Key Points
Following vascular injury, RGS10 tunes the platelet signaling network to ensure the establishment of an effective hemostatic plug.
It does this by limiting Gq- and Gi2-dependent signaling and by agonist-selective effects on responses to thrombin, ADP, and TxA2.
Abstract
Platelets express ≥2 members of the regulators of G protein signaling (RGS) family. Here, we have focused on the most abundant, RGS10, examining its impact on the hemostatic response in vivo and the mechanisms involved. We have previously shown that the hemostatic thrombi formed in response to penetrating injuries consist of a core of fully activated densely packed platelets overlaid by a shell of less-activated platelets responding to adenosine 5′-diphosphate (ADP) and thromboxane A2 (TxA2). Hemostatic thrombi formed in RGS10−/− mice were larger than in controls, with the increase due to expansion of the shell but not the core. Clot retraction was slower, and average packing density was reduced. Deleting RGS10 had agonist-specific effects on signaling. There was a leftward shift in the dose/response curve for the thrombin receptor (PAR4) agonist peptide AYPGKF but no increase in the maximum response. This contrasted with ADP and TxA2, both of which evoked considerably greater maximum responses in RGS10−/− platelets with enhanced Gq- and Gi-mediated signaling. Shape change, which is G13-mediated, was unaffected. Finally, we found that free RGS10 levels in platelets are actively regulated. In resting platelets, RGS10 was bound to 2 scaffold proteins: spinophilin and 14-3-3γ. Platelet activation caused an increase in free RGS10, as did the endothelium-derived platelet antagonist prostacyclin. Collectively, these observations show that RGS10 serves as an actively regulated node on the platelet signaling network, helping to produce smaller and more densely packed hemostatic thrombi with a greater proportion of fully activated platelets.
Introduction
Most platelet agonists, including thrombin, adenosine 5′-diphosphate (ADP), and thromboxane A2 (TxA2), activate platelets through G protein–coupled receptors. G proteins are αβγ heterotrimers, becoming active when the α subunit binds guanosine triphosphate (GTP) and reverting to the inactive state when the GTP is hydrolyzed to guanosine diphosphate (GDP). Signal duration is normally limited by the α subunit’s intrinsic GTPase activity, a relatively slow process that can be greatly accelerated by members of the regulators of G protein signaling (RGS) family.1-3 Human and mouse platelets predominantly express 2 members of this family: RGS10 and RGS18.4,5 RGS18 is primarily expressed in hematopoietic cells.6-8 RGS10 is expressed more widely.9-11 Both proteins have a compact structure that consists primarily of a conserved RGS domain, and each has been shown to accelerate GTP hydrolysis by Gq and Gi but not by GS.6,12-15
The best evidence to date that RGS proteins regulate platelet activation in vivo comes from studies on transgenic mice. Gi2 is the predominant Gi family member in platelets and is especially important for mediating platelet responses to ADP P2Y12 receptors.16,17 Replacing platelet Gi2α with a G184S variant that has diminished ability to interact with RGS proteins produces a gain of function, even in hemizygotes,18,19 as does deleting RGS1820-22 or RGS10.23 These observations suggest that RGS10 and RGS18 normally act as brakes on platelet activation but leave open how they impact hemostatic plug formation in vivo and how their effects might be regulated to allow a robust response to injury while also avoiding premature platelet activation.
An effective hemostatic response requires assembly of a complex 3-dimensional structure. We and other investigators have shown that the hemostatic response produces a core of fully activated and densely packed platelets overlaid by a shell of less-activated and loosely packed platelets.24-27 This structure was originally described in the mouse microvasculature, but it occurs in larger vessels as well.28 Notably, it appears to contribute to and reflect the formation of agonist concentration gradients radiating from the site of injury.29-31 These gradients are agonist specific, determined in part by the hindered transport of molecules in the narrow gaps between platelets, especially in the core region.32 As a result, platelets in different regions of hemostatic plugs are exposed to different combinations and concentrations of agonists. Thrombin and fibrin are limited to the thrombus core, whereas ADP and TxA2 are the major drivers of events in the thrombus shell.24,33,34
Here, we have asked how the signal-limiting ability of platelet RGS proteins contributes to the development of a stable hemostatic plug, focusing on RGS10 because it is the most abundant RGS protein expressed in human and mouse platelets.5,35 Our studies will be presented in 2 parts. In the first, we show that hemostatic plugs formed in RGS10−/− mice are larger than in controls and that this difference is due to expansion of the shell region but not the core. We also show that deleting RGS10 affects signaling events that are Gq and Gi mediated but not those mediated by G13. Most notably, the effects of deleting RGS10 have proved to be agonist selective, causing a considerable increase in the maximal responses to ADP and TxA2 but not to thrombin. In the second part of the study, we show that free RGS10 levels in resting platelets are regulated through binding interactions with 2 scaffold proteins: spinophilin and 14-3-3γ. Platelet activation and platelet suppression (exposure to endothelium-derived prostacyclin [PGI2]) cause an increase in free RGS10. Collectively, these observations indicate that RGS10 is much more than a simple brake on unwanted platelet activation, serving instead as an actively regulated node on the platelet signaling network and helping to shape the architecture of the hemostatic plug.
