Key Points
Late acute and chronic GVHD are experienced differently across the races in terms of incidence, sites, severities, and clinical outcomes.
Japanese patients have more frequent liver and less frequent gastrointestinal involvement with late acute and NIH chronic GVHD.
Abstract
Although differences in the incidence of chronic graft-versus-host disease (GVHD) across the races have been suggested, these have not been systematically investigated. This study compared the incidence, sites, severity, and outcomes of late acute GVHD and chronic GVHD according to National Institutes of Health (NIH) consensus criteria between Japanese (n = 413) and white (n = 708) patients after first allogeneic hematopoietic cell transplantation. Analysis was stratified according to bone marrow transplantation (BMT) or peripheral blood stem cell transplantation (PBSCT). Japanese patients, compared with white patients, had a similar incidence of late acute GVHD (BMT, 19% vs 16%; PBSCT, 19% vs 16%) but experienced more frequent liver late acute GVHD as defined by transaminase elevation (BMT, 79% vs 8%; PBSCT, 92% vs 33%) and less frequent gastrointestinal late acute GVHD (BMT, 11% vs 58%; PBSCT, 20% vs 68%). Japanese patients were more likely to discontinue systemic immunosuppression after late acute GVHD than white patients (hazard ratio, 3.68; 95% confidence interval, 1.96-6.94; P < .001). Japanese patients, compared with white patients, had a lower incidence of chronic GVHD (BMT, 15% vs 30% [P = .002]; PBSCT, 37% vs 45% [P < .001]) and experienced more frequent chronic GVHD of the mouth, eyes, and liver and less frequent gastrointestinal chronic GVHD. The duration of immunosuppressive treatment of NIH chronic GVHD was similar between the races. These differences could not be entirely attributed to practice variation between the centers. This study shows that the incidence, affected sites, severity, and clinical outcomes of late acute GVHD and NIH chronic GVHD differ between Japanese and white patients.
Introduction
Chronic graft-versus-host disease (GVHD) is a common complication after allogeneic hematopoietic cell transplantation (HCT), occurring in as many as 50% of HCT recipients.1 Chronic GVHD has marked impacts on quality of life and is the leading cause of treatment-related mortality among HCT survivors who are otherwise cured of their disease.2,3 The most frequent manifestations at diagnosis include oral and ocular sicca, skin rash, and transaminase elevation.4 More recently, National Institutes of Health (NIH) consensus criteria have been developed and refined to establish standard methods for the diagnosis, grading of severity, and measurement of response to treatment of chronic GVHD, providing opportunities to compare outcomes among different centers and countries.5,6 Late acute (LA) GVHD is recognized as a separate entity from NIH chronic GVHD, with specific clinical features in the skin, gut, and liver.6 Our knowledge of LA GVHD and NIH chronic GVHD is based largely in white populations.7,,-10 Although a previous study showed that Japanese and Scandinavian patients had a lower risk of classic acute GVHD than other races,11 it is largely unknown how the characteristics and outcomes of LA GVHD and NIH chronic GVHD differ among the races.
The current study compared the characteristics and outcomes of LA GVHD and NIH chronic GVHD between Japanese and white patients. The results of this study will help inform physicians who take care of Japanese and possibly other East Asian patients with LA or NIH chronic GVHD.
Patients and methods
The study cohort included 1121 consecutive adult patients who had a first allogeneic HCT for hematological malignancy at 2 centers: 413 Japanese patients who underwent HCT between January 2006 and December 2013 at the National Cancer Center Hospital (NCCH) in Japan and 708 white patients who underwent HCT between January 2006 and December 2010 at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA) in the United States. Race was determined by self-identification. Patients who had bone marrow transplantation (BMT) or peripheral blood stem cell transplantation (PBSCT) were included. Patients who received antithymocyte globulin, sirolimus, or posttransplant cyclophosphamide as GVHD prophylaxis were excluded because the number of these patients was small during the study period and could confound the interpretation of study results.12,-14 Patients had given written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki. This study was approved by the institutional review board of the NCC and the FHCRC.
Definitions
LA GVHD was defined as GVHD that occurred beyond 100 days after HCT but did not meet the diagnostic criteria of NIH chronic GVHD.5 Staging and grading of classic acute GVHD and LA GVHD were made according to the 1994 consensus criteria.15 Chronic GVHD was diagnosed and scored by using the NIH consensus criteria.5 Organ involvement was defined as NIH organ score ≥1 except for the lungs. Lung involvement was defined for patients with bronchiolitis obliterans syndrome according to the NIH consensus criteria.6 Liver staging was based on serum total bilirubin values for LA GVHD and on values of serum total bilirubin, alanine aminotransferase, and alkaline phosphatase for NIH chronic GVHD. Relapse was defined by either morphological or cytogenetic evidence of disease recurrence, or radiologic evidence of recurrent or progressive lymphoma after HCT. Nonrelapse mortality (NRM) was defined as death without recurrent primary disease. Discontinued systemic treatment was defined as the discontinuation and subsequent lack of systemic immunosuppressive treatment of at least 6 months. The intensity of conditioning regimens was defined as described elsewhere.16 The HCT–comorbidity index was scored as described elsewhere.17 Disease risk was defined according to the refined Disease Risk Index.18
Statistical analysis
Analyses were stratified according to stem cell source because this factor has a large influence on outcomes.19 Cumulative incidence frequencies of LA GVHD, NIH chronic GVHD, and discontinued systemic treatment were calculated by using Gray’s method. In the analysis of GVHD, relapse and death before the onset of GVHD were considered as competing events. In the analysis of discontinued systemic treatment after LA GVHD, relapse, NRM, and progression to NIH chronic GVHD were considered as competing events. In the analysis of discontinued systemic treatment after NIH chronic GVHD, relapse and NRM were considered as competing events. Cox proportional hazards models were used for multivariate analysis of overall mortality. Fine-Gray proportional hazards models were used for multivariate analysis of events with competing risks. Logistic regression models were used for examining associations with individual organ involvement. Covariates included race, stem cell source, patient age, patient sex, HCT–comorbidity index, refined Disease Risk Index, donor type, HLA matching, donor–patient sex combination, conditioning intensity, and GVHD prophylaxis. A backward elimination procedure was used in developing final models, using a P value threshold of .05. Race was retained in all models. Two-sided P < .05 was considered statistically significant. Data were analyzed as of June 2017.
Results
Patient characteristics in the whole cohort
Patient characteristics showed several differences between the races (Table 1). BM was the most frequent stem cell source in Japanese patients (283 of 413 [69%]), whereas PBSC was the predominant stem cell source in white patients (639 of 708 [90%]). In the BMT group, Japanese patients had more malignant lymphoma, lower comorbidity at HCT, more HLA-mismatched HCT, and less combination from a female donor to a male patient. Notably, all white BMT recipients had myeloablative conditioning, whereas some Japanese patients had reduced-intensity conditioning. In the PBSCT group, Japanese patients were younger, had more malignant lymphoma, and had lower comorbidity at HCT. Notably, few Japanese patients received mycophenolate mofetil as GVHD prophylaxis for PBSCT, and few received unrelated PBSC grafts during the study period. The median duration of follow-up among survivors was 59 months (range, 3.3-121 months) in Japanese patients and 88 months (range, 22-126 months) in white patients.
