Key Points
Ibrutinib has limited clinical efficacy in patients with relapsed or refractory peripheral T-cell lymphoma or cutaneous T-cell lymphoma.
Ibrutinib inhibits ITK.
Abstract
Ibrutinib has previously been shown to inhibit Bruton’s tyrosine kinase (BTK) and interleukin-2–inducible T-cell kinase (ITK), which mediate B-cell and T-cell receptor signaling, respectively. BTK inhibition with ibrutinib has demonstrated impressive clinical responses in a variety of B-cell malignancies. Whether ibrutinib inhibition of ITK can lead to clinical response in T-cell malignancies is unknown. We hypothesized that ibrutinib-mediated ITK inhibition in T-cell lymphoma would result in decreased signaling through the T-cell receptor pathway and promote antitumor immune response by driving selective cytotoxic Th1 CD4 effector T-cell differentiation. This pilot clinical trial evaluated 2 dose levels of ibrutinib: 560 and 840 mg orally daily. Fourteen patients with relapsed, refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma were enrolled. Both dose levels were safe and well tolerated, and no dose-limiting toxicities were observed. One patient achieved a partial response (overall response rate, 8% [1/13]). ITK occupancy studies demonstrated a mean occupancy of 50% (range, 15%-80%). Higher ITK occupancy of more than 50% correlated with higher serum levels of tumor necrosis factor-α and interferon-γ and favored a Th1 phenotype. Our data suggest that ibrutinib inhibition of ITK has limited clinical activity in T-cell lymphoma. This study is registered at www.clinicaltrials.gov as #NCT02309580.
Introduction
Peripheral T-cell lymphoma (PTCL) and advanced-stage cutaneous T-cell lymphomas (CTCL) are a heterogenous group of non-Hodgkin lymphomas associated with a poor prognosis in the relapsed setting, and novel therapeutic strategies are needed.1
Although ibrutinib was developed as an oral small molecule inhibitor of Bruton tyrosine kinase (BTK), it has also been shown to inhibit interleukin-2–inducible T-cell kinase (ITK).2 ITK is a kinase in the T-cell receptor signaling pathway and regulates T-cell proliferation, differentiation, and activation.3 ITK plays a role in the selective activation of type 2 helper T cells (Th2) and leads to production of Th2-associated cytokines.4 Ibrutinib irreversibly inhibits ITK, and thereby suppresses PLCgamma1 phosphorylation, reduces intracellular calcium elevation, reduces nuclear NFAT1 translocation, and promotes preferential Th1 differentiation and cytokine production in vitro.2
ITK is an attractive target because increased ITK expression has been reported in T-cell lymphomas, and attenuated signaling through the T-cell receptor pathway may decrease malignant T-cell growth and proliferation.5-7 In addition, a bias toward Th2 vs Th1 effector cells has been described in the immune microenvironment in T-cell lymphomas and may facilitate evasion of host immune attack resulting from decreased cytotoxic Th1 T-cell mediated immunity.8-11 In fact, effective therapies in CTCL have been associated with restoration of Th1/Th2 imbalance.9
There is growing evidence that ibrutinib has therapeutic immunomodulatory effects. In combination with anti-PD-L1 antibody and TLR9 ligand therapy in lymphoma mouse models, ibrutinib-mediated ITK inhibition lead to a shift toward the cytotoxic Th1 subset.12-13 Ibrutinib has been shown to have clinical activity in chronic graft-versus-host disease and Hodgkin lymphoma.14-15 Given minimal BTK expression on Hodgkin Reed-Sternberg cells, the therapeutic effect in Hodgkin lymphoma is potentially mediated by ITK inhibition and associated Th1 T-cell skewing in the immune microenvironment.15
On the basis of the hypothesis that ibrutinib-mediated inhibition of ITK would lead to decreased T-cell receptor pathway signaling and increased cytotoxic Th1-based antitumor immunity in T-cell lymphoma, we conducted a pilot study of ibrutinib in patients with relapsed, refractory TCL to determine the optimal biologic dose in this patient population. Secondary objectives included preliminary exploration of therapeutic efficacy and correlative studies to explore the human in vivo effect of ibrutinib-mediated ITK inhibition on the immune microenvironment.