Methods
Materials and additional methods can be found in supplemental Methods.
Vascular injury: platelet and fibrin accumulation
Hemostatic thrombus formation was observed in the cremaster muscle microcirculation of male mice aged 8-12 weeks, as previously described.24 Briefly, Alexa Fluor 568–labeled anti-CD41 antibody F(ab′)2 fragments, Alexa Fluor 647–labeled anti–P-selectin, and Alexa Fluor 647 anti-fibrin antibodies were administered via a catheter in the jugular vein. Arterioles 30-50 μm in diameter were studied. Vascular injury was induced using a pulsed nitrogen dye laser fired through the microscope objective. Thrombus formation was observed for 3 min at 1.9 frames per second and analyzed using SlideBook 6 software (Intelligent Imaging Innovations, Denver, CO). For the embolization studies, anti-CD41–labeled platelets accumulating at the injury site were acquired with 4 × 4 binning and 4-millisecond exposure time; only the red channel was used to increase image acquisition to 35.5 frames per second, with a total of 6300 frames collected in 3 minutes. Data were collected in a region of interest drawn downstream of the thrombus.
Clot retraction
Clot retraction was measured by 2 methods: visual assay and automated light scattering assay.
Visual assay.
Whole mouse blood was drawn in 0.38% sodium citrate and spun at 200g to obtain platelet-rich plasma. Samples were adjusted with platelet-poor plasma to 6 × 108 platelets/mL, recalcified, and stimulated with 10 U/mL thrombin. Clot retraction was recorded at 15-minute intervals at 37°C and analyzed using ImageJ software.
Automated light scattering assay.
Blood was collected from the inferior vena cava of anesthetized mice into 3.8% sodium citrate and activated with 5 U/mL thrombin in the presence of 2 mM CaCl2. Light scatter was measured with a Thrombodynamics Analyser System (HemaCore LLC, Moscow, Russia).36
Measurement of intracellular calcium concentration
Intracellular calcium was measured as described.37 Isolated platelets were suspended in Tyrode’s buffer without Ca++ and loaded with Fura-2/AM (5 μM) in the presence of Pluronic F-127 (0.2 μg/mL) for 20 minutes at 37°C. The platelets were then washed and resuspended in Tyrode’s buffer with no extracellular Ca++. Changes in Fura-2 fluorescence were detected with an SLM Aminco Bowman Series 2 spectrophotometer, with excitation at 340 and 380 nm and emission measurement at 510 nm.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Data were analyzed using the Student t test. P ≤ .05 was considered statistically significant.
Results
In agreement with an earlier report by Hensch et al,23 we found that RGS10−/− mice are grossly normal in appearance. Their blood counts are normal (supplemental Table) as is expression of RGS18 (supplemental Figure 1). We did not observe the hyperglycemia that was reported by investigators at The Jackson Laboratory (http://www.informatics.jax.org/external/ko/lexicon/1405.html), and we found no differences in initial weight gain compared with littermate controls (supplemental Figure 1). Because the RGS10−/− mice were initially on a mixed background, these and subsequent studies were performed on littermates produced by heterozygous RGS10+/− crosses.
The hemostatic response in vivo in RGS10−/− mice
Platelet function in vivo was measured using a laser to make penetrating injuries in cremaster muscle arterioles. We have previously shown that this is a model of hemostasis rather than thrombosis.24 We have also shown that, as long as the injuries fully penetrate the vessel wall, the hemostatic response is the same with a laser or a sharpened probe.24 The resultant hemostatic plug consists of a core of densely packed P-selectin+ platelets overlaid with a shell of loosely packed P-selectin− platelets. P-selectin, in this case, is used as a marker for α-granule exocytosis and to distinguish core from the shell. Thrombin activity and fibrin deposition are limited to the core.33
Figure 1A shows mean platelet accumulation following injury detected with fluorescently tagged F(ab′)2 anti-CD41 (αIIb). Platelet accumulation occurred at the same initial rate in RGS10−/− mice and controls; however, after reaching a maximum, there was a decline in CD41 fluorescence in wild-type (WT) mice that did not occur in the RGS10−/− mice. Measured at the end of the observation period, total platelet accumulation was approximately one-third greater than in the controls (Figure 1B). This increase was due to an expansion of the P-selectin− shell region and not the P-selectin+ core region, which, if anything, was slightly smaller than in controls (Figure 1A,C). Fibrin accumulation was unaffected (Figure 1D).