Patient characteristics
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 283) . | White (n = 69) . | P . | Japanese (n = 130) . | White (n = 639) . | P . | |
| Patient age, median (range), y | 45 (18-69) | 47 (18-67) | .25 | 44 (18-67) | 55 (18-75) | <.001 |
| Sex | .10 | .77 | ||||
| Male | 170 (60) | 49 (71) | 74 (57) | 375 (59) | ||
| Female | 113 (40) | 20 (29) | 56 (43) | 264 (41) | ||
| Disease | <.001 | <.001 | ||||
| AML | 120 (42) | 32 (46) | 49 (38) | 220 (34) | ||
| MDS/MPN | 27 (10) | 10 (14) | 15 (12) | 141 (22) | ||
| ALL | 41 (14) | 14 (20) | 14 (11) | 57 (9) | ||
| CML/CLL | 3 (1) | 12 (17) | 2 (2) | 67 (10) | ||
| Malignant lymphoma | 91 (32) | 1 (1) | 50 (38) | 100 (16) | ||
| Plasma cell neoplasms | 1 (< 1) | 0 (0) | 0 (0) | 54 (8) | ||
| Disease Risk Index | .001 | .15 | ||||
| Low | 23 (8) | 14 (20) | 11 (8) | 88 (14) | ||
| Intermediate | 182 (64) | 29 (42) | 75 (58) | 318 (50) | ||
| High/very high | 78 (28) | 26 (38) | 44 (34) | 233 (36) | ||
| HCT–comorbidity index at transplantation | <.001 | <.001 | ||||
| 0-1 | 232 (82) | 19 (28) | 109 (84) | 135 (21) | ||
| ≥2 | 51 (18) | 47 (68) | 21 (16) | 463 (72) | ||
| Unknown | 0 (0) | 3 (4) | 0 (0) | 41 (6) | ||
| Donor type | 1.0 | <.001 | ||||
| Related | 20 (7) | 4 (6) | 127 (98) | 275 (43) | ||
| Unrelated | 263 (93) | 65 (94) | 3 (2) | 364 (57) | ||
| HLA matching | .01 | 1.0 | ||||
| Match | 152 (54) | 49 (71) | 109 (84) | 533 (83) | ||
| Mismatch | 131 (46) | 20 (29) | 22 (16) | 106 (17) | ||
| Donor–patient sex combination | .008 | .32 | ||||
| Female donor to male patient | 42 (15) | 20 (29) | 36 (28) | 151 (24) | ||
| Others | 241 (85) | 49 (71) | 94 (72) | 488 (76) | ||
| Conditioning intensity | <.001 | .50 | ||||
| Myeloablative | 177 (63) | 69 (100) | 71 (55) | 328 (51) | ||
| Reduced intensity | 106 (37) | 0 (0) | 59 (45) | 311 (49) | ||
| GVHD prophylaxis | .71 | <.001 | ||||
| CNI alone | 6 (2) | 0 (0) | 40 (28) | 8 (1) | ||
| CNI + MTX | 267 (94) | 67 (97) | 86 (66) | 284 (44) | ||
| CNI + MMF | 10 (4) | 2 (3) | 4 (3) | 347 (54) | ||
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 283) . | White (n = 69) . | P . | Japanese (n = 130) . | White (n = 639) . | P . | |
| Patient age, median (range), y | 45 (18-69) | 47 (18-67) | .25 | 44 (18-67) | 55 (18-75) | <.001 |
| Sex | .10 | .77 | ||||
| Male | 170 (60) | 49 (71) | 74 (57) | 375 (59) | ||
| Female | 113 (40) | 20 (29) | 56 (43) | 264 (41) | ||
| Disease | <.001 | <.001 | ||||
| AML | 120 (42) | 32 (46) | 49 (38) | 220 (34) | ||
| MDS/MPN | 27 (10) | 10 (14) | 15 (12) | 141 (22) | ||
| ALL | 41 (14) | 14 (20) | 14 (11) | 57 (9) | ||
| CML/CLL | 3 (1) | 12 (17) | 2 (2) | 67 (10) | ||
| Malignant lymphoma | 91 (32) | 1 (1) | 50 (38) | 100 (16) | ||
| Plasma cell neoplasms | 1 (< 1) | 0 (0) | 0 (0) | 54 (8) | ||
| Disease Risk Index | .001 | .15 | ||||
| Low | 23 (8) | 14 (20) | 11 (8) | 88 (14) | ||
| Intermediate | 182 (64) | 29 (42) | 75 (58) | 318 (50) | ||
| High/very high | 78 (28) | 26 (38) | 44 (34) | 233 (36) | ||
| HCT–comorbidity index at transplantation | <.001 | <.001 | ||||
| 0-1 | 232 (82) | 19 (28) | 109 (84) | 135 (21) | ||
| ≥2 | 51 (18) | 47 (68) | 21 (16) | 463 (72) | ||
| Unknown | 0 (0) | 3 (4) | 0 (0) | 41 (6) | ||
| Donor type | 1.0 | <.001 | ||||
| Related | 20 (7) | 4 (6) | 127 (98) | 275 (43) | ||
| Unrelated | 263 (93) | 65 (94) | 3 (2) | 364 (57) | ||
| HLA matching | .01 | 1.0 | ||||
| Match | 152 (54) | 49 (71) | 109 (84) | 533 (83) | ||
| Mismatch | 131 (46) | 20 (29) | 22 (16) | 106 (17) | ||
| Donor–patient sex combination | .008 | .32 | ||||
| Female donor to male patient | 42 (15) | 20 (29) | 36 (28) | 151 (24) | ||
| Others | 241 (85) | 49 (71) | 94 (72) | 488 (76) | ||
| Conditioning intensity | <.001 | .50 | ||||
| Myeloablative | 177 (63) | 69 (100) | 71 (55) | 328 (51) | ||
| Reduced intensity | 106 (37) | 0 (0) | 59 (45) | 311 (49) | ||
| GVHD prophylaxis | .71 | <.001 | ||||
| CNI alone | 6 (2) | 0 (0) | 40 (28) | 8 (1) | ||
| CNI + MTX | 267 (94) | 67 (97) | 86 (66) | 284 (44) | ||
| CNI + MMF | 10 (4) | 2 (3) | 4 (3) | 347 (54) | ||
CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasms; MTX, methotrexate.
Characteristics of LA GVHD at onset
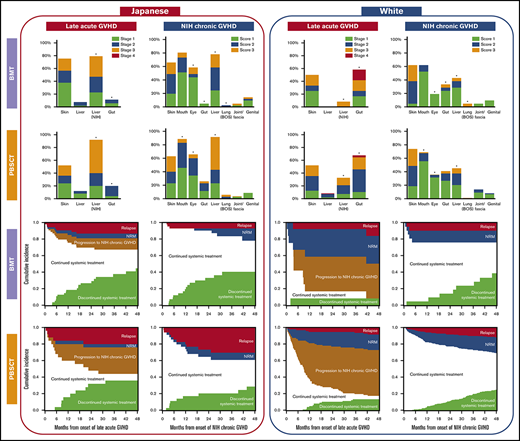
The cumulative incidence of LA GVHD after BMT was 19% (95% confidence interval [CI], 15-24) at 48 months among Japanese patients, which was similar to that among white patients (16%; 95% CI, 8-26; P = .60) (Figure 1). The cumulative incidence of LA GVHD after PBSCT was 19% (95% CI, 13-27) at 48 months among Japanese patients, which was similar to that among white patients (16%; 95% CI, 13-18; P = .25).
