Key Points
Treatments with therapeutic phlebotomy and HU are associated with improved OS and decreased risk of thrombosis in older PV patients.
Phlebotomy and HU are underused in our sample of older PV patients.
Abstract
Current guidelines recommend therapeutic phlebotomy for all polycythemia vera (PV) patients and additional cytoreductive therapy (eg, hydroxyurea [HU]) for high-risk PV patients. Little is known about the impact of these therapies in the real-world setting. We conducted a retrospective cohort study of older adults diagnosed with PV from 2007 to 2013 using the linked Surveillance, Epidemiology, and End Results–Medicare database. Multivariable Cox proportional hazards models were used to assess the effect of phlebotomy and HU on overall survival (OS) and the occurrence of thrombotic events. Of 820 PV patients (median age = 77 years), 16.3% received neither phlebotomy nor HU, 23.0% were managed with phlebotomy only, 19.6% with HU only, and 41.1% with both treatments. After a median follow-up of 2.83 years, 37.2% (n = 305) of the patients died. Phlebotomy (yes/no; hazard ratio [HR] = 0.65; 95% confidence interval [CI], 0.51-0.81; P < .01), increasing phlebotomy intensity (HR = 0.71; 95% CI, 0.65-0.79; P < .01), and a higher proportion of days covered (PDC) by HU were all significantly associated with lower mortality. When thrombosis was the outcome of interest, phlebotomy (yes/no; HR = 0.52; 95% CI, 0.42-0.66; P < .01) and increasing phlebotomy intensity (HR = 0.46; 95% CI, 0.29-0.74; P < .01) were significantly associated with a lower risk of thrombotic events, so was a higher HU PDC. In this population-based study of older adults with PV reflecting contemporary clinical practice, phlebotomy and HU were associated with improved OS and decreased risk of thrombosis. However, both treatment modalities were underused in this cohort of older PV patients.
Introduction
Polycythemia vera (PV), a myeloproliferative neoplasm with a median age at diagnosis of 65 years,1 manifests with overproduction of mature red blood cells and is associated with reduced overall survival.2 The clinical course is characterized by an increased risk of thrombosis, which is the major cause of death among PV patients.3-5 Therefore, prevention of thrombosis is the main goal of PV treatments.
Using therapeutic phlebotomy (to maintain hematocrit below 45%) and low-dose aspirin is recommended for all PV patients.6,7 For patients at high risk for thrombotic events (aged older than 60 years or with a history of thrombosis), additional cytoreductive therapy is indicated with recommendation to use hydroxyurea (HU) or recombinant interferon-α as the first-line treatment.8,9 The recommendation for HU use is based on expert opinion and a limited number of nonrandomized studies for PV patients10-13 and the results of a randomized trial for high-risk patients with essential thrombocythemia.14 A recent analysis of the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) project data observed that among high-risk PV patients, HU only was favored in comparison with phlebotomy only in lowering the rate of fatal and nonfatal cardiovascular events and overall mortality.11 Even though both National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) guidelines recommend HU as the first-line cytoreductive treatment of older PV patients,8,9 the evidence behind this recommendation is considered weak and its merit continues to be questioned by some experts, as, in their view, HU does not prevent thrombosis or prolong survival and hence does not have a role in PV management.15
To evaluate the effectiveness of phlebotomy and HU among older adults with PV in the real-world setting, we conducted a large population-based cohort study in the United States using the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Patients and methods
Data source
The SEER-Medicare database, developed by the National Cancer Institute and the Centers for Medicare and Medicaid Services, links patient-level information on new cancer diagnoses from SEER registry data to Medicare enrollment and claims for inpatient and outpatient physician services, as well as prescription drugs.16 The SEER registries have been shown to be nationally representative, and account for ∼28% of the US population.17 Since 2001, SEER registries have been required to report PV, providing a unique opportunity to access a representative sample of PV patients. The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Study population
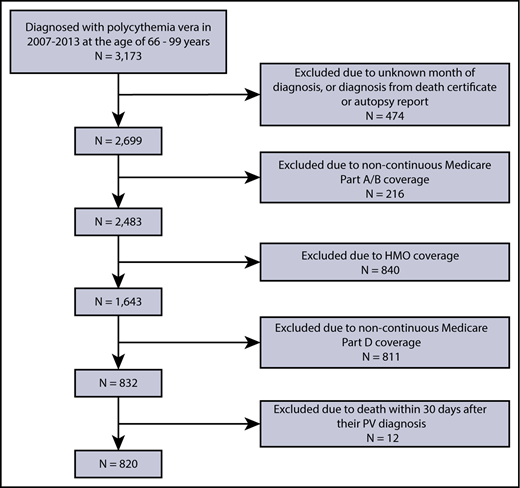
We assembled a retrospective cohort of patients newly diagnosed with PV from 2007 to 2013 using International Classification of Diseases for Oncology, Third Edition code 9950 to identify PV patients from SEER. We initially assigned date of diagnosis as the first day of the SEER-reported month of diagnosis. To address potential reporting delay in SEER,18 we also searched Medicare claims within 1 year before the SEER-reported PV diagnosis date for evidence (1 inpatient claim or at least 2 outpatient claims >30 days apart with an International Classification of Diseases, Ninth Revision, Clinical Modification code of 238.4) of an earlier date of PV diagnosis. All patients were followed through 31 December 2014 or death, whichever occurred first. All patients fulfilled the following eligibility criteria: (1) aged 66 years (because of the need to obtain Medicare claims for 1 year before diagnosis to calculate comorbidity scores and other covariates) to 99 years (to avoid potential changes in the pattern of care for patients ≥100 years19,20 ) at diagnosis; (2) had known month of diagnosis; (3) were not reported from autopsy or death certificate only; and (4) had continuous Medicare fee-for-service coverage (Parts A and B) and were not enrolled in health maintenance organizations (HMOs) from 12 months before diagnosis to the end of follow-up; and (5) were continuously enrolled in Medicare Part D from diagnosis to the end of follow-up; and (6) survived at least 30 days after their PV diagnosis.
