Key Points
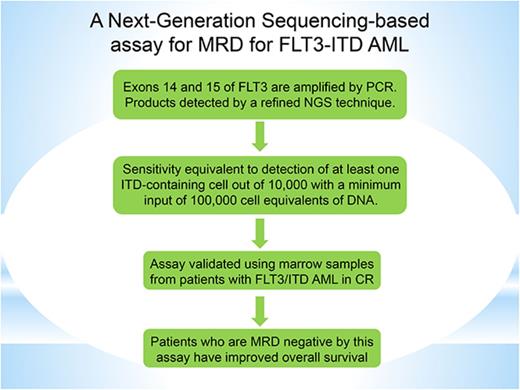
A sensitive and specific assay was developed for detection of MRD in patients with AML who harbor FLT3-ITD mutations.
This standardized assay is readily available and may be used to guide therapy decisions in patients with AML.
Abstract
Internal tandem duplications in fms-like tyrosine kinase 3 (FLT3-ITDs) are common in acute myeloid leukemia (AML) and confer a poor prognosis. A sensitive and specific assay for the detection of minimal residual disease (MRD) in FLT3-ITD mutated AML could guide therapy decisions. Existing assays for MRD in FLT3-ITD AML have not been particularly useful because of limited sensitivity. We developed a sensitive and specific MRD assay for FLT3-ITD mutations using next-generation sequencing. The initial validation of this assay was performed by spiking fixed amounts of mutant DNA into wild-type DNA to establish a sensitivity of detection equivalent to ≥1 FLT3-ITD–containing cell in 10 000, with a minimum input of 100 000 cell equivalents of DNA. We subsequently validated the assay in bone marrow samples from patients with FLT3-ITD AML in remission. Finally, we analyzed bone marrow samples from 80 patients with FLT3-ITD relapsed/refractory AML participating in a trial of a novel FLT3 inhibitor, gilteritinib, and demonstrated a relationship between the mutation burden, as detected by the assay, and overall survival. This novel MRD assay is specific and 2 orders of magnitude more sensitive than currently available polymerase chain reaction– or next-generation sequencing–based FLT3-ITD assays. The assay is being prospectively validated in ongoing randomized clinical trials.
Introduction
The ability to sensitively and accurately detect minimal residual disease (MRD) in patients with leukemia has proven to be useful in the clinical management of select disease subtypes and can potentially facilitate the development of new therapies. For example, BCR-ABL transcript levels are routinely used in the management of chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia in which BCR-ABL transcript levels define optimal treatment response, serve as a marker for therapeutic resistance, and determine the need for treatment changes or more aggressive therapeutic strategies.1-2 To ensure clinical relevance, such measurements require a readily performed, robust, and harmonized assay for MRD detection and universal quantification. Significant progress has been made in the development of clinically useful and generalizable assays for MRD detection in adult acute myeloid leukemia (AML) using flow cytometry– and molecular-based approaches.3-6 However, despite its promise, the widespread use of MRD evaluation in AML remains limited by cross-center validation concerns, technical limitations of next-generation sequencing (NGS) for detection of point mutations, and the heterogeneity and relatively low incidence of specific gene fusions.
Internal tandem duplications in fms-like tyrosine kinase 3 (FLT3-ITDs) are among the most common mutations found in AML and are associated with an aggressive disease phenotype and high relapse rates after traditional cytotoxic chemotherapy, which explains why such patients are referred for allogeneic transplant early in the disease course.7-8 Several FLT3 tyrosine kinase inhibitors (TKIs) are in active development; 1 TKI (midostaurin) has been recently approved for the treatment of FLT3 mutation-positive AML when administered in combination with standard induction chemotherapy.9-13 When administered as monotherapy for relapsed FLT3-mutated AML, FLT3 TKIs can induce differentiation or cytotoxicity in marrow blasts, often resulting in responses that do not conform to the standard International Working Group (IWG) criteria for complete remission (CR).14-15 Indeed, the benefits of the most common type of response induced by FLT3 TKIs, CR with incomplete hematological recovery (CRi), have been difficult to quantify.