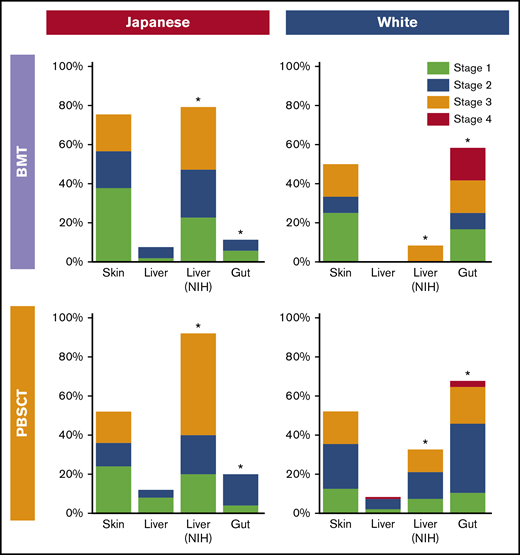
Characteristics of LA GVHD were compared between the races according to stem cell sources (Table 2). In the BMT group, Japanese patients had more frequent liver involvement per the NIH liver score, less frequent gastrointestinal involvement, lower grades of LA GVHD, a greater number of involved sites, and higher Karnofsky Performance Status at onset. In the PBSCT group, Japanese patients had more late onset, less recurrent onset, more frequent liver involvement per the NIH liver score, less frequent gastrointestinal involvement, and lower grades of LA GVHD at onset. Other characteristics did not differ statistically between the 2 races, including the incidence of previous classic acute GVHD, prednisone dose at onset, and the incidence of thrombocytopenia and hyperbilirubinemia. The organ stages of LA GVHD were compared between the races (Figure 2). Compared with white patients, Japanese patients had more severe liver LA GVHD per the NIH liver score both after BMT (P < .001) and PBSCT (P < .001), and less severe gastrointestinal LA GVHD both after BMT (P < .001) and PBSCT (P = .001).
Characteristics of late acute GVHD at onset
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 53) . | White (n = 12) . | P . | Japanese (n = 25) . | White (n = 96) . | P . | |
| Months from transplantation to late acute GVHD, median (IQR) | 5.6 (4.4-8.3) | 5.4 (4.1-6.5) | .51 | 5.8 (4.3-6.9) | 4.7 (3.5-6.5) | .06 |
| Type | .34 | <.001 | ||||
| Late onset | 15 (26) | 2 (17) | 13 (52) | 12 (13) | ||
| Recurrent | 31 (58) | 10 (83) | 9 (36) | 81 (84) | ||
| Persistent | 7 (13) | 0 (0) | 3 (12) | 3 (3) | ||
| Sites involved | ||||||
| Skin | 40 (75) | 6 (50) | .16 | 13 (52) | 50 (52) | 1.0 |
| Liver (per the 1994 consensus) | 4 (8) | 0 (0) | 1.0 | 3 (12) | 8 (8) | .70 |
| Liver (per the 2005 NIH liver score) | 42 (79) | 1 (8) | <.001 | 23 (92) | 31 (33) | <.001 |
| Gastrointestinal tract | 6 (11) | 7 (58) | .001 | 5 (20) | 65 (68) | <.001 |
| Grade of late acute GVHD | .024 | .001 | ||||
| Grade II or lower | 47 (89) | 7 (58) | 20 (80) | 39 (41) | ||
| Grade III or higher | 6 (11) | 5 (42) | 5 (20) | 57 (59) | ||
| No. of involved sites | .025 | .44 | ||||
| 1 | 21 (40) | 10 (83) | 11 (44) | 54 (56) | ||
| 2 | 29 (54) | 2 (17) | 12 (48) | 33 (34) | ||
| 3 | 3 (6) | 0 (0) | 2 (8) | 9 (9) | ||
| Prior IIb-IV classic acute GVHD | 29 (55) | 5 (42) | .53 | 9 (36) | 45 (47) | .37 |
| Karnofsky Performance Status | .028 | .34 | ||||
| 80%-100% | 42 (79) | 6 (50) | 16 (64) | 46 (48) | ||
| <80% | 11 (21) | 5 (42) | 9 (36) | 47 (49) | ||
| Unknown | 0 (0) | 1 (8) | 0 (0) | 3 (3) | ||
| Dose of prednisone, mg/kg | .59 | .72 | ||||
| None | 44 (83) | 9 (75) | 21 (84) | 74 (77) | ||
| >0 but <0.5 | 7 (13) | 3 (25) | 4 (16) | 14 (15) | ||
| 0.5-1.0 | 2 (4) | 0 (0) | 0 (0) | 6 (6) | ||
| >1.0 | 0 (0) | 0 (0) | 0 (0) | 2 (2) | ||
| Platelet count <100 × 109/L | 22 (42) | 5 (42) | 1.0 | 5 (20) | 20 (21) | 1.0 |
| Serum bilirubin level >2 mg/dL | 4 (8) | 0 (0) | 1.0 | 3 (12) | 8 (8) | .70 |
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 53) . | White (n = 12) . | P . | Japanese (n = 25) . | White (n = 96) . | P . | |
| Months from transplantation to late acute GVHD, median (IQR) | 5.6 (4.4-8.3) | 5.4 (4.1-6.5) | .51 | 5.8 (4.3-6.9) | 4.7 (3.5-6.5) | .06 |
| Type | .34 | <.001 | ||||
| Late onset | 15 (26) | 2 (17) | 13 (52) | 12 (13) | ||
| Recurrent | 31 (58) | 10 (83) | 9 (36) | 81 (84) | ||
| Persistent | 7 (13) | 0 (0) | 3 (12) | 3 (3) | ||
| Sites involved | ||||||
| Skin | 40 (75) | 6 (50) | .16 | 13 (52) | 50 (52) | 1.0 |
| Liver (per the 1994 consensus) | 4 (8) | 0 (0) | 1.0 | 3 (12) | 8 (8) | .70 |
| Liver (per the 2005 NIH liver score) | 42 (79) | 1 (8) | <.001 | 23 (92) | 31 (33) | <.001 |
| Gastrointestinal tract | 6 (11) | 7 (58) | .001 | 5 (20) | 65 (68) | <.001 |
| Grade of late acute GVHD | .024 | .001 | ||||
| Grade II or lower | 47 (89) | 7 (58) | 20 (80) | 39 (41) | ||
| Grade III or higher | 6 (11) | 5 (42) | 5 (20) | 57 (59) | ||
| No. of involved sites | .025 | .44 | ||||
| 1 | 21 (40) | 10 (83) | 11 (44) | 54 (56) | ||
| 2 | 29 (54) | 2 (17) | 12 (48) | 33 (34) | ||
| 3 | 3 (6) | 0 (0) | 2 (8) | 9 (9) | ||
| Prior IIb-IV classic acute GVHD | 29 (55) | 5 (42) | .53 | 9 (36) | 45 (47) | .37 |
| Karnofsky Performance Status | .028 | .34 | ||||
| 80%-100% | 42 (79) | 6 (50) | 16 (64) | 46 (48) | ||
| <80% | 11 (21) | 5 (42) | 9 (36) | 47 (49) | ||
| Unknown | 0 (0) | 1 (8) | 0 (0) | 3 (3) | ||
| Dose of prednisone, mg/kg | .59 | .72 | ||||
| None | 44 (83) | 9 (75) | 21 (84) | 74 (77) | ||
| >0 but <0.5 | 7 (13) | 3 (25) | 4 (16) | 14 (15) | ||
| 0.5-1.0 | 2 (4) | 0 (0) | 0 (0) | 6 (6) | ||
| >1.0 | 0 (0) | 0 (0) | 0 (0) | 2 (2) | ||
| Platelet count <100 × 109/L | 22 (42) | 5 (42) | 1.0 | 5 (20) | 20 (21) | 1.0 |
| Serum bilirubin level >2 mg/dL | 4 (8) | 0 (0) | 1.0 | 3 (12) | 8 (8) | .70 |
IQR, interquartile range.