RGS10−/− mice form larger more stable thrombi. Confocal intravital fluorescence microscopy was performed to follow platelet accumulation (A-B), P-selectin expression (A,C), fibrin deposition (D), and platelet embolization (E) after making small penetrating injuries in cremaster muscle arterioles with a laser in RGS10−/− mice and littermate controls. Bar graphs represent the CD41+ (B) and P-selectin+ (C) areas at the end of the 3-minute observation period. N = 78 injuries performed in 11 mice. (D) Fibrin accumulation detected with a fibrin-specific antibody (24 injuries in 3 mice). (E) The mean fluorescence area of platelet clumps passing through a virtual analysis window placed immediately downstream of the injury site. The data were pooled into early (i) and late (ii) time points for analysis. N = 19 injuries for WT and 18 injuries for RGS10−/− in 3 mice. (Fi) The decline over time in the relative porosity of hemostatic thrombi formed in WT and RGS10−/− mice infused with caged FITC-albumin. Data are mean ± SEM, 22 injuries in 4 WT mice and 23 injuries in 4 RGS10−/− mice. (Fii) ∆MFI measured 180 seconds after injury. ∆MFI, increment in mean fluorescence intensity measured over the entire thrombus at 15-second intervals immediately after a light flash that causes the albumin to fluoresce.
RGS10−/− mice form larger more stable thrombi. Confocal intravital fluorescence microscopy was performed to follow platelet accumulation (A-B), P-selectin expression (A,C), fibrin deposition (D), and platelet embolization (E) after making small penetrating injuries in cremaster muscle arterioles with a laser in RGS10−/− mice and littermate controls. Bar graphs represent the CD41+ (B) and P-selectin+ (C) areas at the end of the 3-minute observation period. N = 78 injuries performed in 11 mice. (D) Fibrin accumulation detected with a fibrin-specific antibody (24 injuries in 3 mice). (E) The mean fluorescence area of platelet clumps passing through a virtual analysis window placed immediately downstream of the injury site. The data were pooled into early (i) and late (ii) time points for analysis. N = 19 injuries for WT and 18 injuries for RGS10−/− in 3 mice. (Fi) The decline over time in the relative porosity of hemostatic thrombi formed in WT and RGS10−/− mice infused with caged FITC-albumin. Data are mean ± SEM, 22 injuries in 4 WT mice and 23 injuries in 4 RGS10−/− mice. (Fii) ∆MFI measured 180 seconds after injury. ∆MFI, increment in mean fluorescence intensity measured over the entire thrombus at 15-second intervals immediately after a light flash that causes the albumin to fluoresce.
Embolization and clot retraction in the absence of RGS10
The decline in thrombus size that normally begins ∼1 minute after injury in this model is due in part to embolization from the shell region and in part to clot retraction.24,31 We measured both. To quantify embolization, we observed the passage of CD41+ platelet aggregates downstream from the site of injury. There were fewer emboli in the RGS10−/− mice than in controls during the period (≥100 seconds) when the platelet accumulation curves diverged (Figure 1E). Clot retraction measured in a traditional benchtop assay was slightly slower in the RGS10−/− mice (supplemental Figure 2). Because the difference was small and observed at only a single time point, we repeated the measurements using a method that provides a nearly continuous readout (supplemental Figure 2B).36 Identical results were obtained: loss of RGS10 causes a small, but reproducible, delay in clot retraction. Although small in magnitude, this delay would be expected to contribute to the difference in thrombus size that we observed in Figure 1A.
Platelet-packing density in thrombi formed in RGS10−/− mice
As hemostatic thrombi form, the narrowing of the gaps between platelets and an increase in platelet-packing density create a sheltered environment in which thrombin is effectively trapped and can accumulate.30,31 We have found that this increase in packing density is greater in the core than in the shell. The increased shell size and delayed clot retraction observed in the RGS10−/− mice in the present study suggested that there could be an accompanying decrease in average packing density. To determine whether this is the case, we measured relative thrombus porosity over time by infusing the mice prior to injury with albumin conjugated to caged fluorescein.29,38 Pulses of 405-nm light applied at 15-second intervals were used to uncage the fluorescein, causing a spike in thrombus-associated mean fluorescence intensity with each pulse, followed by a decline in fluorescence as the fluorescent albumin exchanged out of the thrombus. As we have reported previously, the magnitude of this spike declined with each successive light flash, reflecting a decrease in thrombus porosity over time (Figure 1Fi).29 This decline in porosity was not as great in the RGS10−/− mice as in the controls, indicating greater final porosity (lower packing density) in the RGS10−/− thrombi compared with WT (Figure 1Fi-ii).