Treatment assessment
Therapeutic phlebotomy after diagnosis was assessed by Healthcare Common Procedure Coding System code 99195. Phlebotomy users were defined as those who had at least 1 phlebotomy claim from diagnosis to the end of follow-up (end of study or outcome of interest, whichever came first). Phlebotomy intensity was defined as the number of phlebotomies per year. Phlebotomy intensity was treated as a time-dependent variable in the multivariate regression models (updated in the models every 6 months).
Information regarding HU use after diagnosis was obtained via Medicare Part D claims. HU users were defined as those who had at least 1 HU prescription claim from diagnosis to the end of follow-up. HU proportion of days covered (PDC) was calculated as the percentage of days from diagnosis to the end of follow-up covered by HU prescriptions. PDC is commonly used to describe patients’ adherence to medications.21
Definition of outcomes
We evaluated 2 outcomes of interest. The first outcome was overall survival. The second outcome was occurrence of a thrombotic event after PV diagnosis, including venous thrombosis (deep-vein thrombosis, pulmonary embolism, superficial thrombophlebitis), arterial thrombosis (stroke, transient ischemic attack, angina, acute myocardial infarction/acute coronary syndrome, arterial embolism, peripheral arterial thrombosis), or sudden death. We adapted an algorithm from Gupta et al22 to assess thrombotic events (see supplemental Appendix).
Other variables of interest
We obtained information on sociodemographic characteristics: age at diagnosis, sex, race/ethnicity, and Part D low-income subsidy (a proxy for individual socioeconomic status and reduced cost-sharing for oral medications). Influenza vaccination within the 12 months prior to PV diagnosis was included as an indicator for health care access. Because performance status is an important factor in clinical decision-making, we evaluated each patient’s disability status,23 a claims-based proxy of poor performance status before diagnosis. To assess comorbidity, we searched for ICD-9 diagnosis codes in the 12 months prior to PV diagnosis that appeared on any inpatient claims, or at least 2 outpatient/physician claims >30 days apart.24 A modified Elixhauser score was developed by removing prior thrombosis from the original Elixhauser score.25 Thrombosis during the 12 months prior to PV diagnosis was assessed following the previously described algorithm,22 excluding sudden death.
Statistical analysis
Categorical variables were presented using frequencies and percentages. Continuous variables were summarized by median and interquartile range (IQR). Consistent with SEER-Medicare requirement to preserve confidentiality, all categories with 10 or fewer patients were reported as <11. We compared the characteristics of patients who fulfilled our eligibility criteria and were included in the study vs those who were excluded from the study, as well as patients who received treatment of PV (phlebotomy, hydroxyurea, or both) vs those who did not, using χ2 tests for categorical variables (eg, race) and Student t tests for continuous variables (eg, age). Time to event was analyzed with Kaplan-Meier methods and the log-rank test. Multivariable Cox proportional hazards regression models were used to examine the effect of treatments on survival. Multivariable competing risk regression models were used to examine the effect of treatments on thrombosis after diagnosis, with death as the competing risk. The assumption of proportional hazards was checked by Schoenfeld residuals test.26
Based on our a priori understanding of PV, all multivariable models included patient’s age at diagnosis, sex, race, disability status, low-income subsidy, influenza vaccination in the 12 months prior to PV diagnosis, modified Elixhauser comorbidity score, and prior thrombosis as covariates. Influenza vaccination in the 12 months prior to PV diagnosis was included as a proxy for the intensity of interaction between the patient and the health care system because the study outcomes were ascertained by claims and may have been influenced to some extent by access to health care.27
We conducted a sensitivity analysis to compare patients who were treated with only HU with patients who were treated with only phlebotomy. To avoid detection bias at the time of PV diagnosis, another sensitivity analysis with thrombosis as the outcome was performed by excluding patients who had experienced thrombotic events within 30 days of PV diagnosis.5 All tests were 2-sided with an α of 0.05 and were conducted in SAS version 9.4 (SAS Inc, Cary, NC).