Comparison of organ stage of late acute GVHD at onset between the races. *P < .05.
Comparison of organ stage of late acute GVHD at onset between the races. *P < .05.
Characteristics of NIH chronic GVHD at onset
The cumulative incidence of NIH chronic GVHD after BMT was 15% (95% CI, 11-19) at 48 months among Japanese patients, which was lower than that among white patients (30%; 95% CI, 20-42; P = .002) (Figure 1). The cumulative incidence of NIH chronic GVHD after PBSCT was 27% (95% CI, 20-35) at 48 months among Japanese patients, which was lower than among white patients (45%; 95% CI, 41-49; P < 0.001).
Characteristics of NIH chronic GVHD were compared between the races according to stem cell sources (Table 3). In the BMT group, Japanese patients had more frequent involvement with eyes and liver, and less frequent gastrointestinal involvement, and a greater number of involved sites. In the PBSCT group, Japanese patients had more de novo onset; less quiescent onset; more frequent involvement with eyes, mouth, and liver; higher NIH global severity scores; and a greater number of involved sites. The sites contributing to severe global scores were liver (60%), skin (36%), lungs (17%), eyes (14%), mouth (12%), and gut (2%) in Japanese patients, compared with skin (60%), lungs (23%), liver (18%), gut (9%), mouth (3%), and eyes (1%) in white patients. Other characteristics did not differ statistically between the 2 races, including the incidence of previous classic or late acute GVHD, Karnofsky Performance Status, prednisone dose at onset, or the incidence of thrombocytopenia and hyperbilirubinemia. The organ scores of chronic GVHD were compared between the races according to stem cell sources (Figure 3). Compared with white patients, Japanese patients had more severe oral GVHD after PBSCT (P < .001), more severe ocular GVHD both after BMT (P = .022) and PBSCT (P < .001), less severe gastrointestinal GVHD after BMT (P = .008), more severe liver GVHD both after BMT (P = .015) and PBSCT (P < .001), and less severe lung GVHD after BMT (P = .005).
Characteristics of NIH chronic GVHD at onset
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 41) . | White (n = 21) . | P . | Japanese (n = 35) . | White (n = 286) . | P . | |
| Months from transplantation to NIH chronic GVHD, median (IQR) | 6.9 (5.5-9.6) | 7.8 (4.7-10.5) | .91 | 6.0 (4.2-9.5) | 7.2 (4.6-11.7) | .39 |
| Type | .09 | <.001 | ||||
| De novo | 16 (39) | 5 (24) | 19 (54) | 63 (22) | ||
| Quiescent | 21 (51) | 16 (76) | 11 (31) | 197 (69) | ||
| Progressive | 4 (10) | 0 (0) | 5 (14) | 26 (9) | ||
| Sites involved | ||||||
| Skin | 27 (66) | 13 (62) | .79 | 22 (63) | 211 (74) | .23 |
| Mouth | 33 (80) | 13 (62) | .14 | 31 (89) | 197 (69) | .017 |
| Eyes | 24 (59) | 4 (19) | .004 | 23 (66) | 102 (36) | .001 |
| Gastrointestinal tract | 2 (5) | 7 (33) | .005 | 9 (26) | 117 (41) | .10 |
| Liver | 32 (78) | 9 (43) | .01 | 32 (91) | 129 (45) | <.001 |
| Lung (bronchiolitis obliterans) | 1 (2) | 1 (5) | 1.0 | 2 (6) | 3 (1) | .094 |
| Joint/fascia | 1 (2) | 1 (5) | 1.0 | 1 (3) | 39 (14) | .10 |
| Genital | 5 (12) | 2 (10) | 1.0 | 3 (9) | 23 (8) | 1.0 |
| NIH global severity score | .37 | .03 | ||||
| Mild | 2 (5) | 3 (14) | 0 (0) | 11 (4) | ||
| Moderate | 20 (49) | 11 (52) | 12 (34) | 154 (54) | ||
| Severe | 19 (46) | 7 (33) | 23 (66) | 121 (42) | ||
| No. of involved sites | .024 | .025 | ||||
| 1 or 2 | 9 (22) | 12 (57) | 8 (23) | 128 (45) | ||
| 3 | 21 (51) | 6 (29) | 10 (29) | 72 (25) | ||
| ≥4 | 11 (27) | 3 (14) | 17 (49) | 86 (30) | ||
| Prior IIb-IV classic or late acute GVHD | 17 (41) | 8 (38) | 1.0 | 10 (29) | 121 (42) | .15 |
| Karnofsky Performance Status | .37 | .085 | ||||
| 80-100% | 32 (78) | 14 (67) | 28 (80) | 174 (61) | ||
| <80% | 9 (22) | 7 (33) | 7 (20) | 111 (39) | ||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) | ||
| Dose of prednisone, mg/kg | .20 | .78 | ||||
| None | 31 (76) | 19 (90) | 29 (83) | 232 (81) | ||
| >0 but <0.5 | 10 (24) | 2 (10) | 4 (11) | 41 (14) | ||
| 0.5-1.0 | 0 (0) | 0 (0) | 2 (6) | 12 (4) | ||
| >1.0 | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) | ||
| Platelet count <100 × 109/L | 17 (41) | 8 (38) | 1.0 | 11 (31) | 68 (24) | .31 |
| Serum bilirubin level >2 mg/dL | 1 (2) | 0 (0) | 1.0 | 3 (9) | 10 (4) | .16 |
| Characteristic, n (%) . | BMT . | PBSCT . | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 41) . | White (n = 21) . | P . | Japanese (n = 35) . | White (n = 286) . | P . | |
| Months from transplantation to NIH chronic GVHD, median (IQR) | 6.9 (5.5-9.6) | 7.8 (4.7-10.5) | .91 | 6.0 (4.2-9.5) | 7.2 (4.6-11.7) | .39 |
| Type | .09 | <.001 | ||||
| De novo | 16 (39) | 5 (24) | 19 (54) | 63 (22) | ||
| Quiescent | 21 (51) | 16 (76) | 11 (31) | 197 (69) | ||
| Progressive | 4 (10) | 0 (0) | 5 (14) | 26 (9) | ||
| Sites involved | ||||||