Initial response rates to intensive chemotherapy are quite high in patients with FLT3-ITD AML; although a substantial number of these patients will ultimately relapse if not transplanted, this is by no means uniformly seen. Therefore, the availability of a sensitive and specific FLT3-ITD mutation assay to be used for patients in clinical remission that allows for detection of MRD would present a significant advantage for clinicians, because it could help to guide decisions about whether patients should undergo transplantation, as well as potentially whether FLT3 inhibitors should be administered as maintenance therapy following intensive chemotherapy or transplantation. Furthermore, the ability to sensitively and accurately determine the mutated FLT3 allele burden in a given bone marrow sample could be used to better characterize responses to FLT3 TKIs.
Although several groups have reported the development of MRD assays for FLT3-ITD AML, none of these diagnostics have been developed in concert with bioinformatics software under a quality system with the intent of being submitted to regulatory authorities as a harmonized assay available to the international community. Polymerase chain reaction (PCR)–based assays for MRD in FLT3-ITD AML have a detection sensitivity of 1 mutant cell out of 100, at best.16-17 Moreover, having 2 unique templates (ie, a wild-type allele and a longer ITD-mutated allele) within a single reaction tube can potentially result in greater amplification of the shorter wild-type allele. This PCR bias based on template differences is a well-described phenomenon18 that has prevented application of PCR techniques to FLT3-ITD–mutated AML in a manner similar to that of BCR-ABL.
Here, we report the development of an FLT3-ITD assay using an improved PCR methodology with enhanced linearity across the range of FLT3-ITD allele burden, combined with an NGS platform that is sensitive, specific, and can be used to detect the presence of MRD in patients with FLT3 mutation–positive AML achieving morphologic remission. Furthermore, we demonstrate the usefulness of this assay in characterizing clinical responses induced by FLT3 inhibition in patients with FLT3-mutated relapsed/refractory (R/R) AML.
Methods
FLT3-ITD MRD assay
Exons 14 and 15 of the FLT3 gene were amplified by PCR. The PCR primers, which contained gene-specific regions adapted from previously published reports,16 were coupled with sequencing adaptors and proprietary barcodes (Invivoscribe, Inc., San Diego, CA) to lessen amplification bias and to allow amplified products from multiple samples to be run simultaneously on an Illumina MiSeq sequencer (Illumina, San Diego, CA). The PCR products were purified with an Agencourt Ampure XP kit (Beckman Coulter, Pasadena, CA), and the DNA concentration of the purified amplicons was quantified with a LabChip GX system (PerkinElmer, Waltham, MA). Libraries were prepared from purified amplicons and sequenced on a MiSeq sequencer (Illumina), according to the manufacturer’s instructions. Sequencing data were analyzed using proprietary software developed by Invivoscribe. Validation of linearity was performed by testing a serial dilution of fixed amounts of mutant DNA (from cell line A: MV4-11 cells, 30-bp homozygous ITD and cell line B: PL-21 cells, 126-bp heterozygous ITD) that were spiked into wild-type DNA from Jurkat cells. To establish sensitivity sufficient to detect ≥1 FLT3-ITD–containing cell in 10 000, a minimum input of 100 000 cell equivalents of DNA (660 ng) was tested. The DNA input for the linearity validation and the clinical samples was 700 ng.
NPM1 assay
The assay for detection of nucleophosmin (NPM1) mutations was based on a published method.19 RNA was prepared using QIAGEN RNeasy columns (QIAGEN, Valencia, CA) and reverse transcribed. A semiquantitative multiplex PCR reaction was performed using primers specific for mutated NPM1 (Asuragen Signature LTx; Asuragen, Austin, TX).20 The fluorescently labeled PCR products were identified by hybridization to mutation-specific beads, followed by flow cytometry detection of the products (Luminex, Austin, TX). Amplified glyceraldehyde 3-phosphate dehydrogenase mRNA served as a control to verify sample quality.