Results
Patient characteristics and types of treatments received
Of 3173 patients with newly diagnosed PV, we identified 820 patients who met our study criteria (Figure 1). No statistically significant differences were observed between the 820 patients who were included in the study and the 2353 patients who were excluded, in terms of age, sex, and race.
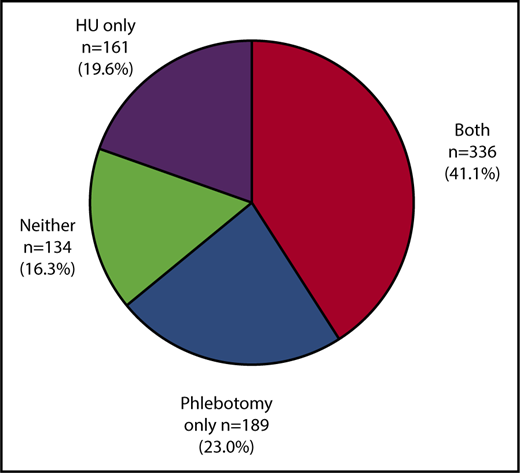
Of the 820 patients included in the study, the median age was 77.0 years (IQR, 71.0-83.0 years). Of the 820 patients, 467 (57.0%) were women, and the majority (91.2%) were white (Table 1). During the study period, 41.1% (n = 336) of patients received both phlebotomy and HU (concurrently or sequentially), 23.0% (n = 189) of patients underwent phlebotomy only, 19.6% (n = 161) received HU only, and 16.3% (n = 134) received neither phlebotomy nor HU (Figure 2). On average, phlebotomy users had a median of 7.0 phlebotomies (IQR, 3.0-12.0) from diagnosis to the end of follow-up and their median phlebotomy intensity (number of phlebotomies per year) was 2.3 (IQR, 1.1-4.1). The median HU PDC was 61.6% (IQR, 35.2%-80.1%) for HU users.
Patient characteristics by treatment among 820 PV patients
| . | All (N = 820), n (%) . | Treated* (n = 686), n (%) . | Untreated* (n = 134), n (%) . | P . |
|---|---|---|---|---|
| Age, median (IQR), y | 77.0 (71.0-83.0) | 77.0 (72.0-83.0) | 77.0 (71.0-85.0) | .33 |
| Sex | ||||
| Female | 467 (57.0) | 395 (57.6) | 72 (53.7) | .41 |
| Male | 353 (43.0) | 291 (42.4) | 62 (46.3) | |
| Race | ||||
| White | 748 (91.2) | 630 (91.8) | 118 (88.1) | .16 |
| Nonwhite | 72 (8.8) | 56 (8.2) | 16 (11.9) | |
| Modified Elixhauser score | ||||
| 0 | 326 (39.8) | 282 (41.1) | 44 (32.8) | <.01 |
| 1 | 215 (26.2) | 189 (27.6) | 26 (19.4) | |
| ≥2 | 279 (34.0) | 215 (31.3) | 64 (47.8) | |
| Prior thrombosis | ||||
| No | 712 (86.8) | 595 (86.7) | 117 (87.3) | .86 |
| Yes | 108 (13.2) | 91 (13.3) | 17 (12.7) | |
| Disability | ||||
| No | 716 (87.3) | 610 (88.9) | 106 (79.1) | <.01 |
| Yes | 104 (12.7) | 76 (11.1) | 28 (20.9) | |
| Low-income subsidy | ||||
| No | 606 (74.0) | 510 (74.3) | 96 (71.6) | .51 |
| Yes | 214 (26.0) | 176 (25.7) | 38 (28.4) | |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||
| No | 357 (43.5) | 292 (41.6) | 65 (48.5) | .20 |
| Yes | 463 (56.5) | 394 (57.4) | 69 (54.5) |
