Key Points
SCD patients with CFA thickness of ≥0.9 mm are 9 times more likely to have had a chronic leg ulcer compared with those with <0.9 mm.
Age, O2 saturation, homocysteine, kidney injury molecule 1, and Cystatin C correlate significantly with CFA IMT.
Abstract
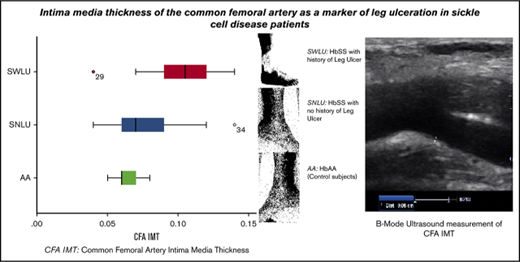
Leg ulceration is a debilitating chronic complication of sickle cell disease (SCD) the pathogenesis of which is yet to be fully elucidated. We hypothesized that SCD patients with histories of previous leg ulcers would have intima hyperplasia of the common femoral artery (CFA). We enrolled 44 SCD patients and 33 age-matched and sex-matched controls with hemoglobin AA. Anthropometric measurements, biochemical parameters, and sonographic intima-media thickness (IMT) of the CFA were determined. The median CFA IMT in SCD limbs with history of leg ulcers (SWLU) was 1.0 mm, whereas it was 0.7 mm in SCD limbs with no history of leg ulcer (SNLU) and 0.60 mm in controls (P < .001). Among the SNLU, 70.3% had CFA IMT <0.9 mm, whereas only 29.7% had CFA IMT ≥0.9 mm. Conversely, only 20.8% of SWLU had CFA IMT <0.9 mm, whereas the remaining 79.2% had CFA IMT ≥0.9 mm. All the controls had CFA IMT <0.9 mm. Binary logistic regression to determine the odds of having leg ulcer among SCD limbs with CFA IMT of ≥0.9 mm yielded an odds ratio of 9, indicating that SCD limbs with CFA IMT ≥0.9 mm had a 9 times greater risk of having leg ulcer compared with those with CFA IMT <0.9 mm. There is a significant increase in the CFA IMT of SCD limbs with ulcer compared with controls and SCD limbs without ulcer, suggesting that arterial vasculopathy plays a major role in the formation of these ulcers.
Introduction
Chronic leg ulceration is a complication of sickle cell disease (SCD) that was first reported as far back as 1910 in North America.1 The proposed pathogenesis of chronic ulcers in SCD is heterogeneous and may include the following: mechanical obstruction by dense sickled red cells, venous incompetence, bacterial infections, autonomic dysregulation, in situ thrombosis, reduced oxygen-carrying capacity from anemia, and impaired endothelial function from reduced nitric oxide bioavailability.2-4 Sickled red and white blood cells (WBC) are abnormally adherent to the vascular endothelium.4,5 This results in intimal hyperplasia, through a complex mechanism, with predilection for sites of arterial bifurcation especially in the carotid circulation.6 Venographic studies have shown that venous insufficiency is not a primary cause of sickle cell ulcerations.7
Although SCD is characterized by a progressive vasculopathy with histopathologic changes that are similar to those seen in atherosclerosis, increased accumulation of cholesterol in arterial wall macrophages (atheromas/foam cells) is not typically present in SCD vasculopathy as in atherosclerosis.8,9
Sickle cell nephropathy has also been associated with hemolysis, which plays a role in the pathogenesis of leg ulceration.10 Cystatin C (CysC) is a nonglycosylated basic protein produced at a constant rate by all investigated nucleated cells. It is freely filtered by the renal glomeruli and primarily catabolized in the tubuli and has been postulated to be an improved marker of glomerular filtration rate compared with serum creatinine.11
Selectins are found on endothelium, platelets, and leukocytes, and they mediate adhesion among these cell types. In addition, all 3 selectins contribute to leukocyte rolling during inflammation.12
We hypothesized that SCD subjects with history of leg ulceration would have intima hyperplasia of the common femoral artery (CFA), which could be evaluated by B-mode ultrasonography. This vasculopathy could be linked with renal impairment, which may be detected by determining serum levels of CysC, creatinine, and kidney injury molecule 1 (KIM1). In addition, it could be associated with alterations in soluble P selectin (sP-selectin), homocysteine (HCY), peripheral oxygen saturation (SpO2), hemoglobin concentration (Hb conc.), and fetal hemoglobin (HbF) levels.