Methods
Patients with histologically confirmed relapsed or refractory PTCL or stage 1B or greater CTCL who had failed at least 1 prior systemic therapy were eligible. Inclusion criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status 2 or lower, absolute neutrophil count 1 × 109/L, platelet count higher than 75 × 109/L (unless secondary to marrow involvement), and adequate organ function. Exclusion criteria included known bleeding diathesis, treatment with vitamin K antagonists, malabsorption, prior use of ibrutinib, central nervous system involvement by lymphoma, allogeneic stem cell transplant within 12 months, autologous stem cell transplant within 6 months, or significant comorbidity. This clinical trial was approved by the institutional review boards at the 2 participating sites (Memorial Sloan Kettering Cancer Center and Ohio State University), and informed consent was obtained from all patients (the complete study protocol is available in the supplemental Data).
Patients received ibrutinib orally at either 560 or 840 mg daily in a 28-day cycle continuously until disease progression, unacceptable toxicity, or patient or investigator decision to end therapy. The study followed a modified 3+3 dose-escalation design, with 6 patients treated at each dose level. Dose-limiting toxicity (DLT) was defined as any drug-related grade 4 hematologic toxicity, any gastrointestinal grade 4 or persistent grade 3 toxicity despite conventional therapy, any grade 3 or higher nonhematologic toxicity, or any dose delay of greater than 10 days. The optimal biologic dose was defined as the highest dose level at which at least 33% of patients experienced a DLT or, in the absence of DLT, the dose level with evidence of higher preliminary efficacy, defined as overall response rate within the first 2 cycles. An expansion cohort of 12 patients was planned.
Adverse events (AEs) were assessed by the National Cancer Institute Common Toxicity Criteria for AEs (version 4.0). Disease response assessments, performed every 2 cycles, were determined by standard criteria.16-17
Blood samples for correlative studies were collected predose on cycle 1, days 1 and 8, and cycle 2, day 1. ITK occupancy was performed as previously reported.2 For T-cell immunophenotyping, peripheral blood mononuclear cells were rested overnight in RPMI media. Surface marker immunophenotyping of Th1 and Th2 cells was performed by flow cytometry.18 For functional analysis, peripheral blood mononuclear cells were restimulated with phorbol myristate acetate and ionomycin in the presence of monensin, and then analyzed for intracellular levels of interferon-γ and interleukin-4.19 Serum cytokine analysis was performed using the V-Plex Proinflammatory Panel 1 kit from Meso Scale Diagnostics.
Results
From January 2015 to September 2016, 14 patients were enrolled: 12 in the dose-escalation phase and 2 in the expansion cohort. Thirteen patients received at least 1 dose of ibrutinib (1 patient progressed before drug administration). Baseline patient characteristics are listed in Table 1.
