 to 4 acute GI GVHD, 10 patients with no GVHD at day 100, and 10 healthy control subjects; the samples were analyzed by using mass spectrometry. Overall, nine (47%) patients had grade 2 GVHD, eight (42%) patients had grade 3 GVHD, and two (11%) patients had grade 4 GVHD; 26% had upper GI, 21% had lower GI, and 53% had both upper and lower GI manifestations. Stepwise canonical discriminant analysis identified 5 VOCs distinguishing patients with and without GI GVHD: 2-propanol, acetaldehyde, dimethyl sulfide, isoprene, and 1-decene (Wilks’ Λ, 0.43; F statistic, 6.08; P = .001). The model correctly classified 89% (17 of 19) and 90% (9 of 10) of patients with and without GI GVHD, respectively. Breath analysis is a feasible and promising noninvasive method to detect acute GI GVHD. Further study of serial breath analysis and the gut microbiome in a larger cohort are ongoing to validate these findings.
Introduction
Gastrointestinal graft-versus-host disease (GI GVHD) remains a significant cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1,2 Early diagnosis and identification of patients with severe symptoms remain challenging. Endoscopy with biopsy is the current standard; however, pathology is often equivocal, cannot distinguish medication effect or infection, and does not predict severity or survival.3 Proteomic profiling has recently identified several blood biomarkers (eg, TNFR1, REG3α, ST2) that can predict therapy response and nonrelapse mortality and have been evaluated in clinical trials.4,5 Their role is less clear, however, as a diagnostic tool for acute GVHD, and there remains a need to quickly identify high-risk disease.
There are increasing data on the role of the intestinal microbiome in GI GVHD.6 Various microorganisms are known to produce volatile metabolites.7,8 Thus, abnormalities in the activity and composition of intestinal microbiota in disease states may alter the organic compounds produced and ultimately expelled in breath.
Mass spectrometry and gas chromatography can identify unique volatile organic compounds (VOCs), and breath analysis may offer a rapid, noninvasive method for detecting and monitoring diseases.8,,-11 Investigation of the breath metabolome in inflammatory bowel disease (IBD) has shown high accuracy in differentiating IBDs and non-IBDs.12,13 We hypothesized that patients with GI GVHD will have a unique breath signature compared with patients without GVHD, and we therefore performed a pilot study of breath analysis in HCT patients.
Methods
This prospective, single-institution pilot study enrolled patients from January 2015 to April 2017. Key inclusion criteria included age ≥12 years, ability to provide consent and an adequate breath sample, and no active respiratory infection. Patients could have received HCT for any diagnosis, with any conditioning, and with any donor source. Patients had a diagnosis of grade 2 to 4 GI acute GVHD (case cohort) or no evidence of any GVHD by day 100 (HCT control cohort). Results of a confirmatory biopsy were preferred, but a clinical diagnosis of grade 3 to 4 acute GVHD was allowed. Breath samples were obtained within 7 days of initiation of systemic front-line therapy within the GVHD cohort. As a comparator, breath samples were also obtained on 10 transplant recipients with no evidence of any GVHD by day 100. Breath samples were obtained within a window of ±14 days of day 100 among control subjects. Breath samples from 10 healthy control subjects, including health care staff and caregivers who were present in a similar environment (eg, inpatient or at home), were also obtained to evaluate potential background environmental factors. This study was approved by the Cleveland Clinic Institutional Review Board.