| Skin | 27 (66) | 13 (62) | .79 | 22 (63) | 211 (74) | .23 |
| Mouth | 33 (80) | 13 (62) | .14 | 31 (89) | 197 (69) | .017 |
| Eyes | 24 (59) | 4 (19) | .004 | 23 (66) | 102 (36) | .001 |
| Gastrointestinal tract | 2 (5) | 7 (33) | .005 | 9 (26) | 117 (41) | .10 |
| Liver | 32 (78) | 9 (43) | .01 | 32 (91) | 129 (45) | <.001 |
| Lung (bronchiolitis obliterans) | 1 (2) | 1 (5) | 1.0 | 2 (6) | 3 (1) | .094 |
| Joint/fascia | 1 (2) | 1 (5) | 1.0 | 1 (3) | 39 (14) | .10 |
| Genital | 5 (12) | 2 (10) | 1.0 | 3 (9) | 23 (8) | 1.0 |
| NIH global severity score | .37 | .03 | ||||
| Mild | 2 (5) | 3 (14) | 0 (0) | 11 (4) | ||
| Moderate | 20 (49) | 11 (52) | 12 (34) | 154 (54) | ||
| Severe | 19 (46) | 7 (33) | 23 (66) | 121 (42) | ||
| No. of involved sites | .024 | .025 | ||||
| 1 or 2 | 9 (22) | 12 (57) | 8 (23) | 128 (45) | ||
| 3 | 21 (51) | 6 (29) | 10 (29) | 72 (25) | ||
| ≥4 | 11 (27) | 3 (14) | 17 (49) | 86 (30) | ||
| Prior IIb-IV classic or late acute GVHD | 17 (41) | 8 (38) | 1.0 | 10 (29) | 121 (42) | .15 |
| Karnofsky Performance Status | .37 | .085 | ||||
| 80-100% | 32 (78) | 14 (67) | 28 (80) | 174 (61) | ||
| <80% | 9 (22) | 7 (33) | 7 (20) | 111 (39) | ||
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) | ||
| Dose of prednisone, mg/kg | .20 | .78 | ||||
| None | 31 (76) | 19 (90) | 29 (83) | 232 (81) | ||
| >0 but <0.5 | 10 (24) | 2 (10) | 4 (11) | 41 (14) | ||
| 0.5-1.0 | 0 (0) | 0 (0) | 2 (6) | 12 (4) | ||
| >1.0 | 0 (0) | 0 (0) | 0 (0) | 1 (< 1) | ||
| Platelet count <100 × 109/L | 17 (41) | 8 (38) | 1.0 | 11 (31) | 68 (24) | .31 |
| Serum bilirubin level >2 mg/dL | 1 (2) | 0 (0) | 1.0 | 3 (9) | 10 (4) | .16 |
Comparison of organ score of NIH chronic GVHD at onset between the races. *P < .05. BOS, bronchiolitis obliterans syndrome.
Comparison of organ score of NIH chronic GVHD at onset between the races. *P < .05. BOS, bronchiolitis obliterans syndrome.
Outcomes after onset of LA GVHD and NIH chronic GVHD
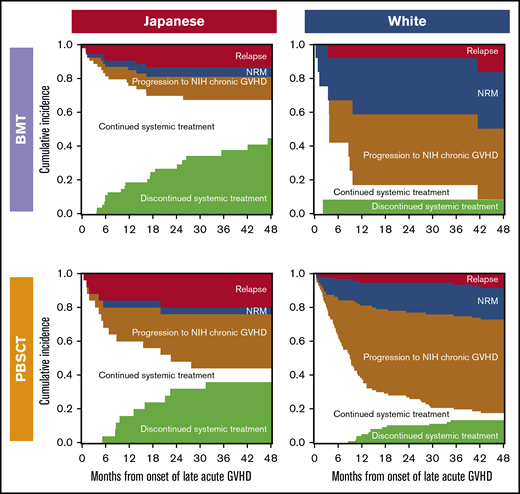
Outcomes after onset of LA GVHD are illustrated according to race and stem cell sources in Figure 4. Compared with white patients, Japanese patients showed lower probabilities of NRM and progression to NIH chronic GVHD, and a higher probability of discontinued systemic treatment after both BMT and PBSCT. The most frequent cause of NRM was infection (75%) in Japanese patients, whereas it was GVHD (50%) in white patients.
Outcomes after onset of late acute GVHD according to races and stem cell sources. Each area represents probabilities of relapse, NRM, progression to NIH chronic GVHD, continued systemic treatment, and discontinued systemic treatment.
Outcomes after onset of late acute GVHD according to races and stem cell sources. Each area represents probabilities of relapse, NRM, progression to NIH chronic GVHD, continued systemic treatment, and discontinued systemic treatment.
Outcomes after onset of NIH chronic GVHD are illustrated in Figure 5. Both Japanese and white BMT patients had similar outcomes after onset of NIH chronic GVHD. Compared with white PBSCT patients, Japanese PBSCT patients showed a higher probability of relapse and a lower probability of NRM. The most frequent cause of NRM was infection in both Japanese patients (38%) and white patients (52%).
Outcomes after onset of NIH chronic GVHD according to races and stem cell sources. Each area represents probabilities of relapse, NRM, continued systemic treatment, and discontinued systemic treatment.
Outcomes after onset of NIH chronic GVHD according to races and stem cell sources. Each area represents probabilities of relapse, NRM, continued systemic treatment, and discontinued systemic treatment.
Multivariate analysis of outcomes in the whole cohort
Multivariate analyses were performed to determine the association of race with individual outcomes (Table 4). The risk of LA GVHD did not differ statistically between the races, whereas Japanese patients had a lower risk of NIH chronic GVHD compared with white patients (hazard ratio [HR], 0.51; 95% CI, 0.37-0.70; P < .001). The risk of relapse after LA GVHD did not differ statistically between the races. Japanese patients had lower risks of NRM and progression to chronic GVHD (HRs of 0.23 [95% CI, 0.08-0.68; P = .007] and 0.42 [95% CI, 0.21-0.84; P = .014], respectively). Japanese patients had a higher likelihood of discontinued systemic treatment after LA GVHD (HR, 3.68; 95% CI, 1.96-6.94; P < .001). The risks of any individual events after onset of NIH chronic GVHD did not differ statistically between the races. In comparing outcomes after HCT with white patients, Japanese patients had a higher risk of relapse (HR, 1.74; 95% CI, 1.42-2.13; P < .001), a lower risk of NRM (HR, 0.51; 95% CI, 0.34-0.75; P = .001), and a similar risk of overall mortality (HR, 1.10; 95% CI, 0.86-1.41; P = .45).