Clinical flow cytometry
Bone marrow aspirates from AML patients with FLT3-ITD mutations treated at Johns Hopkins Hospital (Baltimore, MD) were analyzed by 6-color multiparameter flow cytometry (for a leukemia-associated phenotype), as previously described.14 The estimated sensitivity of this technique was the detection of 1 leukemia cell of 1000 (0.1%).
Patient samples
All patients provided their informed consent, according to the guidelines of the Declaration of Helsinki, and approval from the institutional review board was obtained. Bone marrow aspirates from patients with AML (with and without FLT3-ITD mutations) were collected in heparinized vacutainer tubes. All patients tested were in clinical remission according to IWG criteria.21 DNA was isolated using QIAquick columns (QIAGEN), according to the manufacturer’s instructions. Extracted DNA was analyzed at 2 sites in the United States: the Molecular Diagnostics Laboratory at Johns Hopkins Hospital and Invivoscribe, Inc. The conventional assay for FLT3 mutations, in which DNA was amplified by PCR and separated by capillary electrophoresis (CE), was performed at Johns Hopkins Hospital, as previously described.16 The MRD assay was developed by and performed at Invivoscribe, Inc. The FLT3 mutant/wild-type allelic ratios (derived from the CE assay performed at Johns Hopkins University) were expressed as the mutant fraction of total alleles (ie, ITD/ITD + wild-type × 100%). The site conducting the MRD assay (Invivoscribe, Inc.) was blind to the clinical information regarding the sample. No information was provided regarding the presence or absence of FLT3-ITD mutations, mutation length, or the mutant/wild-type allelic ratio.
Clinical trial samples
Bone marrow samples were obtained from patients who participated in the CHRYSALIS study (www.clinicaltrials.gov NCT02014558), which was a phase 1/2 study of the novel FLT3 TKI gilteritinib (ASP2215) in R/R AML.11 Overall, 137 patients treated in this trial had FLT3-ITD mutations and received FLT3-inhibitory oral doses of 120 or 200 mg/d gilteritinib.11 Of these 137 patients, 80 had bone marrow aspirates collected at baseline and at ≥1 time point after starting treatment with gilteritinib. The primary ITD was defined as the ITD with the highest variant allele frequency (VAF; defined as FLT3 mutant reads: FLT3 total reads) at baseline in a given sample. When multiple ITDs were detected within a sample, the sum of ITDs was used. Responses were classified as CR (ie, bone marrow regenerating normal hematopoietic stem cells and achievement of a morphologic leukemia-free state with no evidence of extramedullary leukemia; absolute neutrophil count >1 × 109/L, a platelet count ≥100 × 109/L, and normal marrow differential with <5% blasts, independent of red blood cell and platelet transfusion), complete remission with incomplete platelet recovery (CRp; ie, response meets all CR criteria except that platelet count is <1 × 109/L), and CRi (ie, response meets all CR criteria except that the subject experiences residual neutropenia [absolute neutrophil count <1 × 109/L], with or without complete platelet recovery, and does not require transfusion independence). Further details regarding the CHRYSALIS study design, patient population, and outcomes are outlined in Perl et al.11
Results
Limit of detection and linearity of contrived samples
The experimental data for the MRD assay are presented in Table 1 and Figure 1. As shown in Figure 1, the linearity of the assay is excellent, in the range of 10−2 to 10−5 (R2 = +0.98-0.99). The reliable limit of detection was 5 × 10−5, although in some cases an ITD mutation was detected at even lower levels. No mutation was detected in the testing of 38 replicates of an FLT3-ITD–negative sample, indicating that the limit of blank is 0. No-template controls, which were included in each amplification run, yielded no-sequencing reads.