Exhaled breath samples were collected as previously described.12 Quantitative assessment of prespecified VOCs, including 2-propanol, acetaldehyde, acetone, acrylonitrile, benzene, carbon disulfide, dimethyl sulfide, ethanol, isoprene, pentane, 1-decene, 1-heptene, 1-nonene, 1-octene, 3-methylhexane, (E)-2-nonene, ammonia, ethane, hydrogen sulfide, triethylamine, and trimethylamine, was performed. Mass scanning peaks were assessed to identify significant peaks that may represent unknown VOCs associated with GVHD. More accurate concentration data were obtained by selected ion monitoring of VOC product ions.8
Baseline characteristics and VOCs were compared between GVHD and no GVHD by using Fisher’s exact test or the Wilcoxon rank sum test. Stepwise linear discriminant analysis was used to identify VOCs that could distinguish GI GVHD and no GVHD, and was incorporated into a canonical discriminant analysis (CDA) model. Logarithmic transformation of each VOC was used for analysis. Results of the model are shown as a scatter plot of the first 2 canonical components. Model performance was assessed by estimating the probability of misclassification and using cross-validation. CDA was also used to distinguish GVHD severity and therapeutic response at day 28. Data were analyzed by using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
There were no significant differences in baseline characteristics between patients with and without GI GVHD (Table 1). One patient enrolled in the GVHD arm was determined not to have GI GVHD and was excluded from analysis; 19 patients were therefore included. Eight patients with GI GVHD had GI symptoms as their sole manifestation, 6 had concurrent liver involvement, 4 had concurrent skin involvement, and 1 patient had both skin and liver involvement. All patients received prophylactic antibiotics per our standard protocols with trimethoprim-sulfamethoxazole from start of conditioning through engraftment, followed by amoxicillin or azithromycin while on immunosuppression. Patients with GI GVHD (n = 9) were more likely to be exposed to broad-spectrum antibiotics (eg, piperacillin-tazobactam [n = 8], meropenem [n = 1], cefepime or ceftazidime [n = 3], vancomycin [n = 5]) at the time of breath sampling compared with those without GVHD (n = 0). Six of these 10 patients without GVHD, however, had relatively recent exposure to broad-spectrum antibiotics within the first 100-day time period.
Patient characteristics
| Variable . | Control Subjects (n = 10) . | No GVHD (n = 10) . | GI GVHD (n = 19) . | P . |
|---|---|---|---|---|
| Sex | .41 | |||
| Male | 1 (10) | 8 (80) | 11 (58) | |
| Female | 9 (90) | 2 (20) | 8 (42) | |
| Race | .53 | |||
| White | 9 (90) | 10 (100) | 17 (89) | |
| Other | 1 (10) | – | 2 (11) | |
| Age at transplant, median (range), y | 55 (43-71) | 58 (13-67) | .89 | |
| Comorbidity index (HCT–comorbidity index) | .54 | |||
| Low risk (0) | 3 (30) | 3 (16) | ||
| Intermediate risk (1-2) | 3 (30) | 9 (47) | ||
| High risk (≥3) | 4 (40) | 7 (37) | ||
| Diagnosis | .15 | |||