Platelet activation in vitro was measured initially by light transmission aggregometry and then by flow cytometry using antibodies to detect integrin αIIbβ3 activation (Jon/A antibody) and α-granule exocytosis (anti–P-selectin). The aggregometry studies (supplemental Figure 3) confirmed the dose/response shift that has been reported previously.23 However, the flow cytometry studies, which were performed in the presence of aspirin and apyrase to reduce secondary signaling events, highlight critical differences between agonists that were not previously appreciated. Platelet activation with the PAR4 thrombin receptor agonist peptide AYPGKF showed a leftward shift in the steep dose/response curves for integrin activation and α-granule exocytosis that was not accompanied by a change in the maximum response (Figure 2A). In contrast, there was a considerable increase in the maximum responses when platelets were stimulated with U46619 (a TxA2 mimetic) or ADP: integrin activation increased 4.1-fold for U46619 and 5.5-fold for ADP, and P-selectin exposure increased 6.7-fold for U46619 and 10.3-fold for ADP (Figure 2B-C). Similar results were obtained when flow cytometric studies were performed on platelets obtained from mice that have been backcrossed for 8 generations into C57BL/6 background. The same results were obtained (supplemental Figure 4). Finally, we note that there was no increase in basal P-selectin exposure or integrin activation on RGS10−/− platelets (Figure 2), and shape change was unaffected (supplemental Figure 5). These observations suggest that the RGS10−/− platelets are not circulating in a preactivated state, and signaling by G12 family members in platelets has been unaffected by deleting RGS10.
Increased integrin activation and α-granule exocytosis in platelets from RGS10−/−mice. Platelets from RGS10−/− and littermate control mice (WT) were stained with fluorophore-conjugated antibodies to P-selectin or activated αIIbβ3 (Jon/A antibody) after incubation with a PAR4 agonist peptide (AYPGKF) (A), a TxA2 mimetic (U46619) (B), or ADP (C) at the concentrations indicated (N = 7). N.S., not significant.
Increased integrin activation and α-granule exocytosis in platelets from RGS10−/−mice. Platelets from RGS10−/− and littermate control mice (WT) were stained with fluorophore-conjugated antibodies to P-selectin or activated αIIbβ3 (Jon/A antibody) after incubation with a PAR4 agonist peptide (AYPGKF) (A), a TxA2 mimetic (U46619) (B), or ADP (C) at the concentrations indicated (N = 7). N.S., not significant.
RGS10 targets Gq- and Gi-mediated responses in platelets
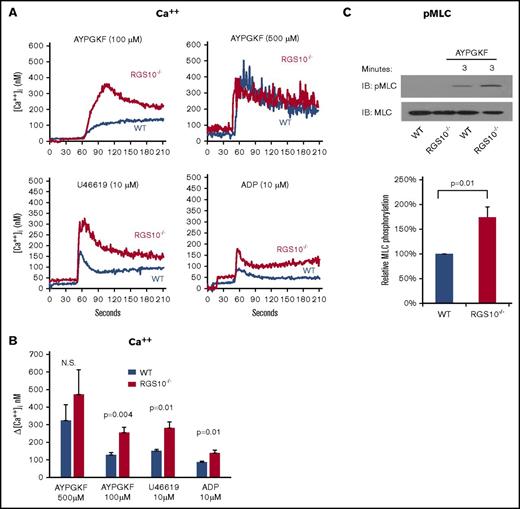
Gq-mediated responses in platelets lead to phosphoinositide hydrolysis, the release of Ca++ from the platelet dense tubular system, and, among other responses, integrin activation and myosin light chain phosphorylation.39,40 In the studies shown in Figure 3A-B, a greater increase in cytosolic Ca++ was observed in RGS10−/− platelets compared with controls. As in the flow cytometry assays, the difference between controls and RGS10−/− platelets disappeared at higher AYPGKF concentrations. The gain of function observed with U46619 persisted to ≥20 µM, the highest concentration tested (data not shown). Myosin light chain phosphorylation was also greater in RGS10−/− platelets than in controls (Figure 3C).
The impact of deleting RGS10 on Ca++mobilization and myosin light chain. (A-B) Ca++ mobilization. Platelets were stimulated with AYPGKF, ADP, or U46619 at the concentrations indicated in the absence of extracellular Ca++. (A) Representative measurements. (B) Summary of the results of 4 experiments (mean ± SEM). (C) Myosin light chain (MLC) phosphorylation. Washed platelets were incubated for 3 minutes with 350 µM AYPGKF to activate PAR-4. Lysates were probed with an antibody specific for MLC phosphorylated on Thr18 and Ser19 before reprobing with anti-MLC. Data are mean ± SEM. N = 4.
The impact of deleting RGS10 on Ca++mobilization and myosin light chain. (A-B) Ca++ mobilization. Platelets were stimulated with AYPGKF, ADP, or U46619 at the concentrations indicated in the absence of extracellular Ca++. (A) Representative measurements. (B) Summary of the results of 4 experiments (mean ± SEM). (C) Myosin light chain (MLC) phosphorylation. Washed platelets were incubated for 3 minutes with 350 µM AYPGKF to activate PAR-4. Lysates were probed with an antibody specific for MLC phosphorylated on Thr18 and Ser19 before reprobing with anti-MLC. Data are mean ± SEM. N = 4.