Patient characteristics (n = 14)
| Characteristics . | Patients, n . |
|---|---|
| Median age (range), y | 59 (44-84) |
| Male sex, n (%) | 11 (79) |
| ECOGstatus, n (%) | |
| 0 | 2 (14) |
| 1 | 11 (79) |
| 2 | 1 (7) |
| Primary histologic diagnosis | |
| Enteropathy-associated T-cell lymphoma, type II | 1 |
| γ-δ hepatosplenic T-cell lymphoma | 1 |
| HTLV-1 associated adult T-cell leukemia/lymphoma | 1 |
| Peripheral T-cell lymphoma, not otherwise specified | 3 |
| Alk-negative anaplastic large cell lymphoma | 1 |
| MF | 2 |
| MF transformed to large cell lymphoma | 2 |
| MF/SS | 2 |
| Cutaneous γ-δ T-cell lymphoma | 1 |
| Disease stage at study entry, n (%) | |
| PTCL (n = 7) | |
| Stage IV | 7 (100) |
| CTCL (n = 7) | |
| MF, stage IB | 2 (14) |
| MF, stage IVA1 | 2 (14) |
| MF transformed to large cell lymphoma, stage IV | 2 (14) |
| Cutaneous γ-δ T-cell lymphoma, stage IV | 1 (7) |
| Refractory to most recent therapy, n (%) | 14 (100) |
| Median time from diagnosis to study entry (range), y | 4.4 (0.4-14.8) |
| Median prior lines of therapy, n (range) | 4 (1-11) |
| For MF patients (n = 6), prior systemic treatment, n (%) | |
| Histone deacetylase inhibitor | 5 (83) |
| Oral retinoid therapy | 6 (100) |
| Oral methotrexate | 4 (67) |
| Interferon | 3 (50) |
| Chemotherapy | 5 (83) |
| Total skin electron beam therapy | 4 (67) |
| Denileukin diftitox | 1 (17) |
| Alemtuzumab | 1 (17) |
| Characteristics . | Patients, n . |
|---|---|
| Median age (range), y | 59 (44-84) |
| Male sex, n (%) | 11 (79) |
| ECOGstatus, n (%) | |
| 0 | 2 (14) |
| 1 | 11 (79) |
| 2 | 1 (7) |
| Primary histologic diagnosis | |
| Enteropathy-associated T-cell lymphoma, type II | 1 |
| γ-δ hepatosplenic T-cell lymphoma | 1 |
| HTLV-1 associated adult T-cell leukemia/lymphoma | 1 |
| Peripheral T-cell lymphoma, not otherwise specified | 3 |
| Alk-negative anaplastic large cell lymphoma | 1 |
| MF | 2 |
| MF transformed to large cell lymphoma | 2 |
| MF/SS | 2 |
| Cutaneous γ-δ T-cell lymphoma | 1 |
| Disease stage at study entry, n (%) | |
| PTCL (n = 7) | |
| Stage IV | 7 (100) |
| CTCL (n = 7) | |
| MF, stage IB | 2 (14) |
| MF, stage IVA1 | 2 (14) |
| MF transformed to large cell lymphoma, stage IV | 2 (14) |
| Cutaneous γ-δ T-cell lymphoma, stage IV | 1 (7) |
| Refractory to most recent therapy, n (%) | 14 (100) |
| Median time from diagnosis to study entry (range), y | 4.4 (0.4-14.8) |
| Median prior lines of therapy, n (range) | 4 (1-11) |
| For MF patients (n = 6), prior systemic treatment, n (%) | |
| Histone deacetylase inhibitor | 5 (83) |
| Oral retinoid therapy | 6 (100) |
| Oral methotrexate | 4 (67) |
| Interferon | 3 (50) |
| Chemotherapy | 5 (83) |
| Total skin electron beam therapy | 4 (67) |
| Denileukin diftitox | 1 (17) |
| Alemtuzumab | 1 (17) |
ECOG, Eastern Cooperative Oncology Group; MF, mycosis fungoides; SS, Sezary syndrome.
There were no DLTs observed at either dose level. The optimal biologic dose of ibrutinib was established at 840 mg daily, based on 1 partial response (PR) in this cohort. Ibrutinib therapy was generally well tolerated. Treatment-related AEs were predominantly grade 1 to 2, and similar to those previously reported with ibrutinib in B-cell lymphomas (Table 2). There were 11 serious AEs; however, only 1 was possibly treatment-related, an invasive Aspergillus pneumonia that developed 1 week after stopping ibrutinib therapy and resolved with antifungal therapy.