| . | All (N = 820), n (%) . | Treated* (n = 686), n (%) . | Untreated* (n = 134), n (%) . | P . |
|---|---|---|---|---|
| Age, median (IQR), y | 77.0 (71.0-83.0) | 77.0 (72.0-83.0) | 77.0 (71.0-85.0) | .33 |
| Sex | ||||
| Female | 467 (57.0) | 395 (57.6) | 72 (53.7) | .41 |
| Male | 353 (43.0) | 291 (42.4) | 62 (46.3) | |
| Race | ||||
| White | 748 (91.2) | 630 (91.8) | 118 (88.1) | .16 |
| Nonwhite | 72 (8.8) | 56 (8.2) | 16 (11.9) | |
| Modified Elixhauser score | ||||
| 0 | 326 (39.8) | 282 (41.1) | 44 (32.8) | <.01 |
| 1 | 215 (26.2) | 189 (27.6) | 26 (19.4) | |
| ≥2 | 279 (34.0) | 215 (31.3) | 64 (47.8) | |
| Prior thrombosis | ||||
| No | 712 (86.8) | 595 (86.7) | 117 (87.3) | .86 |
| Yes | 108 (13.2) | 91 (13.3) | 17 (12.7) | |
| Disability | ||||
| No | 716 (87.3) | 610 (88.9) | 106 (79.1) | <.01 |
| Yes | 104 (12.7) | 76 (11.1) | 28 (20.9) | |
| Low-income subsidy | ||||
| No | 606 (74.0) | 510 (74.3) | 96 (71.6) | .51 |
| Yes | 214 (26.0) | 176 (25.7) | 38 (28.4) | |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||
| No | 357 (43.5) | 292 (41.6) | 65 (48.5) | .20 |
| Yes | 463 (56.5) | 394 (57.4) | 69 (54.5) |
Patients who received neither phlebotomy nor hydroxyurea are categorized as “untreated.” All other patients are categorized as “treated.”
Other cytoreductive treatments in our study were used infrequently, including 17 patients (2%) treated with ruxolitinib and <11 patients (<1.3%) treated with interferons. None of the patients received busulfan. Over a median follow-up time of 2.75 years (IQR, 1.58-4.67 years), evolution to myelofibrosis, acute myeloid leukemia, or either myelofibrosis or acute myeloid leukemia occurred in a small number of patients: 19 (2.3%), 18 (2.2%), and 36 (4.3%), respectively.
Overall survival
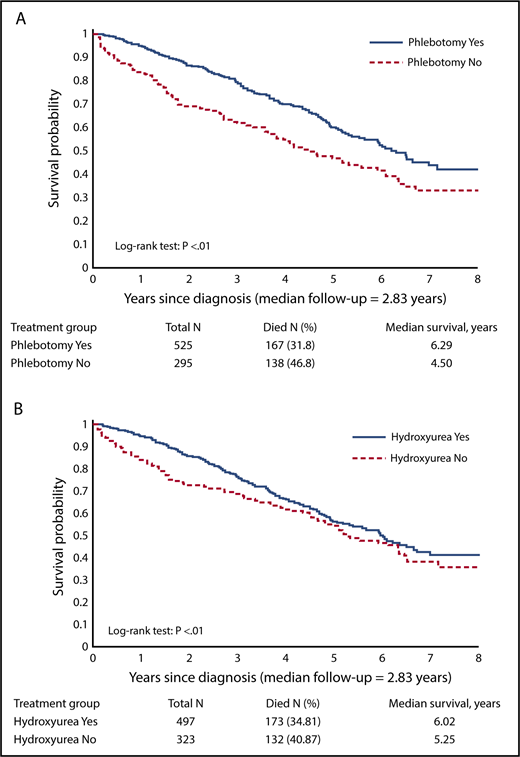
After a median follow-up of 2.83 years, death was recorded in 37.2% (n = 305) of all patients. The median survival was 6.29 years for phlebotomy users and 4.50 years for phlebotomy nonusers (log-rank test, P < .01; Figure 3A). The median survival was 6.02 years for HU users and 5.25 years for HU nonusers (log-rank test, P < .01; Figure 3B).
Kaplan-Meier curves for overall survival. (A) By phlebotomy use. (B) By HU use.
Kaplan-Meier curves for overall survival. (A) By phlebotomy use. (B) By HU use.