Gi-derived Gβγ activates phosphatidylinositol 3-kinase β isoforms, leading to Akt activation.18,41,42 To investigate the impact of RGS10 deletion on Gi-dependent signaling, Akt phosphorylation was measured in platelets stimulated with AYPGKF or ADP in the presence or absence of cangrelor (to block platelet Gi2-coupled ADP P2Y12 receptors) or MRS2500 (to block Gq-coupled ADP P2Y1 receptors). When added alone, ADP caused Akt phosphorylation to a greater extent in RGS10−/− platelets than in controls (Figure 4A-B). Adding MRS2500 at a concentration sufficient to block the ADP-induced Ca++ increase (supplemental Figure 6) had no effect, but cangrelor reduced Akt phosphorylation whether RGS10 was present or not, showing the role of RGS10 in regulating Gi2-dependent signaling. A dose/response curve with AYPGKF showed that deleting RGS10 shifted the curve to the left with, once again, no increase in maximum Akt phosphorylation (Figure 4C-D). Studies with cangrelor or MRS2500 also indicated that the response to AYPGKF was amplified by secreted ADP working through P2Y12, but not P2Y1 (Figure 4A-B), as Kunapuli and colleagues have previously observed in unrelated studies in human and mouse platelets.41 Taken together, these results show that RGS10 normally represses Gi- and Gq-dependent signaling in platelets.
The impact of deleting RGS10 on Akt phosphorylation. (A-B) Gel-filtered platelets from RGS10−/− mice or matched WT controls were incubated with vehicle, 350 μM AYPGKF, or 20 μM ADP for 5 minutes in the presence or absence of the P2Y1 antagonist MRS2500 (50 µM) or the P2Y12 antagonist cangrelor (100 nM), as indicated. Lysates were probed with anti–p-Akt (S473) and then reprobed with anti-Akt. The p-Akt signal was normalized to the Akt loading control and is represented as signal relative to the maximum for WT controls. Data are mean ± SEM, N = 5. (C-D) Platelets were incubated for 5 minutes with AYPGKF at the concentrations shown, lysed, and immunoblotted for p-Akt and Akt as in panel A. Quantification was performed as in panels A and B. Data are mean ± SEM. N = 3 to 5.
The impact of deleting RGS10 on Akt phosphorylation. (A-B) Gel-filtered platelets from RGS10−/− mice or matched WT controls were incubated with vehicle, 350 μM AYPGKF, or 20 μM ADP for 5 minutes in the presence or absence of the P2Y1 antagonist MRS2500 (50 µM) or the P2Y12 antagonist cangrelor (100 nM), as indicated. Lysates were probed with anti–p-Akt (S473) and then reprobed with anti-Akt. The p-Akt signal was normalized to the Akt loading control and is represented as signal relative to the maximum for WT controls. Data are mean ± SEM, N = 5. (C-D) Platelets were incubated for 5 minutes with AYPGKF at the concentrations shown, lysed, and immunoblotted for p-Akt and Akt as in panel A. Quantification was performed as in panels A and B. Data are mean ± SEM. N = 3 to 5.
Regulating free RGS10 levels
We have shown previously that, in resting platelets, RGS18 is bound to the scaffold protein spinophilin (neurabin-II or spinophilin), forming a heterotrimeric complex with the tyrosine phosphatase SHP-1.43 Dissociation of the complex occurs when platelets are activated by thrombin or U46619.43,44 It also occurs when platelets are exposed to PGI2, which causes cyclic adenosine monophosphate (cAMP)-dependent phosphorylation of spinophilin.44 Other investigators have shown that RGS18 can bind to the 14-3-3 family member 14-3-3γ, which is abundant in platelets. In contrast to spinophilin, the binding of RGS18 to 14-3-3γ was reported to increase when platelets are activated.45 In the second part of this study, we probed resting platelets for the presence of RGS10 complexes with spinophilin and 14-3-3γ and established an assay for measuring free RGS10 levels. These studies were performed with human, rather than mouse, platelets to allow comparisons with our previous studies on RGS18. RGS10 was immunoprecipitated from resting platelets and from platelets activated with ADP, collagen, or a PAR-1 thrombin receptor agonist peptide (SFLLRN), U46619. In resting platelets, RGS10 coprecipitated with spinophilin (Figure 5A). SFLLRN and U46619 caused dissociation of the spinophilin/RGS10 complex as did, to a lesser extent, collagen and ADP. Dissociation of the complex by collagen was completely blocked by adding aspirin and apyrase. Dissociation by ADP was reduced, but not eliminated, by adding aspirin (Figure 5B). These results suggest that platelet receptors for thrombin, TxA2, and, to a lesser extent, ADP, are directly coupled to spinophilin and RGS10, whereas collagen receptors are coupled indirectly via released TxA2 and ADP. As we observed previously for RGS18,44 increasing cAMP levels also affect the spinophilin/RGS10 complex. Figure 5C shows that stimulating increased cAMP formation with PGI2 or forskolin causes dissociation of RGS10 from spinophilin. Note the presence of a second band that was seen on the strip and reprobe of the RGS10 immunoprecipitates in Figure 5 that does not align with RGS10 in platelet lysates and was not seen in Figure 6 (see the following paragraphs). The etiology of this band is unknown at present.