This study aimed to compare the CFA IMT of subjects with both SCD and previous history of leg ulcer (SWLU) to that of control subjects and SCD subjects with no history of chronic leg ulcer (SNLU). We also aimed to determine the factors responsible for the intima media thickening in SCD subjects.
Patients, materials, and methods
This cross-sectional observational study included 44 SCD subjects aged 16 years and above in steady state who were recruited consecutively from the hematology outpatient clinic of the institution. Thirty-three age- and sex-matched apparently healthy volunteers (with HbAA genotype confirmed by hemoglobin electrophoresis) were also included as controls. The study was approved by the research and ethics committee of the hospital (Protocol number ERC/2013/03/11), and the study was carried out in compliance with the Declaration of Helsinki of 1964 and its subsequent revisions. Demographic data of all subjects were taken along with their blood pressure, weights, and heights. The body mass index (BMI) was calculated using the standard formula: BMI = weight (kg) ÷ height2 (m).
Excluded from this study were patients with congenital urogenital anomalies, subjects with urinary tract infection, subjects with diabetes mellitus, subjects with systemic hypertension, subjects with dyslipidemias, subjects on oral contraceptive pills and adrenergic drugs, smokers, HIV-positive subjects, subjects on hemodialysis or with massive edema, and patients on any medications that may interfere with renal function, such as cimetidine, probenecid, and angiotensin converting enzyme inhibitors. None of the SCD subjects was on hydroxyurea or Nicosan/Hemoxin.
Sample collection and laboratory analysis
All blood samples taken from participants were collected at 9:00 am and centrifuged (3500g for 5 minutes). The supernatant serum was separated, and samples were frozen at −80°C until assayed within the time limit of analyte stability as specified in the assay standard operating procedures. Quantitative enzyme-linked immunosorbent assay based on competitive immunoassay principles was used to determine HbF, sP-selectin, HCY, CysC, and KIM1 concentrations according to the manufacturer’s instructions (assay kits were procured from Neobiolab, Cambridge, MA). Total cholesterol, triglyceride, and high-density lipoprotein cholesterol assays were done using a point-of-care Cardiochek PA analyzer (equipment and cartridges from PTS Diagnostics Headquarters, Indianapolis, IN). Creatinine was assayed using spectrophotometric method (modified Jaffe-kinetic method). Spectrophotometric assay kits were procured from Agappe Diagnostics Switzerland GmbH (Cham, Switzerland).
The Hb conc., WBC, and platelet count were determined using a Sysmex Hematology Analyzer (Sysmex XP-300, Sysmex Europe GmbH, Norderstedt, Germany), whereas genotype of the sickle cell participants and the controls were determined by hemoglobin gel electrophoresis.
SpO2 was taken using the Nonin GO2 finger pulse oximeter (Nonin Medical Inc, Plymouth, MN) on the right index finger with the hand resting on a table after excessive debris had been removed from the fingers and nails with an alcohol-dampened piece of cloth.
Ultrasonography technique
Sonographic examination was performed with a Mindray real-time ultrasound machine, model DC-6 (Shenzhen Mindray Bio-Medical Electronics, Nanshan, Shenzhen, China) using a linear transducer (frequency of 7.5-12.0 MHz).
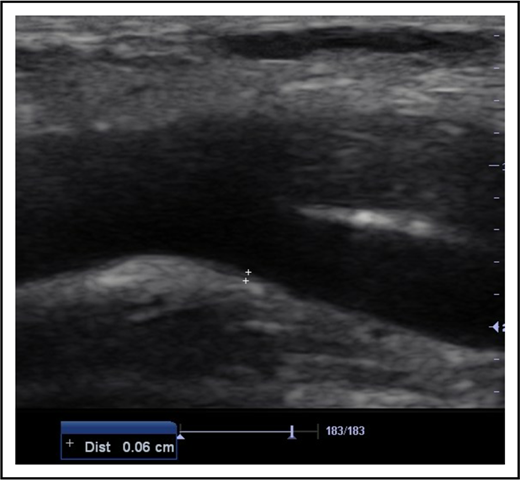
All subjects were examined in the supine position. Deep venous thrombosis of the femoral, popliteal, and calf veins was ruled out in each of the 154 limbs of the participants (88 limbs of SCD subjects and 66 limbs of controls) using compressibility test and venous distension on performance of Valsalva maneuver during B-mode ultrasonography.13 This was done to exclude the possibility of a venous ulcer. Thereafter, the CFAs were scanned in the longitudinal plane. Frozen B-mode images of 3 separate measurements of each CFA IMT were captured in end-diastole at a position on the far wall of the artery where it bifurcated into the femoral and deep femoral arteries (Figure 1). This point was chosen because IMT is most common at points of bifurcation in SCD patients.6 IMT was defined as the distance from the leading edge of the lumen-intima interface to the leading edge of the media-adventitia interface of the far arterial wall. The mean IMT from the 3 separate measurements for each CFA was calculated.14
B-mode longitudinal axis image of the CFA at its point of bifurcation. The measurement point on its far wall for IMT is denoted by the cursors (6 mm in this patient). Dist, distance.