Spike-in validation of MRD assay
| Cell line DNA . | Expected VAF . | Total replicates, N . | Replicates ITD not detected . | Replicates ITD detected . | Sensitivity, % . |
|---|---|---|---|---|---|
| Cell line A (30-bp ITD) | 1E−02 | 4 | 0 | 4 | 100.00 |
| 1E−03 | 4 | 0 | 4 | 100.00 | |
| 1E−04 | 36 | 0 | 36 | 100.00 | |
| 5E−05 | 68 | 0 | 68 | 100.00 | |
| Cell line B (126-bp ITD) | 1E−02 | 4 | 0 | 4 | 100.00 |
| 1E−03 | 4 | 0 | 4 | 100.00 | |
| 1E−04 | 36 | 1 | 35 | 97.22 | |
| 5E−05 | 68 | 19 | 49 | 72.06 | |
| Jurkat cell line (WT) | 0 | 38 | 38 | 0 | N/A |
| Cell line DNA . | Expected VAF . | Total replicates, N . | Replicates ITD not detected . | Replicates ITD detected . | Sensitivity, % . |
|---|---|---|---|---|---|
| Cell line A (30-bp ITD) | 1E−02 | 4 | 0 | 4 | 100.00 |
| 1E−03 | 4 | 0 | 4 | 100.00 | |
| 1E−04 | 36 | 0 | 36 | 100.00 | |
| 5E−05 | 68 | 0 | 68 | 100.00 | |
| Cell line B (126-bp ITD) | 1E−02 | 4 | 0 | 4 | 100.00 |
| 1E−03 | 4 | 0 | 4 | 100.00 | |
| 1E−04 | 36 | 1 | 35 | 97.22 | |
| 5E−05 | 68 | 19 | 49 | 72.06 | |
| Jurkat cell line (WT) | 0 | 38 | 38 | 0 | N/A |
DNA from 2 cell lines with known FLT3-ITD mutations (cell line A: MV4-11, 30 bp homozygous; cell line B: PL-21, 126 bp heterozygous) was diluted into background DNA from an FLT3 wild-type cell line (Jurkat) at the ratios shown (“Expected”). The VAF is the mutation to total reads from the MRD assay and is shown for each cell line used as a source of mutant DNA (“Detected”).
N/A, not applicable; WT, wild-type.
Linearity of NGS-MRD assay. The NGS-MRD assay was performed using mutant DNA spiked into wild-type DNA. Two cell lines, each expressing different ITD mutations, were used (cell line A [MV4-11], 30 bp and cell line B [PL-21], 126 bp). The results of the assay were plotted after linear conversion as detected vs expected. Results of regression analysis are shown.
Linearity of NGS-MRD assay. The NGS-MRD assay was performed using mutant DNA spiked into wild-type DNA. Two cell lines, each expressing different ITD mutations, were used (cell line A [MV4-11], 30 bp and cell line B [PL-21], 126 bp). The results of the assay were plotted after linear conversion as detected vs expected. Results of regression analysis are shown.
Clinical samples
To validate the MRD assay for clinical use, we analyzed bone marrow aspirates from 15 patients with FLT3-ITD AML. These samples were collected at different points during the course of therapy (eg, after induction therapy or after allogeneic transplant). At the time of bone marrow collection for MRD analysis, all 15 patients were in a clinical remission, according to IWG criteria.21 For all 15 samples, the CE assay for the FLT3-ITD mutation was negative, and no cell with a leukemia-associated phenotype could be identified using 6-color multiparameter flow cytometry. The clinical and MRD data for these patients are summarized in supplemental Table 1.
The first 4 patients (supplemental Table 1, patients 1-4) had been diagnosed with FLT3-ITD–mutated AML and had achieved remission after intensive cytarabine-based induction chemotherapy. The bone marrow aspirate samples analyzed were collected at the time of hematologic recovery to confirm CR. Because AML is rarely cured with induction therapy alone, these patients would be expected to have some level of residual disease within the bone marrow. The MRD assay identified FLT3-ITD mutations in all 4 cases, at VAFs ranging from 1.35 × 10−5 to 3.33 × 10−4. The investigators who performed the MRD assay under blinded conditions correctly identified the abnormal insertion sequence matching the exact lengths of the ITD for each patient that had been initially detected by the CE assay at diagnosis, thereby providing confirmation of the specificity of the assay.