In the multivariable Cox model, compared with phlebotomy nonusers, patients who received phlebotomy had a significantly reduced risk of death (hazard ratio [HR] = 0.65; 95% confidence interval [CI], 0.51-0.81; P < .01). Increasing phlebotomy intensity also appeared to be associated with a lower mortality (HR = 0.71; 95% CI, 0.65-0.79; P < .01). Every 10% increase of HU PDC was associated with an 8% to 9% lower risk of death (HR = 0.92; 95% CI, 0.89-0.95; P < .01 in model 1; HR = 0.91; 95% CI, 0.88-0.94; P < .01 in model 2; Table 2). Advanced age, male sex, having >1 comorbidity, and potential underutilization of the health care system as reflected by not receiving influenza vaccination were associated with a significantly increased risk of mortality (Table 2).
Multivariable Cox proportional hazards analysis for overall survival after PV diagnosis by treatments and patient characteristics (n = 820)
| Characteristic* . | Model 1 . | Model 2 . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Phlebotomy | ||||||
| No | 1.00 | |||||
| Yes | 0.65 | 0.51-0.81 | <.01 | |||
| Phlebotomy intensity (times per year) | 0.71 | 0.65-0.79 | <.01 | |||
| HU PDC (every 10%) | 0.92 | 0.89-0.95 | <.01 | 0.91 | 0.88-0.94 | <.01 |
| Age, y | 1.08 | 1.07-1.10 | <.01 | 1.08 | 1.07-1.10 | <.01 |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.36 | 1.06-1.73 | .01 | 1.37 | 1.07-1.75 | .01 |
| Race | ||||||
| White | 1.00 | 1.00 | ||||
| Nonwhite | 1.18 | 0.79-1.76 | .43 | 1.17 | 0.78-1.74 | .45 |
| Modified Elixhauser score | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.19 | 0.88-1.63 | .26 | 1.24 | 0.91-1.68 | .18 |
| ≥2 | 1.46 | 1.10-1.94 | .01 | 1.38 | 1.04-1.84 | .03 |
| Prior thrombosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.04 | 0.75-1.45 | .80 | 0.98 | 0.71-1.36 | .91 |
| Disability | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.31 | 0.93-1.85 | .12 | 1.30 | 0.92-1.82 | .13 |
| Low-income subsidy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.23 | 0.94-1.60 | .13 | 1.18 | 0.90-1.54 | .23 |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.78 | 0.62-0.99 | .04 | 0.79 | 0.63-1.00 | .05 |
| Characteristic* . | Model 1 . | Model 2 . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Phlebotomy | ||||||
| No | 1.00 | |||||
| Yes | 0.65 | 0.51-0.81 | <.01 | |||
| Phlebotomy intensity (times per year) | 0.71 | 0.65-0.79 | <.01 | |||
| HU PDC (every 10%) | 0.92 | 0.89-0.95 | <.01 | 0.91 | 0.88-0.94 | <.01 |
| Age, y | 1.08 | 1.07-1.10 | <.01 | 1.08 | 1.07-1.10 | <.01 |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 1.36 | 1.06-1.73 | .01 | 1.37 | 1.07-1.75 | .01 |
| Race | ||||||
| White | 1.00 | 1.00 | ||||
| Nonwhite | 1.18 | 0.79-1.76 | .43 | 1.17 | 0.78-1.74 | .45 |
| Modified Elixhauser score | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.19 | 0.88-1.63 | .26 | 1.24 | 0.91-1.68 | .18 |
| ≥2 | 1.46 | 1.10-1.94 | .01 | 1.38 | 1.04-1.84 | .03 |
| Prior thrombosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.04 | 0.75-1.45 | .80 | 0.98 | 0.71-1.36 | .91 |
| Disability | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.31 | 0.93-1.85 | .12 | 1.30 | 0.92-1.82 | .13 |
| Low-income subsidy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.23 | 0.94-1.60 | .13 | 1.18 | 0.90-1.54 | .23 |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.78 | 0.62-0.99 | .04 | 0.79 | 0.63-1.00 | .05 |
All variables in the table were simultaneously included in the same model. The only difference between models 1 and 2 was that model 1 included phlebotomy as a binary variable and model 2 included the intensity of phlebotomy (number of phlebotomies each year).