B-mode longitudinal axis image of the CFA at its point of bifurcation. The measurement point on its far wall for IMT is denoted by the cursors (6 mm in this patient). Dist, distance.
Three subgroups/categories of participants were analyzed: Controls with HbAA, SCD with no history of leg ulcer (SNLU), and SCD with history of leg ulcer (SWLU). Ultrasonography was performed in a blinded manner with regards to the subject category by the sonologist.
The study data were entered and analyzed with the SPSS, version 22 for Windows (SPSS Inc, Chicago, IL). Normality of the data was determined using the Kolmogorov-Smirnov test. The results were presented as median with interquartile ranges (IQRs). The Mann-Whitney U test was used as appropriate. Pearson χ2 test was applied to compare 2 categorical variables, whereas the Kruskal-Wallis test was used when continuous variables were compared across >2 groups. Logarithmic transformation of the skewed data was done as necessary for HCY and KIM1 in the construction of a regression model. The relationships between CFA IMT and subject characteristics as well as CFA IMT and presence of leg ulcers were explored using Spearman’s correlational analysis and linear and logistic regression where appropriate. The level of statistical significance was set at P < .05. For original data, please contact O.O.A. at oluwagbemiga.ayoola@npmcn.edu.ng.
Results
Eighty-eight limbs of 44 SCD subjects and 66 limbs of 33 controls were evaluated (ratio 4:3). The median age of the subjects (24.50 years; [IQR], 19.5-32 years) was not significantly different from that of the controls (24.00 years [IQR, 21-27 years]) (P = .145). The SCD group was composed of 23 men (52.3%) and 21 women (47.7%), whereas the control group consisted of 16 (48.5%) men and 17 (51.5%) women.
The median Hb conc. of the SCD subjects was 8.33 g/dL (IQR 7.33-8.67), whereas the median WBC count was 9.5 × 109/L (IQR, 7.3 × 109/L to 11.7 × 109/L).
The statistical comparison of BMI, SpO2, sP-selectin, HbF, HCY, KIM1, CysC, and CFA IMT between SCD subjects and controls is shown in Table 1. HbF, HCY, KIM1, and CysC were higher in SCD subjects than in controls, whereas BMI and SpO2 were significantly lower in SCD subjects than in controls.
Comparison of SCD patients and controls
| . | SCD subjects (n = 44) . | Controls (n = 33) . | P . |
|---|---|---|---|
| BMI, kg/m2 | |||
| Median (IQR) | 18.1 (16.77-20.40) | 21.6 (19.91-25.71) | <.001 |
| O2saturation, % | |||
| Median (IQR) | 95.0 (92-98) | 98.0 (96-99) | <.001 |
| sP-selectin, ng/mL | |||
| Median (IQR) | 78.70 (29.40-93.10) | 72.7 (14.10-89.50) | <.001 |
| HbF, ng/mL | |||
| Median (IQR) | 373.30 (316.80-448.28) | 273.90 (212.23-332.23) | <.001 |
| HCY, μmol/L | |||
| Median (IQR) | 16.4 (10.18-28.83) | 8.3 (5.00-18.65) | <.001 |
| KIM1, ng/mL | |||
| Median (IQR) | 0.45 (0.32-0.62) | 0.13 (0.12-0.18) | <.001 |
| CysC, mg/L | |||
| Median (IQR) | 4.8 (1.6-7.9) | 1.1 (0.5-1.6) | <.001 |
| CFA IMT, mm | |||
| Median (IQR) | 0.8 (0.63-1.0) | 0.6 (0.60-0.70) | <.001 |
| . | SCD subjects (n = 44) . | Controls (n = 33) . | P . |
|---|---|---|---|
| BMI, kg/m2 | |||
| Median (IQR) | 18.1 (16.77-20.40) | 21.6 (19.91-25.71) | <.001 |
| O2saturation, % | |||
| Median (IQR) | 95.0 (92-98) | 98.0 (96-99) | <.001 |
| sP-selectin, ng/mL | |||