Treatment-related AEs (reported at a frequency of >10% [≥2/13 patients])
| AE . | Grades 1 and 2, n (%) . | Grades 3 and 4, n (%) . |
|---|---|---|
| Anemia | 2 (15) | |
| Thrombocytopenia | 6 (46) | 2 (15) |
| Neutropenia | 1 (8) | 2 (15) |
| Fatigue | 3 (23) | |
| Diarrhea | 4 (31) |
| AE . | Grades 1 and 2, n (%) . | Grades 3 and 4, n (%) . |
|---|---|---|
| Anemia | 2 (15) | |
| Thrombocytopenia | 6 (46) | 2 (15) |
| Neutropenia | 1 (8) | 2 (15) |
| Fatigue | 3 (23) | |
| Diarrhea | 4 (31) |
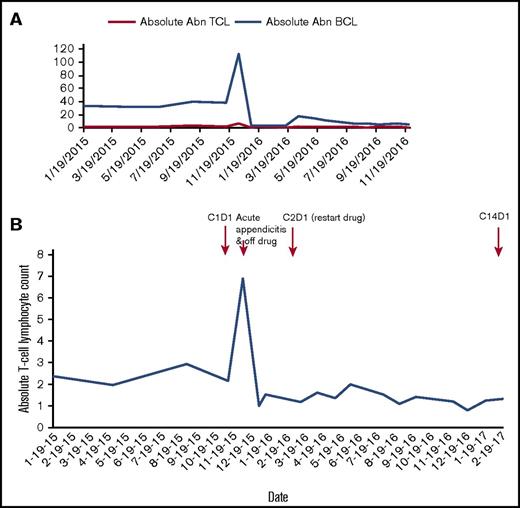
Of the 13 patients treated with ibrutinib, 1 patient with MF/SS treated at 840 mg daily achieved a PR, whereas 10 patients had progression of disease. Two patients discontinued therapy before response assessment because of serious AEs: 1 had aspiration pneumonia and respiratory failure and 1 had a bowel perforation secondary to bowel involvement with enteropathy-associated T-cell lymphoma type II; neither was attributed to ibrutinib. The median duration on ibrutinib therapy was 39 days (range, 14-373 days). Only 1 patient with MF/SS and chronic lymphocytic leukemia (CLL) who achieved a PR remains on study as of 1 March 2017, and has received ibrutinib for 14 cycles. This patient had a Sezary population at baseline with an absolute abnormal T-cell population of 2370 cells/mm3 that decreased by 50% or more with ibrutinib therapy (Figure 1). With no clinically meaningful responses in 14 patients, we estimated the probability of achieving an overall response rate of 20% was less than 5%, so the study was closed prematurely. Eleven patients have died, with no deaths while on study.
Response in 1 patient who achieved a partial response to ibrutinib therapy with SS/MF and CLL. (A) Ibrutinib therapy resulted in significant decrease in both abnormal (Abn) B-cell (CLL) and Abn T-cell (Sezary) populations. (B) The patient’s Sezary population at baseline was 2370 cells/mm3; after ibrutinib therapy, his Sezary population has ranged from 803 to 2000 cells/mm3, most recently being 1325 cells/mm3. He had a marked rise in white blood cell count in the setting of acute appendicitis in December 2015. Ibrutinib was held for 84 days during appendicitis treatment (antibiotics and surgery) and recovery. Ibrutinib was restarted March 2016, and the patient has continued on therapy to date.
Response in 1 patient who achieved a partial response to ibrutinib therapy with SS/MF and CLL. (A) Ibrutinib therapy resulted in significant decrease in both abnormal (Abn) B-cell (CLL) and Abn T-cell (Sezary) populations. (B) The patient’s Sezary population at baseline was 2370 cells/mm3; after ibrutinib therapy, his Sezary population has ranged from 803 to 2000 cells/mm3, most recently being 1325 cells/mm3. He had a marked rise in white blood cell count in the setting of acute appendicitis in December 2015. Ibrutinib was held for 84 days during appendicitis treatment (antibiotics and surgery) and recovery. Ibrutinib was restarted March 2016, and the patient has continued on therapy to date.