Multivariate analysis results
| Outcome . | Factor . | n . | HR (95% CI) . | P . |
|---|---|---|---|---|
| Incidence of late acute GVHD | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.97 (0.69-1.37) | .88 | |
| Patient sex | ||||
| Male | 668 | 1.00 (reference) | ||
| Female | 453 | 0.66 (0.49-0.90) | .009 | |
| Disease Risk Index | ||||
| Low | 136 | 1.34 (0.90-1.99) | .15 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 0.46 (0.31-0.67) | <.001 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.38 (0.77-2.45) | .28 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 0.61 (0.42-0.89) | .011 | |
| Relapse after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 1.98 (0.88 −4.47) | .10 | |
| NRM after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 0.23 (0.08-0.68) | .007 | |
| Discontinued systemic treatment after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 3.68 (1.96-6.94) | <.001 | |
| Female donor to male patient | 47 | 0.35 (0.16-0.78) | .01 | |
| Conditioning intensity | ||||
| Myeloablative | 111 | 1.00 (reference) | ||
| Reduced intensity | 75 | 1.88 (1.04-3.40) | .036 | |
| Progression from late acute GVHD to NIH chronic GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 0.42 (0.21-0.84) | .014 | |
| Graft source | ||||
| BM | 65 | |||
| PBSC | 121 | 2.15 (1.01-4.61) | .048 | |
| Conditioning intensity | ||||
| Myeloablative | 111 | |||
| Reduced intensity | 75 | 0.56 (0.34-0.91) | .02 | |
| Incidence of NIH chronic GVHD | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.51 (0.37-0.70) | <.001 | |
| Graft source | ||||
| BM | 62 | 1.00 (reference) | ||
| PBSC | 321 | 1.83 (1.31-2.56) | <.001 | |
| Disease Risk Index | ||||
| Low | 136 | 1.19 (0.89-1.60) | .25 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 0.65 (0.51-0.82) | <.001 | |
| Relapse after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 1.57 (0.85-2.92) | .15 | |
| Donor type | ||||
| Related | 172 | 1.00 (reference) | ||
| Unrelated | 211 | 0.54 (0.31-0.93) | .027 | |
| Conditioning intensity | ||||
| Myeloablative | 219 | 1.00 (reference) | ||
| Reduced intensity | 164 | 0.36 (0.19-0.69) | .002 | |
| NRM after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 0.84 (0.34-2.05) | .70 | |
| Graft source | ||||
| BM | 62 | 1.00 (reference) | ||
| PBSC | 321 | 1.23 (1.01-1.50) | .037 | |
| GVHD prophylaxis | ||||
| CNI alone | 15 | 1.15 (0.22-6.13) | .87 | |
| CNI + MTX | 215 | 1.00 (reference) | ||
| CNI + MMF | 153 | 2.34 (1.40-3.91) | .001 | |
| Discontinued systemic treatment after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 1.19 (0.74-1.92) | .47 | |
| Female donor to male patient | 98 | 0.55 (0.36-0.84) | .005 | |
| Relapse after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 1.74 (1.42-2.13) | <.001 | |
| Disease Risk Index | ||||
| Low | 136 | 0.92 (0.64-1.32) | .64 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 2.12 (1.71-2.63) | <.001 | |
| NRM after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.51 (0.34-0.75) | .001 | |
| HCT–comorbidity index at transplantation | ||||
| 0-1 | 495 | 1.00 (reference) | ||
| ≥2 | 582 | 1.37 (1.00-1.88) | .048 | |
| Unknown | 44 | 0.75 (0.37-1.52) | .43 | |
| Disease Risk Index | ||||
| Low | 136 | 1.29 (0.93-1.80) | .13 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 1.51 (1.17-1.95) | .002 | |
| Donor type | ||||
| Related | 426 | 1.00 (reference) | ||
| Unrelated | 695 | 1.57 (1.20-2.05) | .001 | |
| HLA matching | ||||
| Match | 843 | 1.00 (reference) | ||
| Mismatch | 278 | 1.49 (1.12-1.99) | .006 | |
| Female donor to male patient | 249 | 1.43 (1.10-1.85) | .007 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.82 (0.96-3.45) | .065 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 1.95 (1.49-2.55) | <.001 | |
| Overall mortality after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 1.10 (0.86-1.41) | .45 | |
| Patient age per decade | 1121 | 1.10 (1.02-1.18) | .009 | |
| HCT–comorbidity index at transplantation | ||||
| 0-1 | 495 | 1.00 (reference) | ||
| ≥2 | 582 | 1.39 (1.12-1.72) | .003 | |
| Unknown | 44 | 1.02 (0.64-1.63) | .93 | |
| Disease Risk Index | ||||
| Low | 136 | 1.04 (0.79-1.38) | .76 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 2.29 (1.93-2.73) | <.001 | |
| Donor type | ||||
| Related | 426 | 1.00 (reference) | ||
| Unrelated | 695 | 1.21 (1.01-1.45) | .038 | |
| HLA matching | ||||
| Match | 843 | 1.00 (reference) | ||
| Mismatch | 278 | 1.24 (1.00-1.52) | .04 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.65 (1.11-2.46) | .013 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 1.35 (1.10-1.65) | .004 |
| Outcome . | Factor . | n . | HR (95% CI) . | P . |
|---|---|---|---|---|
| Incidence of late acute GVHD | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.97 (0.69-1.37) | .88 | |
| Patient sex | ||||
| Male | 668 | 1.00 (reference) | ||
| Female | 453 | 0.66 (0.49-0.90) | .009 | |
| Disease Risk Index | ||||
| Low | 136 | 1.34 (0.90-1.99) | .15 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 0.46 (0.31-0.67) | <.001 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.38 (0.77-2.45) | .28 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 0.61 (0.42-0.89) | .011 | |
| Relapse after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 1.98 (0.88 −4.47) | .10 | |
| NRM after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 0.23 (0.08-0.68) | .007 | |
| Discontinued systemic treatment after late acute GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 3.68 (1.96-6.94) | <.001 | |
| Female donor to male patient | 47 | 0.35 (0.16-0.78) | .01 | |
| Conditioning intensity | ||||
| Myeloablative | 111 | 1.00 (reference) | ||
| Reduced intensity | 75 | 1.88 (1.04-3.40) | .036 | |
| Progression from late acute GVHD to NIH chronic GVHD | Race | |||
| White | 108 | 1.00 (reference) | ||
| Japanese | 78 | 0.42 (0.21-0.84) | .014 | |
| Graft source | ||||
| BM | 65 | |||
| PBSC | 121 | 2.15 (1.01-4.61) | .048 | |
| Conditioning intensity | ||||
| Myeloablative | 111 | |||
| Reduced intensity | 75 | 0.56 (0.34-0.91) | .02 | |
| Incidence of NIH chronic GVHD | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.51 (0.37-0.70) | <.001 | |
| Graft source | ||||
| BM | 62 | 1.00 (reference) | ||
| PBSC | 321 | 1.83 (1.31-2.56) | <.001 | |
| Disease Risk Index | ||||
| Low | 136 | 1.19 (0.89-1.60) | .25 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 0.65 (0.51-0.82) | <.001 | |
| Relapse after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 1.57 (0.85-2.92) | .15 | |
| Donor type | ||||
| Related | 172 | 1.00 (reference) | ||
| Unrelated | 211 | 0.54 (0.31-0.93) | .027 | |
| Conditioning intensity | ||||
| Myeloablative | 219 | 1.00 (reference) | ||
| Reduced intensity | 164 | 0.36 (0.19-0.69) | .002 | |
| NRM after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 0.84 (0.34-2.05) | .70 | |