| Median (IQR) | 78.70 (29.40-93.10) | 72.7 (14.10-89.50) | <.001 |
| HbF, ng/mL | |||
| Median (IQR) | 373.30 (316.80-448.28) | 273.90 (212.23-332.23) | <.001 |
| HCY, μmol/L | |||
| Median (IQR) | 16.4 (10.18-28.83) | 8.3 (5.00-18.65) | <.001 |
| KIM1, ng/mL | |||
| Median (IQR) | 0.45 (0.32-0.62) | 0.13 (0.12-0.18) | <.001 |
| CysC, mg/L | |||
| Median (IQR) | 4.8 (1.6-7.9) | 1.1 (0.5-1.6) | <.001 |
| CFA IMT, mm | |||
| Median (IQR) | 0.8 (0.63-1.0) | 0.6 (0.60-0.70) | <.001 |
All control subjects had CFA IMT <0.9 mm. Among the 88 limbs of the SCD subjects, 56.8% (n = 50) had CFA IMT <0.9 mm, whereas 43.2% (n = 38) had CFA IMT ≥0.9 mm. None of the controls had leg ulcer in the past or currently. Of the 44 SCD subjects, none had active ulcer, whereas 16 (36.4%) individuals had a history of previous chronic leg ulcer. Out of the 88 SCD limbs, 64 (72.7%) had no history of previous leg ulcer, whereas 24 (27.3%) had a history of ulcer. Five (11.4%) SCD subjects had left leg ulcer, whereas 3 (6.8%) had right leg ulcer. Bilateral leg ulcer was as common as unilateral leg ulcer (n = 8 [18.2%]). Overall, leg ulcer was more common in male subjects (9) compared with female subjects (7), resulting in a male:female ratio of 1.3:1. However, more women had right leg ulcer (2 vs 1), whereas more men had left leg ulcer (4 vs 1). The median duration period of ulcers in SWLU subjects was 10.5 months (IQR 8.3-14.0 months).
Table 2 shows the categories of subjects and controls based on the presence or absence of leg ulcer and comparison of their BMI, Hb conc., WBC, platelet count, SpO2, sP-selectin, HbF, HCY, KIM1, CysC, creatinine, and CFA IMT values. There was significant negative weak point biserial correlation between history of ulcer and SpO2 (rpb = −0.26; P = .013). Correlation analysis done to determine the relationship between CFA IMT and several parameters in the SCD subjects is shown in Table 3. Table 4 shows the results of multiple regressions done to assess predictability of CFA IMT from several parameters in the SCD subjects.
Categories of patients and controls based on the presence or absence of leg ulcer
| . | HbAA (Gp 1) . | SNLU (Gp 2) . | SWLU (Gp 3) . | P (Gp 1 vs 2 vs 3) . | P (Gp 1 vs 2) . | P (Gp 1 vs 3) . | P (Gp 2 vs 3) . |
|---|---|---|---|---|---|---|---|
| n (limbs) | 66 | 64 | 24 | ||||
| BMI, median, kg/m2 | 21.64 | 18.08 | 19.84 | <.001* | <.001† | .002† | .298† |
| Hb conc., median, g/dL | ND | 8.33 | 7.67 | NA | NA | NA | .003† |
| WBCs, median, ×109/L | ND | 9.20 | 9.80 | NA | NA | NA | .136† |
| Platelets, median, ×109/L | ND | 285.50 | 305.00 | NA | NA | NA | .851† |
| O2 saturation, median, % | 98.00 | 95.50 | 93.00 | <.001* | <.001† | <.001† | .033† |
| sP-selectin, median, ng/mL | 72.70 | 78.15 | 79.40 | .001* | .001† | .003† | .399† |
| HbF, median, ng/mL | 273.90 | 369.90 | 397.25 | <.001* | <.001† | <.001† | .626† |
| HCY, median, μmol/L | 8.30 | 13.85 | 13.72 | <.001* | <.001† | <.001† | .154† |
| KIM1, median, pg/mL | 125.00 | 445.00 | 460.00 | <.001* | <.001† | <.001† | .771† |
| CysC, median, mg/L | 1.10 | 4.50 | 5.70 | <.001* | <.001† | <.001† | .047† |
| Creatinine, median, μmol/L | ND | 80.0 | 90.5 | NA | NA | NA | .368† |
| CFA IMT, median, mm | 0.60 | 0.70 | 1.0 | <.001* | <.001† | <.001† | <.001† |