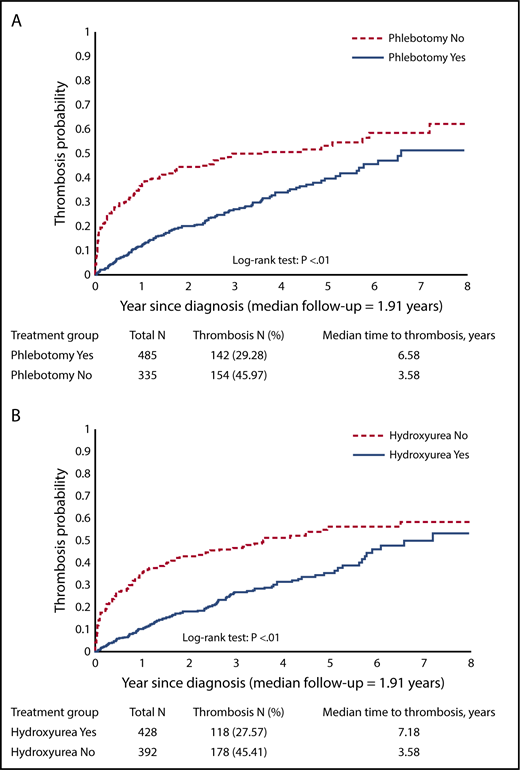
Occurrence of thrombotic events
Thrombotic events were observed in 296 patients (36.1%). The percentage of patients developing thrombotic events was 46.0% (n = 154) among phlebotomy nonusers and 29.3% (n = 142) among phlebotomy users. The percentage of patients developing thrombotic events was 45.4% (n = 178) among HU nonusers and 27.6% (n = 118) among HU users. Kaplan-Meier curves depicting the incidence of thrombotic events were significantly different by phlebotomy use (log-rank test, P < .01; Figure 4A) and by HU use (log-rank test, P < .01; Figure 4B). In the multivariable competing risk model, compared with phlebotomy nonusers, those who received phlebotomy had a significantly lower risk of thrombosis. Increasing phlebotomy intensity also appeared to be associated with a lower risk of thrombotic events after diagnosis (HR = 0.52; 95% CI, 0.42-0.66; P < .01). Every 10% increase of HU PDC was associated with an 8% lower risk of thrombosis (HR = 0.92; 95% CI, 0.89-0.96; P < .01 in model 1; HR = 0.92; 95% CI, 0.88-0.95; P < .01 in model 2; Table 3). Other factors that were associated with higher risk of thrombosis included having 2 or more comorbidities, and receiving low-income subsidy (Table 3).
Kaplan-Meier curves for thrombosis after diagnosis. (A) By phlebotomy use. (B) By HU use.
Kaplan-Meier curves for thrombosis after diagnosis. (A) By phlebotomy use. (B) By HU use.
Competing risk models for thrombosis after PV diagnosis by treatments and patient characteristics (n = 820)
| Characteristic* . | Model 1 . | Model 2 . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Phlebotomy | ||||||
| No | 1.00 | |||||
| Yes | 0.52 | 0.42-0.66 | <.01 | |||
| Phlebotomy intensity, times per year | 0.46 | 0.29-0.74 | <.01 | |||
| HU PDC, every 10% | 0.92 | 0.89-0.96 | <.01 | 0.92 | 0.88-0.95 | <.01 |
| Age, y | 1.01 | 0.99-1.02 | .55 | 1.01 | 0.99-1.03 | .27 |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 0.94 | 0.74-1.20 | .61 | 0.98 | 0.77-1.24 | .86 |
| Race | ||||||
| White | 1.00 | 1.00 | ||||
| Nonwhite | 0.74 | 0.46-1.20 | .23 | 0.85 | 0.53-1.38 | .52 |
| Modified Elixhauser score | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.28 | 0.94-1.75 | .11 | 1.30 | 0.95-1.77 | .10 |
| ≥2 | 1.37 | 1.01-1.86 | .05 | 1.38 | 1.03-1.86 | .03 |
| Prior thrombosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.17 | 0.82-1.67 | .40 | 1.01 | 0.71-1.43 | .98 |
| Disability | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.98 | 0.66-1.45 | .92 | .01 | 0.69-1.47 | .98 |
| Low-income subsidy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.48 | 1.10-1.99 | .01 | 1.38 | 1.03-1.85 | .03 |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.99 | 0.78-1.26 | .96 | 0.98 | 0.78-1.25 | .88 |
| Characteristic* . | Model 1 . | Model 2 . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Phlebotomy | ||||||
| No | 1.00 | |||||
| Yes | 0.52 | 0.42-0.66 | <.01 | |||
| Phlebotomy intensity, times per year | 0.46 | 0.29-0.74 | <.01 | |||
| HU PDC, every 10% | 0.92 | 0.89-0.96 | <.01 | 0.92 | 0.88-0.95 | <.01 |
| Age, y | 1.01 | 0.99-1.02 | .55 | 1.01 | 0.99-1.03 | .27 |
| Sex | ||||||
| Female | 1.00 | 1.00 | ||||
| Male | 0.94 | 0.74-1.20 | .61 | 0.98 | 0.77-1.24 | .86 |
| Race | ||||||
| White | 1.00 | 1.00 | ||||
| Nonwhite | 0.74 | 0.46-1.20 | .23 | 0.85 | 0.53-1.38 | .52 |
| Modified Elixhauser score | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.28 | 0.94-1.75 | .11 | 1.30 | 0.95-1.77 | .10 |
| ≥2 | 1.37 | 1.01-1.86 | .05 | 1.38 | 1.03-1.86 | .03 |
| Prior thrombosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.17 | 0.82-1.67 | .40 | 1.01 | 0.71-1.43 | .98 |
| Disability | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.98 | 0.66-1.45 | .92 | .01 | 0.69-1.47 | .98 |
| Low-income subsidy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.48 | 1.10-1.99 | .01 | 1.38 | 1.03-1.85 | .03 |
| Influenza vaccination within 12 mo prior to PV diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.99 | 0.78-1.26 | .96 | 0.98 | 0.78-1.25 | .88 |
All variables in the table were simultaneously included in the same model. The only difference between models 1 and 2 was that model 1 included phlebotomy as a binary variable and model 2 included the intensity of phlebotomy (number of phlebotomies each year).