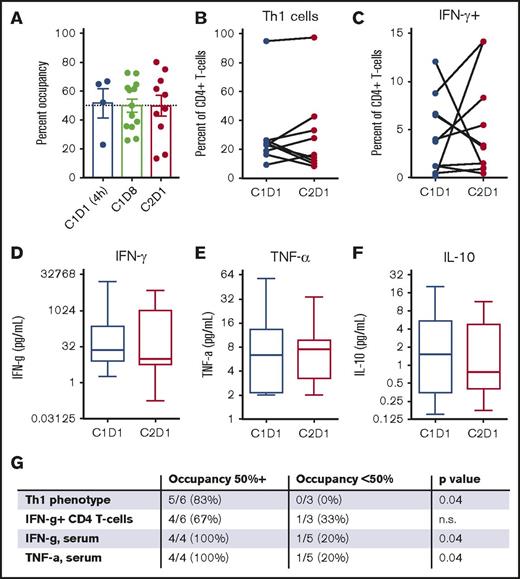
The results of correlative analyses are shown in Figure 1. The mean ITK occupancy in peripheral blood mononuclear cells was 50% (range, 15%-80%; Figure 2A), with similar results across both dose levels. Analysis of Th1/Th2 balance as assessed by surface immunophenotyping (Figure 2B) or cytokine production (Figure 2C) of peripheral blood T cells or serum cytokine levels (Figure 2D-F) did not demonstrate that ibrutinib induced a shift toward Th1 dominance across patients. An unplanned secondary analysis demonstrated that ibrutinib did promote Th1 skewing in patients in whom treatment was associated with high ITK occupancy (50% or higher), correlating with higher serum levels of tumor necrosis factor-α and interferon-γ (Figure 2G). Of note, however, higher ITK occupancy was not associated with superior clinical outcomes.
Correlative analyses of patients with T-cell lymphoma treated with ibrutinib. (A) Ibrutinib therapy achieved 50% ITK occupancy within 4 hours, which was stable over time. (B) Ibrutinib did not increase the percentage of Th1 cells (CD4+/CXCR3+/CCR6−) in the peripheral blood. (C) Ibrutinib did not increase the percentage of cells producing interferon-γ (IFN-γ) in response to restimulation. (D-F) Ibrutinib did not significantly alter serum levels of interferon-γ (Th1), tumor necrosis factor-α (TNF-α; Th1), or interleukin-10 (IL-10; Th2). (G) Patients in whom ibrutinib therapy resulted in higher ITK occupancy (50% or higher, as measured on C2D1) were more likely to increase Th1 skewing in the peripheral blood in response to ibrutinib monotherapy as compared with patients in whom ibrutinib achieved lower ITK occupancy. P value by unpaired Student t test.
Correlative analyses of patients with T-cell lymphoma treated with ibrutinib. (A) Ibrutinib therapy achieved 50% ITK occupancy within 4 hours, which was stable over time. (B) Ibrutinib did not increase the percentage of Th1 cells (CD4+/CXCR3+/CCR6−) in the peripheral blood. (C) Ibrutinib did not increase the percentage of cells producing interferon-γ (IFN-γ) in response to restimulation. (D-F) Ibrutinib did not significantly alter serum levels of interferon-γ (Th1), tumor necrosis factor-α (TNF-α; Th1), or interleukin-10 (IL-10; Th2). (G) Patients in whom ibrutinib therapy resulted in higher ITK occupancy (50% or higher, as measured on C2D1) were more likely to increase Th1 skewing in the peripheral blood in response to ibrutinib monotherapy as compared with patients in whom ibrutinib achieved lower ITK occupancy. P value by unpaired Student t test.
Discussion
This is the first reported clinical experience of ibrutinib in relapsed, refractory T-cell lymphoma. Ibrutinib was well tolerated, and the optimal biologic dose was 840 mg daily, based on 1 PR at this dose level. Unfortunately, there was a low overall response rate of 8% (1/13). The only responder had both MF/SS and CLL. It has been described in CLL that the leukemia tumor microenvironment is important for promoting maintenance and expansion of the CLL clone via various mechanisms, including suppression of T-cell and NK-cell antitumor cytotoxicity and secretion of chemokines that promote tumor growth, resistance to apoptosis, and tissue homing and adhesion to stromal cells.20 It is possible that in this responding patient, the CLL created a tumor microenvironment that favored proliferation of the Sezary clone. Thus, the ibrutinib-mediated dismantling of the tumor-promoting microenvironment may have lead to concomitant reduction of both the CLL and Sezary clones. In addition, one could speculate that ibrutinib may be more effective at eradicating cancer cells circulating in the peripheral blood vs in the skin, lymph nodes, or other tissue, perhaps because of a higher plasma drug concentration, and thus may explain the clinical activity in a patient with 2 leukemic disease clones.