| . | HbAA (Gp 1) . | SNLU (Gp 2) . | SWLU (Gp 3) . | P (Gp 1 vs 2 vs 3) . | P (Gp 1 vs 2) . | P (Gp 1 vs 3) . | P (Gp 2 vs 3) . |
|---|---|---|---|---|---|---|---|
| n (limbs) | 66 | 64 | 24 | ||||
| BMI, median, kg/m2 | 21.64 | 18.08 | 19.84 | <.001* | <.001† | .002† | .298† |
| Hb conc., median, g/dL | ND | 8.33 | 7.67 | NA | NA | NA | .003† |
| WBCs, median, ×109/L | ND | 9.20 | 9.80 | NA | NA | NA | .136† |
| Platelets, median, ×109/L | ND | 285.50 | 305.00 | NA | NA | NA | .851† |
| O2 saturation, median, % | 98.00 | 95.50 | 93.00 | <.001* | <.001† | <.001† | .033† |
| sP-selectin, median, ng/mL | 72.70 | 78.15 | 79.40 | .001* | .001† | .003† | .399† |
| HbF, median, ng/mL | 273.90 | 369.90 | 397.25 | <.001* | <.001† | <.001† | .626† |
| HCY, median, μmol/L | 8.30 | 13.85 | 13.72 | <.001* | <.001† | <.001† | .154† |
| KIM1, median, pg/mL | 125.00 | 445.00 | 460.00 | <.001* | <.001† | <.001† | .771† |
| CysC, median, mg/L | 1.10 | 4.50 | 5.70 | <.001* | <.001† | <.001† | .047† |
| Creatinine, median, μmol/L | ND | 80.0 | 90.5 | NA | NA | NA | .368† |
| CFA IMT, median, mm | 0.60 | 0.70 | 1.0 | <.001* | <.001† | <.001† | <.001† |
Gp, group; NA, not applicable; ND, not determined.
Kruskal-Wallis test applied.
Mann-Whitney U test applied.
Correlation of CFA IMT with SCD subject parameters
| . | Correlation coefficient . | P . |
|---|---|---|
| Age, y | 0.49 | <.001 |
| Weight, kg | 0.00 | .97 |
| BMI, kg/m2 | −0.08 | .37 |
| O2 saturation, % | −0.19 | .03 |
| Hb conc., g/dL | −0.13 | .24 |
| WBCs, ×109/L | 0.17 | .12 |
| Platelets, ×109/L | 0.11 | .33 |
| sP-selectin, ng/mL | 0.15 | .06 |
| HbF, ng/mL | 0.11 | .19 |
| HCY, μmol/L | 0.28 | <.001 |
| KIM1, pg/mL | 0.19 | .02 |
| CysC, mg/L | 0.31 | <.001 |
| Creatinine, μmol/L | −0.10 | .34 |
| . | Correlation coefficient . | P . |
|---|---|---|
| Age, y | 0.49 | <.001 |
| Weight, kg | 0.00 | .97 |
| BMI, kg/m2 | −0.08 | .37 |
| O2 saturation, % | −0.19 | .03 |
| Hb conc., g/dL | −0.13 | .24 |
| WBCs, ×109/L | 0.17 | .12 |
| Platelets, ×109/L | 0.11 | .33 |
| sP-selectin, ng/mL | 0.15 | .06 |
| HbF, ng/mL | 0.11 | .19 |
| HCY, μmol/L | 0.28 | <.001 |
| KIM1, pg/mL | 0.19 | .02 |
| CysC, mg/L | 0.31 | <.001 |
| Creatinine, μmol/L | −0.10 | .34 |
Multiple regression of the statistically significant values in Table 3
| CFA IMT . | Coef. . | Std. err. . | t . | P . | 95% CI . | |
|---|---|---|---|---|---|---|
| LL . | UL . | |||||
| Age, y | 0.001 | 0.000 | 6.74 | <.001 | 0.001 | 0.002 |
| O2 saturation, % | 0.000 | 0.000 | 0.64 | .53 | −0.001 | 0.001 |
| Log HCY, μmol/L | 0.005 | 0.001 | 3.28 | <.001 | 0.002 | 0.008 |
| Log KIM1, pg/mL | 0.007 | 0.002 | 2.98 | <.001 | 0.002 | 0.011 |
| CysC, mg/L | 0.001 | 0.000 | 1.39 | .17 | 0.000 | 0.001 |
| Constant | −0.040 | 0.043 | −0.93 | .35 | −0.124 | 0.045 |
| CFA IMT . | Coef. . | Std. err. . | t . | P . | 95% CI . | |
|---|---|---|---|---|---|---|
| LL . | UL . | |||||
| Age, y | 0.001 | 0.000 | 6.74 | <.001 | 0.001 | 0.002 |
| O2 saturation, % | 0.000 | 0.000 | 0.64 | .53 | −0.001 | 0.001 |
| Log HCY, μmol/L | 0.005 | 0.001 | 3.28 | <.001 | 0.002 | 0.008 |
| Log KIM1, pg/mL | 0.007 | 0.002 | 2.98 | <.001 | 0.002 | 0.011 |
| CysC, mg/L | 0.001 | 0.000 | 1.39 | .17 | 0.000 | 0.001 |
| Constant | −0.040 | 0.043 | −0.93 | .35 | −0.124 | 0.045 |
R2 = 0.4195.