Release of RGS10 from binding sites on spinophilin leads to a rise in free RGS10 levels in platelets incubated with agonists or PGI2. (A) Human platelets were incubated for 3 minutes with a PAR1 agonist peptide (SFLLRN 50 µM), a TxA2 mimetic (U46619 10 µM), ADP (10 µM), or collagen (10 µg/mL) in the absence or presence of 100 μM aspirin (ASA) and 1 U/mL apyrase (APY), as indicated. Lysates were precipitated with anti-RGS10 and probed for spinophilin before reprobing with anti-RGS10 (data are mean ± SEM, N = 3). (B) Human platelets were incubated for 3 minutes with ADP (10 µM) in the absence or presence of 100 μM aspirin (ASA). Lysates were precipitated with anti-RGS10 and probed for spinophilin (SPL) before reprobing with anti-RGS10 (data are mean ± SEM, N = 4). (C) Human platelets were incubated with 20 µM forskolin (Forsk) or 15 µM PGI2, with or without 1 µM okadaic acid, as indicated. Proteins were precipitated with anti-RGS10 or nonimmune immunoglobulin (Ig) and then probed with anti-spinophilin before reprobing with anti-RGS10 (data mean ± SEM, N = 4). P values are relative to resting platelets. (D) Lysates were prepared from resting platelets and from platelets incubated with PGI2 (15 µM) or the PAR1 agonist peptide SFLLRN (50 µM). The lysates were then incubated with GST-Gi2α coupled to glutathione beads in the presence of GDP plus AlF4− or GDP alone, as indicated. Bound proteins were subjected to electrophoresis and probed with anti-RGS10 and Gi2α antibodies to detect RGS10 and GST–Gi2α fusion protein, respectively (upper panels). Summary of 3 experiments expressed as the percentage of the result obtained with resting platelets (data are mean ± SEM) (lower panel). P values are relative to resting platelets.
Release of RGS10 from binding sites on spinophilin leads to a rise in free RGS10 levels in platelets incubated with agonists or PGI2. (A) Human platelets were incubated for 3 minutes with a PAR1 agonist peptide (SFLLRN 50 µM), a TxA2 mimetic (U46619 10 µM), ADP (10 µM), or collagen (10 µg/mL) in the absence or presence of 100 μM aspirin (ASA) and 1 U/mL apyrase (APY), as indicated. Lysates were precipitated with anti-RGS10 and probed for spinophilin before reprobing with anti-RGS10 (data are mean ± SEM, N = 3). (B) Human platelets were incubated for 3 minutes with ADP (10 µM) in the absence or presence of 100 μM aspirin (ASA). Lysates were precipitated with anti-RGS10 and probed for spinophilin (SPL) before reprobing with anti-RGS10 (data are mean ± SEM, N = 4). (C) Human platelets were incubated with 20 µM forskolin (Forsk) or 15 µM PGI2, with or without 1 µM okadaic acid, as indicated. Proteins were precipitated with anti-RGS10 or nonimmune immunoglobulin (Ig) and then probed with anti-spinophilin before reprobing with anti-RGS10 (data mean ± SEM, N = 4). P values are relative to resting platelets. (D) Lysates were prepared from resting platelets and from platelets incubated with PGI2 (15 µM) or the PAR1 agonist peptide SFLLRN (50 µM). The lysates were then incubated with GST-Gi2α coupled to glutathione beads in the presence of GDP plus AlF4− or GDP alone, as indicated. Bound proteins were subjected to electrophoresis and probed with anti-RGS10 and Gi2α antibodies to detect RGS10 and GST–Gi2α fusion protein, respectively (upper panels). Summary of 3 experiments expressed as the percentage of the result obtained with resting platelets (data are mean ± SEM) (lower panel). P values are relative to resting platelets.