| AML | 3 (30) | 11 (58) | ||
| MDS | 3 (30) | 3 (16) | ||
| ALL | – | 3 (16) | ||
| Other | 4 (40) | 2 (10) | ||
| Donor | .25 | |||
| MUD | 3 (30) | 10 (53) | ||
| MSD | 6 (60) | 4 (21) | ||
| Haploidentical | 1 (10) | 3 (16) | ||
| Cord | – | 2 (11) | ||
| Conditioning intensity | .43 | |||
| Myeloablative | 7 (70) | 9 (47) | ||
| Reduced intensity | 3 (30) | 10 (53) | ||
| Source of stem cells | .32 | |||
| Peripheral blood | 4 (40) | 11 (58) | ||
| Bone marrow | 6 (60) | 6 (32) | ||
| Cord blood | – | 2 (11) | ||
| GVHD prophylaxis | .34 | |||
| CSA or FK/MMF | 4 (40) | 10 (53) | ||
| FK/MMF/posttransplant cyclophosphamide | 1 (10) | 3 (16) | ||
| FK/MTX | – | 2 (11) | ||
| Clinical trial* | 5 (50) | 4 (21) | ||
| Grade of GVHD | – | |||
| 0 (none) | 10 (100) | – | ||
| Grade II | – | 9 (47) | ||
| Grade III | – | 8 (42) | ||
| Grade IV | – | 2 (11) | ||
| GVHD site | – | – | ||
| Lower | 4 (21) | |||
| Upper | 5 (26) | |||
| Both | 10 (52) | |||
| Time to GVHD onset, median (range), d | – | 43 (19-160) | – |
| Variable . | Control Subjects (n = 10) . | No GVHD (n = 10) . | GI GVHD (n = 19) . | P . |
|---|---|---|---|---|
| Sex | .41 | |||
| Male | 1 (10) | 8 (80) | 11 (58) | |
| Female | 9 (90) | 2 (20) | 8 (42) | |
| Race | .53 | |||
| White | 9 (90) | 10 (100) | 17 (89) | |
| Other | 1 (10) | – | 2 (11) | |
| Age at transplant, median (range), y | 55 (43-71) | 58 (13-67) | .89 | |
| Comorbidity index (HCT–comorbidity index) | .54 | |||
| Low risk (0) | 3 (30) | 3 (16) | ||
| Intermediate risk (1-2) | 3 (30) | 9 (47) | ||
| High risk (≥3) | 4 (40) | 7 (37) | ||
| Diagnosis | .15 | |||
| AML | 3 (30) | 11 (58) | ||
| MDS | 3 (30) | 3 (16) | ||
| ALL | – | 3 (16) | ||
| Other | 4 (40) | 2 (10) | ||
| Donor | .25 | |||
| MUD | 3 (30) | 10 (53) | ||
| MSD | 6 (60) | 4 (21) | ||
| Haploidentical | 1 (10) | 3 (16) | ||
| Cord | – | 2 (11) | ||
| Conditioning intensity | .43 | |||
| Myeloablative | 7 (70) | 9 (47) | ||
| Reduced intensity | 3 (30) | 10 (53) | ||
| Source of stem cells | .32 | |||
| Peripheral blood | 4 (40) | 11 (58) | ||
| Bone marrow | 6 (60) | 6 (32) | ||
| Cord blood | – | 2 (11) | ||
| GVHD prophylaxis | .34 | |||
| CSA or FK/MMF | 4 (40) | 10 (53) | ||
| FK/MMF/posttransplant cyclophosphamide | 1 (10) | 3 (16) | ||
| FK/MTX | – | 2 (11) | ||
| Clinical trial* | 5 (50) | 4 (21) | ||
| Grade of GVHD | – | |||
| 0 (none) | 10 (100) | – | ||
| Grade II | – | 9 (47) | ||
| Grade III | – | 8 (42) | ||
| Grade IV | – | 2 (11) | ||
| GVHD site | – | – | ||
| Lower | 4 (21) | |||
| Upper | 5 (26) | |||
| Both | 10 (52) | |||
| Time to GVHD onset, median (range), d | – | 43 (19-160) | – |
Data are presented as n (%) unless otherwise indicated.
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CSA, cyclosporine; FK, tacrolimus; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MSD, matched sibling donor; MUD, matched unrelated donor; MTX, methotrexate.