Patients in the next group (supplemental Table 1, patients 5-7) were considered clinically more high risk. Bone marrow aspirate samples were collected while these patients were in their second or third remission. FLT3-ITD mutations were detected in all 3 patients at VAFs ranging from 1.38 × 10−6 to 1.11 × 10−4. Patient 5 underwent a second allogeneic hematopoietic stem cell transplant (HSCT) following sample collection (ie, the bone marrow biopsy performed to confirm remission immediately prior to the transplant) and remained in remission while on sorafenib maintenance therapy 2.5 years after transplantation. Patients 6 and 7 received no further therapy, and both relapsed and died.
For the next set of patients (supplemental Table 1, patients 8-13), we sought to determine whether an FLT3-ITD mutation could persist during a durable remission. Each of these patients had undergone allogeneic HSCT 24-48 months prior to sample collection; in each case, the MRD assay was negative. These results suggest that the MRD assay can be used to identify patients in whom durable remission has been achieved.
Finally, we selected 2 patients (supplemental Table 1, patients 14 and 15) with FLT3-ITD AML who had relapsed following allogeneic HSCT. We obtained banked DNA that had been isolated from bone marrow aspirates collected at 2 months (patient 14) and 6 months (patient 15) after transplantation. It should be noted that, for both of these cases, the CE assay was negative for FLT3-ITD mutations, and the chimerism in the unfractionated bone marrow and peripheral blood T-cell compartments was 100% donor. The MRD assay correctly identified the length of the corresponding FLT3-ITD mutation at VAFs of 1.04 × 10−4 to 3.67 × 10−3. Both patients relapsed 2 months after these samples were collected.
NPM1 mutations had been identified at diagnosis in 11 of the 15 patients. By using the same NPM1 mutation assay that was performed at diagnosis in these 11 patient samples, an NPM1 mutation was identified at a low level in 1 patient (supplemental Table 1, patient 7) but was not detected in the other 9 patients (patients 1-4, 8, 10-12, and 14).
Relationship between MRD and survival
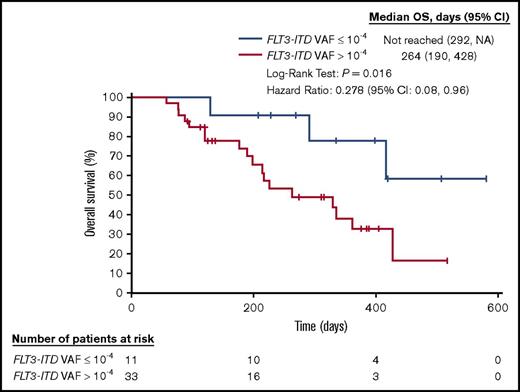
The presence of detectable MRD in patients with AML who are in remission correlates with an increased risk for relapse and reduced overall survival (OS).3-6 We analyzed bone marrow aspirates collected from patients with FLT3-ITD mutation-positive AML who were treated with the novel oral FLT3 inhibitor, gilteritinib, in a clinical trial (2215-CL-0101; NCT02014558).11 In this trial, adult patients with R/R AML were treated with oral gilteritinib as a single agent in 1 of 7 dose-escalation cohorts.11 Doses of 120 and 200 mg/d resulted in complete inhibition of FLT3 ex vivo, as measured by the plasma-inhibitory assay.22 Of the 137 FLT3-ITD–mutated patients who had received gilteritinib doses of 120 or 200 mg/d, 80 patients who had bone marrow aspirates at baseline and ≥1 additional time point were included in this analysis. Thirty-six of these 80 patients had >1 ITD. The composite CR rate (CRc; defined as CR plus CRi plus CRp) for these 80 patients was 55% (44 patients). MRD (defined as an FLT3-ITD VAF >10−4) was detected in 33 of these 44 patients (summarized in supplemental Table 2); 15 of these 44 patients who had achieved CRc had >1 ITD. The rate of MRD negativity was the same when ITDs were summed and when only the primary ITDs were used. Figure 2 shows that the survival of these MRD-positive patients is significantly worse than in those with a VAF ≤10−4, which demonstrates that gilteritinib can induce deep molecular responses, as measured by a highly sensitive MRD assay, in this patient population.