Dissociation of RGS10 from binding sites on 14-3-3γ during platelet activation. (A) Lysates were prepared from human platelets stimulated with 1 U/mL thrombin, after which 14-3-3γ was precipitated with anti-RGS10 antibody or nonimmune immunoglobulin (Ig) and probed with anti–14-3-3γ before reprobing with anti-RGS10 antibody. The bar graph summarizes results from 2 experiments. (B) Lysates were prepared from CHO cells transfected with HA-tagged RGS10 and Myc-tagged 14-3-3γ. Proteins were precipitated with an anti-HA antibody or nonimmune Ig and probed for Myc–14-3-3γ before reprobing with anti-RGS10 antibody (data are mean ± SEM, N = 3).
Dissociation of RGS10 from binding sites on 14-3-3γ during platelet activation. (A) Lysates were prepared from human platelets stimulated with 1 U/mL thrombin, after which 14-3-3γ was precipitated with anti-RGS10 antibody or nonimmune immunoglobulin (Ig) and probed with anti–14-3-3γ before reprobing with anti-RGS10 antibody. The bar graph summarizes results from 2 experiments. (B) Lysates were prepared from CHO cells transfected with HA-tagged RGS10 and Myc-tagged 14-3-3γ. Proteins were precipitated with an anti-HA antibody or nonimmune Ig and probed for Myc–14-3-3γ before reprobing with anti-RGS10 antibody (data are mean ± SEM, N = 3).
In Figure 5D, a pull-down assay was used to detect free RGS10 in platelets. In this assay, free RGS10 is captured by a glutathione S-transferase (GST)–Gi2α fusion protein and detected with anti-RGS10.44 GDP and AlF4− are added to mimic the transition state of Gi2α, which is recognized by RGS domains. The results show that levels of free RGS10 rise when human platelets are stimulated with SFLLRN or PGI2.
Finally, we asked whether RGS10 binds to the scaffold protein 14-3-3γ. The results show that, in resting human platelets, 14-3-3γ coprecipitates with RGS10 (Figure 6A). Activation of the platelets with thrombin caused dissociation of RGS10 from 14-3-3γ. Dissociation was complete within 1 minute, a time course similar to what we have previously observed for dissociation of spinophilin/RGS18 complexes.43 Because dissociation from 14-3-3γ during platelet activation is opposite to what has previously been reported for RGS18,22 we performed a comparison study in CHO cells expressing epitope-tagged RGS10 and 14-3-3γ. A similar result was obtained: RGS10 and 14-3-3γ were associated in resting CHO cells but not in CHO cells incubated with thrombin (Figure 6B). Thus, the results in the second part of this study show that RGS10 is associated with ≥2 scaffold proteins in resting platelets, forming complexes that dissociate when platelets are activated and leading to an increase in free RGS10 able to interact with Gα-GTP.
Discussion
There is increasing recognition that the hemostatic response to penetrating injuries does more than pile up activated platelets and fibrin.46 Once platelets begin to accumulate, they create a sheltered local environment in which solute transport is restricted and agonist concentration gradients are established. Depending on their location within the mass, platelets are exposed to different combinations of agonists and packed together to different extents. Nodal points within the platelet signaling network provide opportunities to regulate the pace and extent of platelet activation. Heterotrimeric G proteins and their regulators provide 1 such potential nodal point. Although G proteins are essentially on/off switches, the time until they turn off can be shortened by members of the RGS protein family. The evidence that RGS proteins impact platelet activation has been explored through the use of RGS protein knockouts and a Gi2α(G184S) variant that does not bind to RGS proteins. In each case, a gain of function has been observed.18,20,21,23 Mice lacking RGS10 or RGS18 have a shorter bleeding time and a reduced time to occlusive thrombus formation in vivo in the FeCl3 injury model.20,21,23 Here, we have attempted to go beyond the concept of RGS proteins as brakes, focusing on the ways in which they can potentially shape the hemostatic response to penetrating injuries and the extent to which they do so. In the first part of these studies, we examined the impact of RGS10 on the hemostatic response in vivo and explored the mechanisms underlying the observed effects. In the second part, we asked whether interactions with other proteins regulate free RGS10 levels in platelets, demonstrating cross talk between G protein–dependent activation pathways and cAMP-dependent inhibitory pathways.