CI, confidence interval; Coef., coefficient of regression; LL, lower limit; Std. err., standard error of regression; UL, upper limit.
Of the 64 limbs in SNLU group, 70.3% (n = 45) had CFA IMT <0.9 mm, whereas only 29.7% (n = 19) had CFA IMT ≥0.9 mm. Only 20.8% (n = 5) of the 24 limbs in the SWLU group had CFA IMT <0.9 mm, whereas the remaining 79.2% (n = 19) had CFA IMT ≥0.9 mm. CFA IMT was significantly different (Table 2) between SWLU and SNLU (P < .001). There was a moderately strong correlation between the presence of leg ulcer and the CFA IMT (rpb = 0.50; P < .001).
Binary logistic regression done to determine the odds of having leg ulcer among SCD patients with CFA IMT of ≥0.9 mm (Table 5) showed an odds ratio of 9 indicating that SCD patients with CFA IMT ≥0.9 mm had a 9 times greater risk of having leg ulcer compared with those with CFA IMT <0.9 mm.
Logistic regression of CFA IMT vs leg ulcer
| Variable . | OR . | Std. err. . | z . | P . | 95% CI . | |
|---|---|---|---|---|---|---|
| LL . | UL . | |||||
| CFAIMT, mm | ||||||
| <0.9 | 1.00 | |||||
| ≥0.9 | 9.00 | 5.15 | 3.84 | <.001 | 2.93 | 27.63 |
| Constant | 0.11 | 0.05 | −4.66 | <.001 | 0.04 | 0.28 |
| Variable . | OR . | Std. err. . | z . | P . | 95% CI . | |
|---|---|---|---|---|---|---|
| LL . | UL . | |||||
| CFAIMT, mm | ||||||
| <0.9 | 1.00 | |||||
| ≥0.9 | 9.00 | 5.15 | 3.84 | <.001 | 2.93 | 27.63 |
| Constant | 0.11 | 0.05 | −4.66 | <.001 | 0.04 | 0.28 |
OR, odds ratio.
Discussion
Leg ulceration in SCD has a variable prevalence, being low before the age of 10 years.4 It is most common in HbSS and seen less often in HbSC disease or HbS-β thalassemia.4 It also has a variable geographical distribution, affecting 75% of HbSS patients in Jamaica but only 8% to 10% of North American patients.4 It is a common manifestation of SCD in Nigeria with a reported prevalence of 1.7% to 29.4%.15-18 Leg ulcers are said to be more common in men with a male-to-female ratio of 1.3:1,17 which is exactly what was obtained in this study.
SCD is associated with a chronic arterial vasculopathy said to result from complex endothelial dysfunction. This has various cardiovascular manifestations, such as ischemic stroke, pulmonary hypertension, autosplenectomy, priapism, retinopathy, nephropathy, fetal growth restriction, and leg ulceration.19-23 CFA IMT is a marker for cardiovascular disease in asymptomatic adults.24 It is a better predictor of peripheral arterial disease than carotid IMT in patients with coronary arterial disease.25 In this study, CFA IMT was significantly higher in SWLU than control subjects and SNLU. Furthermore, there was statistically significant moderately strong correlation between the presence of leg ulcer and the CFA IMT. The odds ratio of 9 corroborates the theory that vasculopathy plays a major role in the pathogenesis of ulcer in SCD patients.