Clinical trial: 3 patients in the non-GVHD group and 3 patients in the GVHD group received FK/MMF/mini-dose MTX on a clinical trial; 2 patients in the non-GVHD group received FK/MTX/bortezomib on the BMT CTN 1203 trial, and 1 patient in the GVHD group received FK/MTX/maraviroc on the BMT CTN 1203 trial.
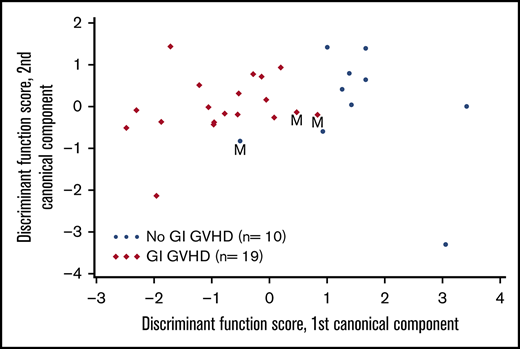
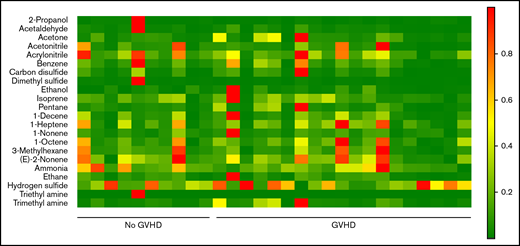
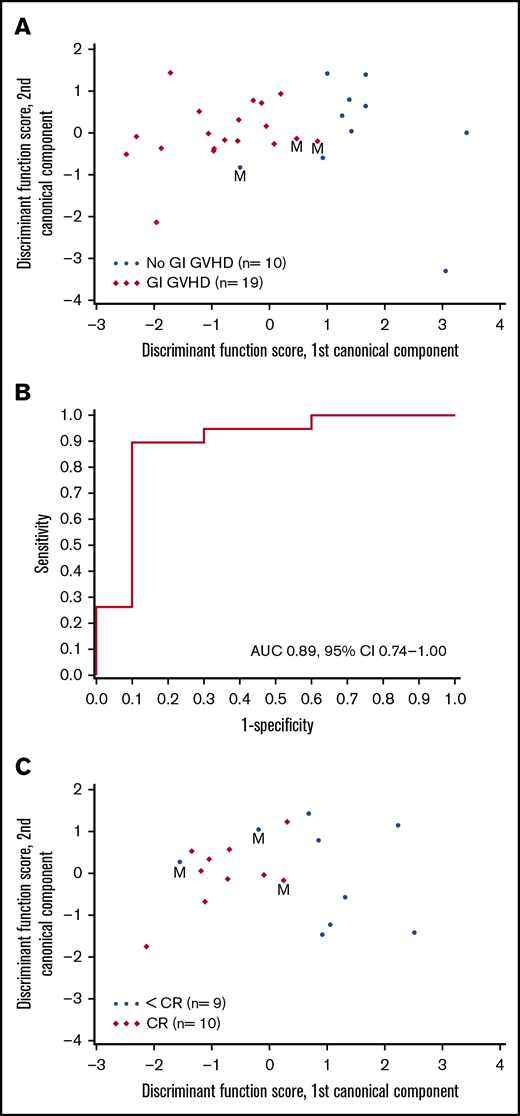
VOCs were compared among patients with GI GVHD, without GVHD, and healthy volunteers. Relative concentrations of 22 VOCs were compared among patients with and without GVHD (Figure 1). Discriminant analysis was performed to assess the combination of a set of VOCs to distinguish GVHD. According to CDA, 5 VOCs differentiated between GI GVHD and no GVHD: 2-propanol, acetaldehyde, dimethyl sulfide, isoprene, and 1-decene (Wilks’ Λ, 0.431; F statistic, 6.08; P = .001). The model correctly classified 89% (17 of 19) and 90% (9 of 10) of patients with and without GI GVHD, respectively (Figure 2A-B). Considering sample size limitation, we assessed the correlation between VOCs and GVHD severity. No VOC concentrations were found to have a significant association with GVHD grade (range, 0.24-0.32; P ≥ .09).
Heat map of mass scans for the relative concentrations of 22 VOCs in 19 patients with GI GVHD and 10 without GI GVHD. Green indicates low concentrations and red indicates higher concentrations.
Heat map of mass scans for the relative concentrations of 22 VOCs in 19 patients with GI GVHD and 10 without GI GVHD. Green indicates low concentrations and red indicates higher concentrations.
Canonical discriminant analysis of VOCs to distinguish GVHD and response. (A) Canonical discriminant analysis used 5 VOCs to classify patients as GI GVHD or no GI GVHD. Discriminant function scores based on linear combinations of these 5 VOCs are shown for 29 patients. Three patients were misclassified (M) by using this discriminant model. (B) Receiver-operating characteristic curve for the discriminant analysis model that used the 5 VOCs to classify patients as GI GVHD or no GVHD. (C) CDA used 2 VOCs to classify day 28 response in patients with GI GVHD. Discriminant function scores based on linear combinations of these 2 VOCs are shown for 19 patients. Three patients were misclassified (M) by using this discriminant model. AUC, area under the curve; CI, confidence interval.
Canonical discriminant analysis of VOCs to distinguish GVHD and response. (A) Canonical discriminant analysis used 5 VOCs to classify patients as GI GVHD or no GI GVHD. Discriminant function scores based on linear combinations of these 5 VOCs are shown for 29 patients. Three patients were misclassified (M) by using this discriminant model. (B) Receiver-operating characteristic curve for the discriminant analysis model that used the 5 VOCs to classify patients as GI GVHD or no GVHD. (C) CDA used 2 VOCs to classify day 28 response in patients with GI GVHD. Discriminant function scores based on linear combinations of these 2 VOCs are shown for 19 patients. Three patients were misclassified (M) by using this discriminant model. AUC, area under the curve; CI, confidence interval.