| Graft source | ||||
| BM | 62 | 1.00 (reference) | ||
| PBSC | 321 | 1.23 (1.01-1.50) | .037 | |
| GVHD prophylaxis | ||||
| CNI alone | 15 | 1.15 (0.22-6.13) | .87 | |
| CNI + MTX | 215 | 1.00 (reference) | ||
| CNI + MMF | 153 | 2.34 (1.40-3.91) | .001 | |
| Discontinued systemic treatment after NIH chronic GVHD | Race | |||
| White | 307 | 1.00 (reference) | ||
| Japanese | 76 | 1.19 (0.74-1.92) | .47 | |
| Female donor to male patient | 98 | 0.55 (0.36-0.84) | .005 | |
| Relapse after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 1.74 (1.42-2.13) | <.001 | |
| Disease Risk Index | ||||
| Low | 136 | 0.92 (0.64-1.32) | .64 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 2.12 (1.71-2.63) | <.001 | |
| NRM after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 0.51 (0.34-0.75) | .001 | |
| HCT–comorbidity index at transplantation | ||||
| 0-1 | 495 | 1.00 (reference) | ||
| ≥2 | 582 | 1.37 (1.00-1.88) | .048 | |
| Unknown | 44 | 0.75 (0.37-1.52) | .43 | |
| Disease Risk Index | ||||
| Low | 136 | 1.29 (0.93-1.80) | .13 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 1.51 (1.17-1.95) | .002 | |
| Donor type | ||||
| Related | 426 | 1.00 (reference) | ||
| Unrelated | 695 | 1.57 (1.20-2.05) | .001 | |
| HLA matching | ||||
| Match | 843 | 1.00 (reference) | ||
| Mismatch | 278 | 1.49 (1.12-1.99) | .006 | |
| Female donor to male patient | 249 | 1.43 (1.10-1.85) | .007 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.82 (0.96-3.45) | .065 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 1.95 (1.49-2.55) | <.001 | |
| Overall mortality after transplantation | Race | |||
| White | 708 | 1.00 (reference) | ||
| Japanese | 413 | 1.10 (0.86-1.41) | .45 | |
| Patient age per decade | 1121 | 1.10 (1.02-1.18) | .009 | |
| HCT–comorbidity index at transplantation | ||||
| 0-1 | 495 | 1.00 (reference) | ||
| ≥2 | 582 | 1.39 (1.12-1.72) | .003 | |
| Unknown | 44 | 1.02 (0.64-1.63) | .93 | |
| Disease Risk Index | ||||
| Low | 136 | 1.04 (0.79-1.38) | .76 | |
| Intermediate | 604 | 1.00 (reference) | ||
| High/very high | 381 | 2.29 (1.93-2.73) | <.001 | |
| Donor type | ||||
| Related | 426 | 1.00 (reference) | ||
| Unrelated | 695 | 1.21 (1.01-1.45) | .038 | |
| HLA matching | ||||
| Match | 843 | 1.00 (reference) | ||
| Mismatch | 278 | 1.24 (1.00-1.52) | .04 | |
| GVHD prophylaxis | ||||
| CNI alone | 54 | 1.65 (1.11-2.46) | .013 | |
| CNI + MTX | 704 | 1.00 (reference) | ||
| CNI + MMF | 363 | 1.35 (1.10-1.65) | .004 |
Associations of practice differences between the 2 centers with outcomes
We identified 3 major practice differences between the 2 centers: (1) reduced-intensity BMT was performed predominantly at NCCH in Japan but not at FHCRC/SCCA in the United States; (2) GVHD prophylaxis with mycophenolate mofetil was used predominantly at FHCRC/SCCA but not at NCCH; and (3) PBSCT from unrelated donors was performed predominantly at FHCRC/SCCA but not at NCCH. The associations of these practice differences with outcomes of interest are summarized in Table 5. In comparing conditioning intensity among patients who had BMT at NCCH, reduced-intensity conditioning was associated with a lower risk of mouth and liver involvement with chronic GVHD (odds ratio [OR], 0.13; 95% CI, 0.02-0.74; P = .022; OR, 0.10; 95% CI, 0.02-0.56; P = .009, respectively). In comparing GVHD prophylaxis among patients who had PBSCT at FHCRC/SCCA, mycophenolate mofetil was associated with a lower risk of liver involvement with LA GVHD and NIH chronic GVHD (ORs of 0.35 [95% CI, 0.14-0.88; P = .026] and 0.61 [95% CI, 0.38-1.00; P = .044]) and a higher risk of gastrointestinal involvement with LA GVHD (OR, 2.55; 95% CI, 1.00-6.48; P = .05). Donor relation was not statistically associated with any outcomes among patients who had PBSCT at FHCRC/SCCA.
Associations of practice differences with outcomes
| Outcome . | NCCH-BMT RIC (vs MAC) . | FHCRC/SCCA-PBSCT CNI + MMF (vs CNI + MTX) . | FHCRC/SCCA-PBSCT Unrelated (vs Related) . | |||
|---|---|---|---|---|---|---|
| HR/OR (95% CI) . | P . | HR/OR (95% CI) . | P . | HR/OR (95% CI) . | P . | |
| Cumulative incidence of NIH chronic GVHD | 0.99 (0.52-1.87) | .97 | 0.90 (0.71-1.14) | 0.38 | 0.83 (0.66-1.05) | .12 |
| Liver involvement with late acute GVHD per the NIH liver score | 1.81 (0.42-7.83) | .43 | 0.35 (0.14-0.88) | 0.026 | 0.79 (0.32-1.93) | .61 |
| Gut involvement with late acute GVHD | 1.76 (0.32-9.73) | .51 | 2.55 (1.00-6.48) | 0.05 | 1.40 (0.57-3.42) | .47 |
| Mouth involvement with NIH chronic GVHD | 0.13 (0.02-0.74) | .022 | 1.09 (0.66-1.81) | 0.73 | 1.19 (0.72-1.96) | .50 |
| Eye involvement with NIH chronic GVHD | 0.71 (0.20-2.58) | .61 | 0.87 (0.54-1.42) | 0.59 | 1.16 (0.71-1.89) | .55 |
| Gut involvement with NIH chronic GVHD | 1.79 (0.10-30.8) | .69 | 1.56 (0.96-2.51) | 0.072 | 1.37 (0.85-2.21) | .19 |
| Liver involvement with NIH chronic GVHD | 0.10 (0.02-0.56) | .009 | 0.61 (0.38-1.00) | 0.044 | 0.71 (0.44-1.13) | .15 |
| Discontinued systemic treatment after late acute GVHD | 1.67 (0.72-3.89) | .23 | 0.88 (0.30-2.57) | 0.81 | 0.94 (0.32-2.78) | .91 |
| NRM after late acute GVHD | NA* | NA* | 2.24 (0.84-6.00) | 0.11 | 1.41 (0.51-3.94) | .51 |
| Progression from late acute GVHD to NIH chronic GVHD | 0.26 (0.03-2.18) | .21 | 0.69 (0.40-1.21) | 0.20 | 0.94 (0.54-1.63) | .82 |
| Outcome . | NCCH-BMT RIC (vs MAC) . | FHCRC/SCCA-PBSCT CNI + MMF (vs CNI + MTX) . | FHCRC/SCCA-PBSCT Unrelated (vs Related) . | |||
|---|---|---|---|---|---|---|
| HR/OR (95% CI) . | P . | HR/OR (95% CI) . | P . | HR/OR (95% CI) . | P . | |
| Cumulative incidence of NIH chronic GVHD | 0.99 (0.52-1.87) | .97 | 0.90 (0.71-1.14) | 0.38 | 0.83 (0.66-1.05) | .12 |
| Liver involvement with late acute GVHD per the NIH liver score | 1.81 (0.42-7.83) | .43 | 0.35 (0.14-0.88) | 0.026 | 0.79 (0.32-1.93) | .61 |
| Gut involvement with late acute GVHD | 1.76 (0.32-9.73) | .51 | 2.55 (1.00-6.48) | 0.05 | 1.40 (0.57-3.42) | .47 |
| Mouth involvement with NIH chronic GVHD | 0.13 (0.02-0.74) | .022 | 1.09 (0.66-1.81) | 0.73 | 1.19 (0.72-1.96) | .50 |
| Eye involvement with NIH chronic GVHD | 0.71 (0.20-2.58) | .61 | 0.87 (0.54-1.42) | 0.59 | 1.16 (0.71-1.89) | .55 |
| Gut involvement with NIH chronic GVHD | 1.79 (0.10-30.8) | .69 | 1.56 (0.96-2.51) | 0.072 | 1.37 (0.85-2.21) | .19 |
| Liver involvement with NIH chronic GVHD | 0.10 (0.02-0.56) | .009 | 0.61 (0.38-1.00) | 0.044 | 0.71 (0.44-1.13) | .15 |
| Discontinued systemic treatment after late acute GVHD | 1.67 (0.72-3.89) | .23 | 0.88 (0.30-2.57) | 0.81 | 0.94 (0.32-2.78) | .91 |
| NRM after late acute GVHD | NA* | NA* | 2.24 (0.84-6.00) | 0.11 | 1.41 (0.51-3.94) | .51 |
| Progression from late acute GVHD to NIH chronic GVHD | 0.26 (0.03-2.18) | .21 | 0.69 (0.40-1.21) | 0.20 | 0.94 (0.54-1.63) | .82 |
MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
Not applicable due to no event in the RIC group.