OS for subjects achieving remission in the CHRYSALIS study (ASP2215-CL-0101) according to detectable MRD. Kaplan-Meier analysis of survival for subjects treated with FLT3-inhibitory doses of gilteritinib (ASP2215) who achieved a CR, CRi, or CRp, according to the presence or absence of MRD detected by the NGS-MRD assay after treatment. MRD is defined as FLT3-ITD VAF ≥10−4. CI, confidence interval; NA, not achieved.
OS for subjects achieving remission in the CHRYSALIS study (ASP2215-CL-0101) according to detectable MRD. Kaplan-Meier analysis of survival for subjects treated with FLT3-inhibitory doses of gilteritinib (ASP2215) who achieved a CR, CRi, or CRp, according to the presence or absence of MRD detected by the NGS-MRD assay after treatment. MRD is defined as FLT3-ITD VAF ≥10−4. CI, confidence interval; NA, not achieved.
Relationship between FLT3 variant allele frequency and survival
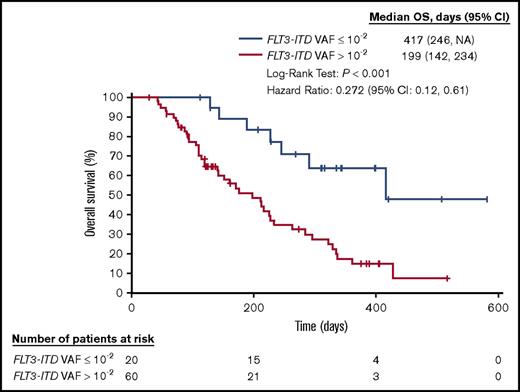
The NGS-based MRD assay for FLT3-ITD mutations was used to further characterize the molecular responses of patients treated with gilteritinib by examining the correlation between FLT3-ITD VAF and survival. Of the 80 patients from whom pre- and posttreatment samples were obtained, 20 (25%) had an FLT3-ITD VAF (mutant to total reads) ≤10−2. When we empirically examined multiple cutoff points from 10−1 to 10−4, we noted that a reduction in FLT3-ITD VAF (mutant to total reads) down to ≤10−2 was associated with a longer median OS. Therefore, we defined ≤10−2 as a molecular response. This level also approximates the lower limit of detectability for the CE assay, as performed at different institutions throughout the world.16 Of these 20 patients, 18 had an FLT3-ITD VAF ≤10−3 (major molecular response), and 13 had an FLT3-ITD VAF ≤10−4 (MRD-negative status). The median time to achieve the minimum VAF was 57 days. Patients who had a molecular response, as defined by an FLT3-ITD VAF ≤10−2, 10−3, or 10−4, had significantly longer median OS than those who did not (P < .001, Table 2; Figure 3).
OS for subjects in the CHRYSALIS study (ASP2215-CL-0101)
| Molecular response . | Achieved a molecular response . | Did not achieve a molecular response . | P . | ||
|---|---|---|---|---|---|
| n . | Median OS (95% CI), d . | n . | Median OS (95% CI), d . | ||
| ITD VAF ≤10−2 | 20 | 417 (246–NA) | 60 | 199 (142–234) | <.001 |
| ITD VAF ≤10−3 | 18 | 417 (228–NA) | 62 | 213 (143–264) | .003 |
| ITD VAF ≤10−4 (MRD negative) | 13 | 417 (228–NA) | 67 | 213 (144–264) | .002 |
| Molecular response . | Achieved a molecular response . | Did not achieve a molecular response . | P . | ||
|---|---|---|---|---|---|
| n . | Median OS (95% CI), d . | n . | Median OS (95% CI), d . | ||
| ITD VAF ≤10−2 | 20 | 417 (246–NA) | 60 | 199 (142–234) | <.001 |
| ITD VAF ≤10−3 | 18 | 417 (228–NA) | 62 | 213 (143–264) | .003 |
| ITD VAF ≤10−4 (MRD negative) | 13 | 417 (228–NA) | 67 | 213 (144–264) | .002 |
Pre- and posttreatment bone marrow samples were available from 80 patients with FLT3-ITD AML treated with FLT3-inhibitory doses of gilteritinib (ASP2215; 120 or 200 mg/d). A comparison was made between patients achieving a molecular response (FLT3-ITD VAF ≤10−2, ≤10−3, or negative as defined by ITD VAF ≤10−4) by the MRD assay and those not achieving a molecular response by the MRD assay. The P values were determined by the log-rank test.