VOCs were also analyzed to evaluate their association with day 28 response. Of 19 patients, 10 patients achieved complete response (CR), 3 patients had a partial response (PR), and 6 had no response. CDA identified 2 VOCs (pentane and ammonia) distinguishing patients with CR and less than CR (Wilks’ Λ, 0.570; F statistic, 6.05; P = .011). This model correctly classified 90% (9 of 10) of patients with CR and 78% (7 of 9) of patients with less than CR (Figure 2C). CDA identified 1 VOC (acetone) distinguishing patients with any response (CR + PR) and no response. Patients with higher levels of log(acetone) were less likely to have a response (odds ratio, 0.22 per 1 unit increase; 95% confidence interval, 0.06-0.82; P = .024).
Discussion
The current pilot study provides preliminary evidence that breath VOCs may correlate with GI GVHD diagnosis and response. VOCs are a diverse and abundant group of carbon-based volatile chemicals. They are emitted from a variety of body excreta (eg, breath, feces, urine, blood) and have been studied as biomarkers in several diseases.14,15 VOCs are believed to be endogenously metabolized and released by both human and bacterial cells, or produced from exogenous sources (eg, medications, gaseous exposures).15 Major metabolic themes arising from VOC identification are bacterial fermentation, fatty acid and carbohydrate metabolism, and changes induced by reactive oxygen species.16 Because the GI microbiota contributes to many metabolic functions,17 the resulting gas produced could be a reflection of microbial metabolic activity and may serve as a specific biomarker of intestinal disease. Indeed, increasing data have shown that microbial dysbiosis is associated with GVHD,18 and microbial metabolites, reflected by breath VOCs, may play an important role in identifying GVHD.19 Although the significance of “GVHD VOCs” identified in the current study (2-propanol, isoprene, acetaldehyde, dimethyl sulfide, and 1-decene) has yet to be fully elucidated, previous data suggest that several compounds may be a product of cholesterol metabolism generated by intestinal microbiota (eg, isoprene).12,20
There are several limitations to acknowledge. This analysis was a single-institution pilot study showing the feasibility of obtaining breath VOCs in patients with and without GI GVHD. VOCs were determined at a single cross-sectional time point. Data have shown that microbial diversity changes throughout the transplant course at different time points. Given the limitations and small numbers enrolled in this prospective pilot study, however, we were unable to match control, non-GVHD time points to case, GI GVHD time points. Because the clustering of cohorts in the CDA models regardless of time point at which GVHD developed, we believe results of this study provide sufficient evidence for further future investigation that will entail serial, longitudinal collection of samples.
In addition, although we obtained and compared breath samples from control subjects and from different settings (inpatient/outpatient), we may not have fully accounted for all confounding exposures. The contribution of 2-propanol, for instance, must be further confirmed because this compound is known to be found throughout the environment (eg, cleaning products, hand sanitizers, lotions). Although we found no significant differences between cohorts, breath samples were all obtained within our health care settings. Although the significance of 2-propanol remains unclear, its inclusion provided a better predictive model than any other model excluding 2-propanol (data not shown), and it thus requires additional study. Furthermore, given this was a pilot study with small numbers, we were unable to formally analyze the impact of different medications (in particular, antimicrobial agents), which may also significantly influence differences in VOCs.21,22 We acknowledge that approximately one-half of patients with GI GVHD in our cohort were exposed to broad-spectrum antibiotics at the time of breath sampling. In contrast, no patients without GVHD had an indication for the use of extended-spectrum antibiotics, although approximately one-half of these patients (n = 6) also had relatively recent exposure to piperacillin-tazobactam. The impact of antimicrobial agents on VOCs also requires further investigation in a larger study.
In conclusion, this pilot study shows the feasibility of obtaining breath samples in HCT patients and provides preliminary evidence that the presence of a VOC footprint may hold potential as a surrogate marker for GVHD. A prospective study is planned that will collect longitudinal breath and stool samples in transplant recipients to confirm these findings and correlate breath VOCs with intestinal dysbiosis.
Acknowledgment
This work was supported by a Lerner Research Institute of the Cleveland Clinic Research Programs Grant.
Authorship
Contribution: B.K.H. designed the study, analyzed and interpreted data, and wrote the manuscript; N.S.M. and R.A.D. designed the study, interpreted data, and critically reviewed the manuscript; L.A.R. provided statistical support and reviewed the manuscript; D.G. processed samples and analyzed and interpreted results; C.F., J.S., B.H., J.E., V.W., and D.C. collected samples and performed data collection; A.T.G., R.H., M.E.K., and R.M.S. contributed patients to the study and critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Betty K. Hamilton, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: hamiltb2@ccf.org.