Chronic hypoxia has been implicated in the pathogenesis of vasculopathy in SCD. It increases the secretion of vascular endothelial-derived growth factor and thrombospondin-1, both of which have mitogenic effects on endothelial smooth muscle and connective tissue leading to muscle hypertrophy and structural remodeling.26 In this study, SpO2 was significantly lower in SCD subjects than controls. It was also significantly lower in SWLU than in control subjects and SNLU. SpO2 had a significant correlation with CFA IMT, although it was not a strong predictor of thickened CFA IMT when other factors were considered in the multiple regression model.
Elevated plasma concentration of HCY is an established risk factor for venous thrombosis and arterial occlusion.27 In this study, HCY was significantly higher in SCD subjects than control subjects. The median HCY was also higher in SWLU than in SNLU.
Platelet activation has been implicated in atherothrombosis in SCD patients.28 Although platelet count was higher in SWLU than SNLU, it was not statistically significant. Similarly, sP-selectin, which is a marker of platelet activation with increased expression in patients with SCD,17 was also slightly higher in SWLU than in SNLU, but this was also not statistically significant. The reasons for these were not immediately evident.
Leg ulceration in SCD has been suggested to be an early and visible sign of end organ disease, including renal.29 CysC, which is an early biochemical marker of renal impairment, was significantly higher in SCD subjects than in controls. It was also significantly higher in SWLU than in both controls and SNLU. Although serum creatinine was higher in SWLU than in SNLU, this was not statistically significant. In addition, it showed no significant correlation with CFA IMT. This may be due to the fact that CysC is independent of age, sex, muscle mass, volume status, and hepatic dysfunction (which may be seen in SCD) in contrast to serum creatinine.11,30 Furthermore, it has been shown to be a better marker of renal function than serum creatinine level.30-33 Indeed, use of serum creatinine for estimating renal function often leads to suboptimal assessment in SCD patients.34 The drawback of CysC is that it is expensive.30
There are conflicting reports on the role of HbF in the pathogenesis of leg ulcer in SCD. Earlier studies showed that increased HbF levels was protective against the development of leg ulcers in SCD patients.35,36 However, recent studies showed no relationship between HbF and leg ulcers.7,37 In this study, although HbF levels were higher in SWLU than in SNLU, it was not statistically significant. Furthermore, no significant correlation was noted between HbF levels and CFA IMT.
Low Hb conc. levels are associated with increased incidence of leg ulcer formation.35 In this study, the Hb conc. was significantly lower in SWLU than SNLU, although there was no significant correlation between Hb conc. and CFA IMT.
Elevated white cell count has also been proposed to play a role in the pathogenesis of ulcers in SCD due to its potential to affect blood flow through vessels.38 This study showed a higher white cell count in SWLU than in SNLU subjects, although not statistically significant. In addition, no significant correlation was noted between WBC and CFA IMT.
In conclusion, there is a paucity of studies on the role of vasculopathy in the pathogenesis of leg ulcer in SCD disease. Ultrasonography of CFA IMT could aid in the elucidation of this role. This study demonstrated a significant increase in the CFA IMT of SWLU compared with controls and SNLU, suggesting that arterial vasculopathy plays a major role in the formation of these ulcers. We also found that patients with elevated CFA IMT ≥0.9 mm had a 9 times higher risk of having had chronic leg ulcer than those with lesser values.
Acknowledgment
The authors acknowledge the financial contribution made by the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Osun State, Nigeria.
Authorship
Contribution: O.O.A. conceptualized the study, carried out all sonographic examinations, wrote a portion of final manuscript, and proofread the final manuscript; R.A.B. screened and recruited study subjects, carried out the hematological investigations, and proofread the final manuscript; U.U.O. proof-read the final manuscript; T.A.A. carried out the biochemical investigations and proofread the final manuscript; C.C.O. conducted the statistical analysis and wrote a portion of the final manuscript; and B.M.I. wrote a portion of the final manuscript, proofread the final manuscript, and is the corresponding author.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bukunmi M. Idowu, Department of Radiology, Union Diagnostics and Clinical Services Plc, No 37 Tejuosho St, Yaba, Lagos 930001, Nigeria; e-mail: ibmcontacts@gmail.com.