Sensitivity analysis
Multivariable Cox regression analysis suggested that there was no significant difference in overall survival between patients who received only HU and patients who received only phlebotomy (P = .52). In addition, there was no significant difference in thrombosis between these 2 groups (P = .28) as shown by our sensitivity analysis using multivariable competing risk model.
In another sensitivity analysis, we excluded those who had thrombotic events within 30 days after their PV diagnosis. Therefore, the sample size was reduced to 754 patients. Results from a multivariable competing risk model showed that phlebotomy (yes/no) was still associated with a lower risk of thrombosis after diagnosis, however, not significantly (HR = 0.79; 95% CI, 0.61-0.1.03; P = .09). Increasing phlebotomy intensity (HR = 0.50; 95% CI, 0.31-0.82; P = .01) and HU PDC (HR = 0.96; 95% CI, 0.92-0.99; P = .03 in the model with phlebotomy [yes/no]; HR = 0.95; 95% CI, 0.92-0.99; P = .02 in the model with phlebotomy intensity) were still associated with a significant lower risk of thrombosis after diagnosis.
Discussion
In this population-based cohort study reflecting contemporary clinical practice, we observed improved overall survival and decreased risk of thrombosis in older PV patients treated with phlebotomy and HU. However, both treatment modalities were underused in this population of 820 older patients, as only 64.0% underwent therapeutic phlebotomy, and 60.6% received HU. These findings suggest that patients in our study cohort were undertreated according to ELN and NCCN guidelines.8,9 Same guidelines recommend second-line cytoreductive treatment of patients who are refractory to or intolerant of HU, including ruxolitinib, interferons, and busulfan. The fact that very few patients received these medications in our study further illustrates the underutilization of cytoreductive treatments. Ruxolitinib was approved by the US Food and Drug Administration as a second-line treatment of PV patients refractory to or intolerant of HU in December 2014 and its use could not be adequately evaluated by our study, which ended in December 2014.
The effectiveness of phlebotomy with/without HU has been studied previously in clinical trials.7,10,11,28 We observed similar results using real-world data. The CYTO-PV study7 demonstrated that using phlebotomy, HU, or both to maintain the hematocrit below 45% significantly decreased cardiovascular death and occurrence of major thrombosis. Nearly half of the patients in the CYTO-PV study were recruited >2 years after PV diagnosis, and the median age of all patients was 64.5 years. In our study, only newly diagnosed PV patients were included, and information on all cytoreductive treatments since diagnosis was retrieved and analyzed. Additionally, our study focused on older patients (at least 66 years old at the time of diagnosis), who usually are underrepresented in clinical trials.29
In our study, increasing HU PDC was associated with a reduced incidence of thrombotic events among older PV patients, which is consistent with a recent reanalysis of the ECLAP project data,11 showing that treatment with HU was associated with a reduced risk of thrombosis. In addition, we observed improved survival among patients treated with HU alone or in combination with phlebotomy. Patients receiving combined treatment with HU and phlebotomy may belong to a subgroup that has a disease with an increased proliferative capacity requiring a higher treatment intensity to achieve hematocrit control. These patients in our study had a significant reduction of thrombosis and survival benefit, and the benefit appeared to be more pronounced for those who underwent more phlebotomies per year. This observation is consistent with the findings of a phlebotomy intensity analysis that included PV patients from ECLAP and CYTO-PV studies and showed that increasing phlebotomy frequency was not associated with increased risk of thrombosis and in fact a decreased risk of thrombosis in a subgroup of patients, suggesting that a higher risk of thrombosis described among patients receiving >3 phlebotomies per year in a large retrospective study of PV patients30 might be related to an uncontrolled hematocrit value, rather than the use of phlebotomies per se.31
Advancing age, being male, and having 2 or more comorbidities were associated with worse survival. Having ≥2 comorbidities was also associated with more thrombotic events after diagnosis. Although more patients with ≥2 comorbidities were left untreated in our study, multivariable models controlled for the impact of the above covariates while accessing the association between treatments of our interest and outcomes.