The results show that deleting RGS10 changes platelet reactivity in a Gq- and Gi-dependent manner, reshaping thrombus structure and altering thrombus stability. Hemostatic thrombi formed in RGS10−/− mice have a larger shell region without an increase in the core region. As a result, they have a lower average packing density (greater porosity) that we were able to measure by studying the exchange of fluorescent albumin. Among the agonists studied, PAR4 activation produced the greatest response in assays of integrin activation, α-granule secretion, and cytosolic Ca++, with a steep dose/response curve in each case. Deleting RGS10 caused a modest leftward shift in the steep dose/response curve to a PAR4 agonist peptide and no increase in the maximum response. In contrast, platelet responses to ADP and U46619 were increased from fourfold to 10-fold. One way of summarizing these observations is that RGS10 plays a critical inhibitory role in platelets activated with some weaker agonists and with low concentrations of some stronger agonists. How RGS10 inhibition is bypassed at higher PAR4 agonist concentrations should be investigated in a future study. However, because ADP and TxA2 are the main drivers in the shell region,34 we suggest that these differential effects underlie the observed growth of the shell. In contrast, thrombin is the main driver of platelet activation in the thrombus core where close packing of the platelets traps thrombin and raises its local concentration.31-33 The lack of an effect of the knockout on fibrin accumulation suggests that the thrombin concentration in the core has not been markedly affected. We infer from the lack of an effect of deleting RGS10 on the core that the local thrombin concentration is sufficient to produce a maximum PAR4-mediated response. At the same time, we found no evidence for an increase in basal activation in circulating RGS10−/− platelets, which indicates that loss of RGS10 is insufficient to trigger platelet activation in the absence of injury. It remains to be seen whether removing RGS18, as well as RGS10, will have a greater effect.
What is the molecular basis for differences in the agonist-specific response of platelets following RGS10 deletion? The data suggest that it is not due to differences at the level of the G proteins that couple to the receptors for each agonist. Prior work in cells other than platelets indicates that RGS10 can accelerate GTP hydrolysis by Gq and Gi family members.6,12-15 There is no evidence that it regulates G12 family members. The present studies indicate that this is also the case in platelets because shape change is unaffected by deleting RGS10. Knocking out RGS10 increased intracellular Ca++ responses and Akt phosphorylation. The former is mediated by Gq; the latter is mediated largely by Gi2. Other factors that may determine the agonist-selective effects of removing RGS10 include the location of receptors and RGS proteins within the platelet, how many receptors of each class exist, how efficiently they are coupled to each G protein, and the localization of other RGS10-binding proteins. These hypotheses remain to be tested.
Once signal transduction is initiated in platelets, critical events, such as integrin engagement and granule exocytosis, can occur. An unexpected finding in our studies is that clot retraction occurs more slowly in RGS10−/− platelets than in controls. Robust clot retraction requires integrin activation, outside-in signaling through the integrin, and myosin phosphorylation. Data presented here show that deleting RGS10 increases integrin activation. Myosin phosphorylation is also enhanced, as is the increase in cytosolic Ca++ that promotes it. Why, then, does clot retraction occur more slowly? At present the answer is unknown, but we note that conventional clot retraction assays are performed at very high thrombin concentrations and occur over a time period much longer than used in the studies that we performed in vivo.
Finally, the data show that, in resting platelets, RGS10 is at least partly held in complexes with spinophilin and 14-3-3γ. The ability of RGS10, like RGS18, to bind to scaffold proteins in an activation-sensitive manner places both RGS family members at a regulated nodal point in the platelet signaling network that balances the need for platelets to resist inappropriate activation while retaining the ability to activate quickly in response to injury. Platelet agonists cause dissociation of spinophilin/RGS complexes and a rise in free RGS protein levels, as does PGI2, although by a different mechanism. We have shown previously that agonists cause SHP-1–dependent dephosphorylation of critical tyrosine residues in spinophilin.43 PGI2 causes protein kinase A–dependent phosphorylation of spinophilin Ser94 and release of RGS18.44 The present studies show that PGI2 also causes an increase in free RGS10. This means that, in addition to helping to shape the response to injury, both RGS proteins contribute to the inhibitory effects of PGI2.
Summing up, the available evidence suggests that the regulated agonist-specific interaction of RGS proteins with Gq and Gi2 in platelets provides a mechanism not only for limiting the growth of hemostatic thrombi, but also for shaping critical features of their architecture. Hemostatic thrombi formed after penetrating injuries in mice lacking RGS10 have a larger than normal shell region and, partly as a result, a lower average packing density. Fewer platelets detach from the shell region in the absence of RGS10, suggesting that they are more activated. Removing RGS10 causes the shell to expand, whereas blocking ADP receptors with cangrelor or introducing Hermansky–Pudlak syndrome mutations to prevent ADP release causes the shell region to contract.18,24,47 When RGS10 is present, the hemostatic response produces a smaller more compact plug with a greater proportion of fully activated platelets.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by American Heart Association grants 14SDG20380473 (P.M.) and 16PRE30260002 (V.T.) and National Institutes of Health, National Heart, Lung, and Blood Institute grant P01 HL40387 (L.F.B. and T.J.S.).
Authorship
Contribution: P.M., S.G., S.S., T.J.S., D.D. and L.F.B. designed experiments; P.M., S.G., S.S., V.T., A.T., and D.D. performed experiments; and P.M. and L.F.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peisong Ma, Department of Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, 1020 Locust St, Room 394, Philadelphia, PA 19107; e-mail: peisong.ma@jefferson.edu; and Lawrence F. Brass, Department of Medicine, University of Pennsylvania, 815 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: brass@pennmedicine.upenn.edu.