Results of the correlative analyses suggest that ibrutinib monotherapy resulted in similar ITK occupancy, as previously reported in patients with CLL at the 420-mg daily dose, with maximal occupancy failing to exceed 70% to 80%, likely because of multimerization of ITK molecules in the cytoplasm, which serves as an ITK regulatory mechanism.2 Ibrutinib therapy did not result in Th1 skewing across all patients, but may promote Th1 differentiation in patients in whom ibrutinib achieves high occupancy (>50%). That patients with high ITK occupancy did not have superior outcomes with ibrutinib may suggest that Th1 skewing by ibrutinib alone is inadequate to induce antitumor responses in this heterogeneous population.
The patients treated in this study were heavily pretreated and were a clinically and biologically diverse group. Thus, it is possible that an efficacy signal was not appreciated, in the absence of a predictive biomarker for response. Given the fact that TCL compromises many different histologic subsets with diverse biology and relatively few subtypes of TCL were adequately represented in this small pilot study (few patients were enrolled with the most common subsets of PTCL, including PTCL-not otherwise specified, Alk-anaplastic large cell lymphoma, and angioimmunoblastic T-cell lymphoma), the study does not rule out that ITK inhibition could be effective in some PTCL subsets. Potential mechanisms of resistance to ibrutinib reported in TCLs may include mutations downstream of ITK, activation of the JAK-STAT pathway, and absence of cell surface T-cell receptor or proximal T-cell receptor signaling molecules.21-23 It is also possible that ITK-mediated signaling in the T-cell receptor pathway may not be required for TCL proliferation. Most patients had aggressive disease and progressed quickly on therapy, so the kinetics of immune modulation with single-agent ibrutinib may not be rapid enough to achieve disease control, and combinatorial strategies may be more effective. Ibrutinib may potentiate alternate immunotherapies, such as checkpoint blockade or CAR T-cell therapy, but alone may be ineffective at dramatically augmenting the cytotoxic Th1-based antitumor immune response, particularly in T-cell lymphomas in which defective nonmalignant T-cell responses have been observed.24-25 Recent proteomics-based analysis to characterize the target spectrum of ibrutinib and other kinase drugs suggests that ibrutinib is highly selective for BTK and not a potent ITK inhibitor.26 Based on the correlative analyses that demonstrate more Th1 skewing with higher ITK occupancy, perhaps a more potent ITK inhibitor would be associated with increased efficacy.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in this study. The authors are grateful to Stella Chang and Karl Eckert for their important contributions to this manuscript, and acknowledge that their collaboration was essential for the completion of the ITK occupancy analyses.
Direct funding for this research was issued by Pharmacyclics, Inc., through joint financial support of Pharmacyclics, Inc., and AbbVie, Inc. A.K. received funding for this research from the Lymphoma Research Foundation. This research was funded in part through the National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA008748.
Authorship
Contribution: A.K., S.V., A.Y., and S.M.H. designed the research; A.K., S.V., A.J.M., P.P., A.D., J.A.D., M.J.M., Z.Z., A.Y., and S.M.H. contributed to the study design, patient recruitment, data collection, and data analysis; and the draft report was written by A.K. and S.V. and was commented on and revised by all authors.
Conflict-of-interest disclosure: A.K. and S.M.H. have received research funding from Pharmacyclics. A.Y. has received honorarium and research funding from Janssen. J.A.D. is employed at Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Anita Kumar, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: kumara2@mskcc.org.