Discussion
The current study elucidated differences in the incidence, affected sites, severities, and clinical outcomes of LA GVHD and NIH chronic GVHD between Japanese and white patients. The incidence of LA GVHD was similar between the races, whereas the incidence of NIH chronic GVHD was lower in Japanese patients compared with white patients. These observations were consistent after both BMT and PBSCT. In addition, the incidence of NIH chronic GVHD was higher after PBSCT compared with BMT, and race and stem cell source were independent factors associated with onset of NIH chronic GVHD in multivariate analysis. The increased incidence of chronic GVHD after PBSCT is consistent with published data.19,-21 Published data are limited for LA GVHD, but several studies have shown that the incidence of classic acute GVHD is similar between BMT and PBSCT.19,20,22 These results may indicate that mechanisms of LA GVHD are similar to those leading to classic acute GVHD and differ from those leading to NIH chronic GVHD.
Japanese patients had more frequent transaminase elevation and less frequent gastrointestinal involvement compared with white patients in both LA GVHD and NIH chronic GVHD. Methods of diagnosis, staging, and grading of GVHD between the centers could affect the results, but evaluation was made in a standardized way by using the NIH consensus criteria.5 Thus, it is unlikely that staging or grading methods affected the outcomes in this study. Diagnostic practices such as liver biopsy and endoscopy were similar between the centers. The proportions of patients with positive serology for hepatitis B virus and hepatitis C virus were similarly low in both races (1.2% and 0.5%, respectively, in the Japanese cohort, and 1.7% and 0.7%, respectively, in the white cohort).
Despite the higher global score and a greater number of sites involved with chronic GVHD at onset in Japanese patients, the duration of immunosuppression and the probability of NRM were similar between the 2 races. The differences in attributable sites to severe global scores may account for this observation. Liver GVHD may be responsive to therapy with calcineurin inhibitors, whereas gastrointestinal GVHD is clinically more challenging.3 In multivariate analysis, overall mortality was comparable, but Japanese patients had a higher risk of relapse and a lower risk of NRM compared with white patients, suggesting the presence of racial differences in GVHD characteristics leading to these outcomes.
The results should be interpreted with caution because practices differed between the 2 centers in 3 major respects. First, reduced-intensity conditioning was used for BMT only at NCCH and correlated with less frequent involvement of mouth and liver with chronic GVHD. The frequency of mouth and liver involvement, however, was higher in NCCH patients than in FHCRC/SCCA patients after BMT. Second, few patients at NCCH received mycophenolate mofetil after PBSCT. The use of mycophenolate mofetil was associated with less frequent liver involvement and more frequent gastrointestinal involvement after PBSCT at FHCRC/SCCA. The frequency of liver and gastrointestinal involvement, however, was still higher in NCCH patients after BMT where the majority received methotrexate at both centers. Third, few NCCH patients had PBSCT from unrelated donors, but outcomes were similar between related and unrelated donors among patients who had PBSCT at FHCRC/SCCA. We acknowledge that these major practice differences between the centers may be difficult to adjust in the analysis, but practice differences alone do not fully explain the different outcomes.
Several mechanisms could explain the different incidence frequencies and outcomes of GVHD among the races. Eckrich et al23 reported higher incidence frequencies of both classic acute and extensive chronic GVHD in African-American subjects compared with white patients after controlling for HLA mismatching, indicating that factors other than HLA mismatching play a critical role in determining immune-mediated outcomes. A lesser frequency of classic acute GVHD among Japanese patients has been hypothesized to be linked to founder effects in an island population, resulting in greater population homogeneity for minor histocompatibility antigens and haplotypes.24,25 A greater degree of HLA variation among white patients could also be relevant to immune mechanisms, leading to the higher incidence of chronic GVHD.26,-28 Alternatively, certain haplotypes, more common in specific racial groups, may be associated with a predisposition to chronic GVHD.29 In addition to HLA and haplotype, a growing body of research has identified cytokine gene polymorphisms associated with chronic GVHD.30,31 It is possible that genetic variations in regulatory function of immune cells, chemokines, and cytokines between the 2 groups influence manifestations of LA GVHD and chronic GVHD.
This study has many limitations. First, this was a retrospective study at 2 centers in different countries. Although we tried to interpret the results carefully by taking practice differences between the centers into account, confirmation of the results in a multicenter cohort with less practice variation is necessary. We were also not able to consider other potential confounders such as food, alcohol, and smoking exposures. Second, the retrospective study design did not allow us to compare treatment details, treatment response, and quality of life between the races. Third, the sample size and statistical power were limited in several subgroups, and P values were not corrected for multiple comparisons. The wide CIs in several analyses would not exclude true values within the range. Larger numbers of patients would be needed to draw definitive conclusions. Despite these limitations, this study provides important information regarding the differences in GVHD characteristics across racial groups. The suggested differences in affected sites and outcomes may support specific treatment approaches for the various racial groups.
Acknowledgments
The authors thank Chris Davis, Kevin Bray, and Aaron Johnson for their help in retrieving and assembling the data for the FHCRC/SCCA study cohort.
This study was supported by grants from the Japan Society for the Promotion of Science (18K08345) and the National Cancer Research and Development Fund (26-A-26). This study was also supported by grants from the National Institutes of Health, National Cancer Institute (CA018029, P30 CA015704, and CA078902) and National Heart, Lung, and Blood Institute (HL122173).
Authorship
Contribution: Y.I., J.W., and M.E.D.F. designed the study, analyzed data, and wrote the paper; Y.I. performed statistical analysis; R.I., P.J.M., G.F., A.I., T.T., S.K., S.-W.K., M.B., M.L.S., B.M.S., S.J.L., and T.F. wrote the paper; and all authors approved the manuscript for publication.
Conflict-of-interest disclosure: M.L.S. has participated in advisory board and has received honorarium from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Yoshihiro Inamoto, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo, Tokyo 104-0045, Japan; e-mail: yinamoto@ncc.go.jp.
References
Author notes
Y.I. and J.W. contributed equally to this work.