OS for subjects in the CHRYSALIS study (ASP2215-CL-0101) according to molecular response. Kaplan-Meier analysis of survival for subjects treated with FLT3-inhibitory doses of gilteritinib (ASP2215), according to ITD VAF detected by the NGS-MRD assay after treatment.
OS for subjects in the CHRYSALIS study (ASP2215-CL-0101) according to molecular response. Kaplan-Meier analysis of survival for subjects treated with FLT3-inhibitory doses of gilteritinib (ASP2215), according to ITD VAF detected by the NGS-MRD assay after treatment.
Discussion
We have described the development of a novel NGS-based sensitive and highly specific MRD assay to detect FLT3-ITD mutations in patients with AML. This assay differs from other widely used NGS assays for AML mutations in that it starts with a PCR amplification step and is followed by NGS using a unique software program. Unlike most AML driver mutations, FLT3-ITD mutations consist of variable-length inserts of nucleotide sequences, which are not readily amenable to analysis by the conventional NGS algorithms. PCR, by itself, lacks the necessary sensitivity because of competition from the wild-type allele (unless patient-specific primers are used). This NGS-based MRD assay detected FLT3-ITD mutations in patients with AML who were in remission, including patients with FLT3-ITD allele burdens that fell below the range of linearity of the assay. It should also be noted that the assay was found to be more sensitive than conventional reverse-transcription PCR–based assays for NPM1 performed at Johns Hopkins Hospital using a published methodology.19
The MRD assay described here fulfills the requirements for a test that can be used by any clinician treating an AML patient with an FLT3-ITD mutation. This MRD assay is sensitive, specific, standardized, and is commercially available and accessible to any practitioner.23 Although there have been a number of other reported assays for detecting and monitoring FLT3-ITD mutations, these have the disadvantages of lacking sensitivity, being overly cumbersome, or not being publicly available.17,24-26 Midostaurin, a TKI with activity against FLT3,27 was recently granted regulatory approval in the United States for the treatment of FLT3-mutated AML,28 and several FLT3 inhibitors have entered pivotal trials. Therefore, the introduction of this assay is timely, because the future use of these agents could be guided by the presence or absence of detectable MRD (or VAF in patients with incomplete responses) in patients with FLT3-ITD mutations. In fact, this assay will be used to measure MRD as a secondary end point in a pivotal trial of the novel FLT3 inhibitor gilteritinib administered as maintenance therapy after allogeneic HSCT (BMT-CTN 1506/Morpho; NCT02997202). The primary end point of BMT-CTN1506 is relapse-free survival, which necessitates a prolonged clinical study. The study will assess whether there is a relationship between MRD and survival. These types of studies may substantiate further use of MRD measurement as an end point, thereby offering a potentially expeditious route to the development and approval of new drugs for FLT3-ITD AML.
With regard to the important issue of whether an FLT3-ITD mutation is a suitable target for monitoring MRD, there is a general consensus that FLT3-ITD mutations tend to occur relatively late in leukemogenesis29-30 and can be “unstable,” such that their presence between diagnosis and relapse may be discordant.31-32 Alternatively, so-called “founder mutations,” such as DNMT3a or TET2, are less useful as markers of MRD, because they often define clonal hematopoiesis but not necessarily malignant hematopoiesis.33-34 Furthermore, lesions in DNMT3a and TET2 are often missense mutations that may also be present as background noise in an amplification reaction. NPM1 mutations have clear potential for MRD assessment,6,35 but only about half of the patients with an FLT3-ITD mutation have an NPM1 mutation. When comparing FLT3-ITD mutations and other mutations as an MRD target, an apparent advantage is that each patient’s FLT3-ITD mutation is a unique length. Detecting an FLT3-ITD mutation that is the exact sequence found in the diagnostic sample confers a distinct degree of specificity in this assay. If the FLT3-ITD mutation that was present at diagnosis is detected in a bone marrow sample, it likely represents the disease.