We also evaluated the feasibility of using evolution to myelofibrosis or acute myeloid leukemia as another outcome of interest. Because only 35 patients were identified with such an evolution during the follow-up, we did not have sufficient statistical power to conduct the analysis.
A major strength of our study is the large, population-based cohort of older (ie, high-risk) PV patients treated in the real-world setting. The nationwide Medicare claims data covered a variety of health services, regardless of where the patients sought their care, therefore providing comprehensive information on the treatments received by patients. Furthermore, the linked SEER-Medicare database also enabled us to control for many other factors that may influence PV treatment decisions and risk of thrombotic events after PV diagnosis, such as sociodemographic factors, comorbidity, and disability status.
Although our study generated a number of important findings, limitations exist. First, we were unable to capture agents not covered by Medicare, such as aspirin, because we relied exclusively on Medicare claims to evaluate PV treatment. In addition, the SEER-Medicare database did not contain information on the results of laboratory tests, such as hematocrit level and leukocyte count, so we could not incorporate these important clinical parameters into the analysis. Another limitation is the need to exclude a large number of patients without continuous Medicare coverage as well as HMO participants, limiting the number of analyzed patients to 820 of 3173 (26%), although those who were excluded were not different from those who were included in terms of age, sex, and race. Also, we could not compare patients who used phlebotomy and HU concurrently with those who used these 2 treatments sequentially due to small sample sizes. Furthermore, we do not know why some patients received both phlebotomy and HU or why patients changed treatment modalities during the course of the study. Lastly, our study is observational in design and may be subject to potential selection bias related to unobserved factors that may affect treatment and outcomes of interest. However, our analysis included extensive controls for health status (both comorbidity and disability status), prior thrombosis, sociodemographic factors, and receipt of preventive health care (influenza vaccination), which should reduce the potential for bias.
Overall, our findings confirm clinical utility for both therapeutic phlebotomy and hydroxyurea in the management of older PV patients who are at high risk for thrombotic events, supporting recommendations of ELN and NCCN guidelines.8,9 However, our population-based study suggests that both therapeutic phlebotomy and hydroxyurea are underused. Improved dissemination and implementation of the guidelines may translate to better patient outcomes.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute, Office of Research, Development and Information, Centers for Medicare and Medicaid Services, Information Management Services, Inc, and the SEER Program tumor registries in the creation of the SEER-Medicare database.
This work was supported by the Frederick A. Deluca Foundation. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Institutes of Health, National Cancer Institute’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors. The sources of support were not used for any portion of the current study.
Authorship
Contribution: N.A.P. acquired funding, conceptualized the study, wrote the original draft of the manuscript, and reviewed and edited the manuscript; M.Z. provided formal analysis, conceptualized the study, wrote the original draft of the manuscript, and reviewed and edited the manuscript; A.M.Z. conceptualized the study and reviewed and edited the manuscript; R.W. established the methodology, conceptualized the study, and reviewed and edited the manuscript; X.W., A.J.D., S.F.H., S.G., and S.D.G. reviewed and edited the manuscript; and X.M. conceptualized the study, wrote the original draft of the manuscript, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: N.A.P. consulted for and received honoraria from Alexion and Pfizer, and received research funding from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Celator, Pfizer, Astex Pharmaceuticals, CTI Biopharma, Celgene, Genentech, and LAM Therapeutics. A.M.Z. has consulted for and received honoraria from AbbVie, Ostuka, Pfizer, Gilead, Celgene, Ariad, Incyte, Agios, Novartis, Takeda, Daiichi Sankyo, Boehringer Ingelheim, and Takeda, and has served on a speaker’s bureau for, and received research funding from, Celgene, Pfizer, Incyte, ADC Therapeutics, Medimmune, Takeda, AbbVie, and Boehringer Ingelheim. A.J.D. received research funding from Celgene. S.F.H. has received honoraria from Pharmacyclics and consulted for Celgene and Janssen. S.D.G. has consulted for and receives research funding from Celgene. X.M. consulted for Celgene and Incyte. The remaining authors declare no competing financial interests.
Correspondence: Nikolai A. Podoltsev, Department of Internal Medicine, Section of Hematology, School of Medicine, Yale University, 37 College St, New Haven, CT 06510; e-mail: nikolai.podoltsev@yale.edu.