Finally, although flow cytometry–based assays are well established and have been used to successfully detect MRD in AML patients (including in FLT3-ITD AML),36-37 expanding their use in standardized fashion across multiple institutions in different countries (as required in a multicenter clinical trial) has proven to be challenging. The MRD assay described herein will be prospectively tested as a secondary end point in an international multicenter trial.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was funded by Astellas Pharma Global Development, Inc. Editorial support for this manuscript was provided by Succinct Choice Medical Communications (Chicago, IL) and was funded by Astellas Pharma, Inc. M.J.L. was supported by a grant from the National Institutes of Health, National Cancer Institute (NCI Leukemia SPORE P50 CA100632).
The FLT3-ITD MRD assay was developed and performed by Invivoscribe, Inc.
Authorship
Contribution: M.J.L., T.T.S., and J.E.M. designed the study, performed experiments, analyzed the data, and wrote the manuscript; A.E.P., J.K.A., and E.B. analyzed data and edited the manuscript; J.H. and C.L. analyzed the data, performed statistical analyses, and edited the manuscript; and C.D.G., Z.X., A.R.C., and V.M. performed experiments, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: M.J.L. has received consultancy fees and research funding from Astellas and Novartis, honoraria from Daiichi-Sankyo and Novartis, and research funding from Fujifilm and Millenium Pharmaceuticals/Takeda Pharmaceuticals. A.E.P. has received consultancy fees from Astellas, Daiichi-Sankyo, Novartis, Pfizer, and Arog Pharmaceuticals and has participated in advisory boards for Actinium Pharmaceuticals and Asana Biosciences. J.K.A has received consultancy fees from Astellas, Syros, Bristol-Myers Squibb, Celgene, Ceplene, Novartis, and Janssen Pharmaceuticals and has served as an educational speaker for the American Society of Hematology and the National Comprehensive Cancer Network. J.H., C.L., and E.B. are employees of Astellas Pharma, Inc. J.H. has ownership of Ligacept, LLC and patents (US7852995B2 [issued] and W02013163419A1 [pending]). A.R.C., J.E.M., T.T.S., V.M., and Z.X. are employees of Invivoscribe, Inc. C.D.G. declares no competing financial interests.
Correspondence: Mark J. Levis, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, 1650 Orleans St, Room 2M44, Baltimore, MD 21287; e-mail: levisma@jhmi.edu.

![Figure 1. Linearity of NGS-MRD assay. The NGS-MRD assay was performed using mutant DNA spiked into wild-type DNA. Two cell lines, each expressing different ITD mutations, were used (cell line A [MV4-11], 30 bp and cell line B [PL-21], 126 bp). The results of the assay were plotted after linear conversion as detected vs expected. Results of regression analysis are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/8/10.1182_bloodadvances.2018015925/3/m_advances015925f1.jpeg?Expires=1769083733&Signature=VEbmLyutxFqHjgscGKZ~Y6Wk9oXNlQ8EuWHeX2TIdpXaOJyPysSKB3vFbqMczUDUX7xNPmXPlI2A9Zle4FaWqRFPGm4oAZjkzvudRxA5qbBQg-YNb5lN2ULhJA6cYyx9iZdCDheIYu1z9T21Uy7~GYWDRjERqlQ9HC0yFeCkZwAe2CDSOPyeoGYRJU1JOlqBavwHTWMCtjwcLfTrs3XqHRHcUNBSYKoHtuQw5At7r-LMyroJY7iXJYaIkgC3f51hGnKi0EoV-NP0fJBr3M7qIDPTFaH4~OI78i5N7OXlwbkDwoB159KGvZo7ko10yyXJGMPXl5-SI8oodFwLJ4CUqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)