Key Points
Coordinated thromboxane A2 and ADP/P2Y12 signaling is required for platelet accumulation in the outer shell region of hemostatic plugs.
Platelet activation within the hemostatic plug core region is predominantly mediated by thrombin.
Abstract
The local microenvironment within an evolving hemostatic plug shapes the distribution of soluble platelet agonists, resulting in a gradient of platelet activation. We previously showed that thrombin activity at a site of vascular injury is spatially restricted, resulting in robust activation of a subpopulation of platelets in the hemostatic plug core. In contrast, adenosine 5′-diphosphate (ADP)/P2Y12 signaling contributes to the accumulation of partially activated, loosely packed platelets in a shell overlying the core. The contribution of the additional platelet agonists thromboxane A2 (TxA2) and epinephrine to this hierarchical organization was not previously shown. Using a combination of genetic and pharmacologic approaches coupled with real-time intravital imaging, we show that TxA2 signaling is critical and nonredundant with ADP/P2Y12 for platelet accumulation in the shell region but not required for full platelet activation in the hemostatic plug core, where thrombin activity is highest. In contrast, epinephrine signaling is dispensable even in the presence of a P2Y12 antagonist. Finally, dual P2Y12 and thrombin inhibition does not substantially inhibit hemostatic plug core formation any more than thrombin inhibition alone, providing further evidence that thrombin is the primary driver of platelet activation in this region. Taken together, these studies show for the first time how thrombin, P2Y12, and TxA2 signaling are coordinated during development of a hierarchical organization of hemostatic plugs in vivo and provide novel insights into the impact of dual antiplatelet therapy on hemostasis and thrombosis.
Introduction
The hemostatic response to vascular injury is a dynamic process requiring the rapid adhesion and activation of platelets to form a plug that prevents continued blood loss. The signaling pathways responsible for platelet activation have largely been worked out by in vitro studies of platelet responses to individual agonists alone and in combination. From these studies, it has long been recognized that stimulation of platelets with combinations of agonists that activate both Gq- and Gi-coupled G protein–coupled receptors can result in synergistic effects, especially when each agonist is used at submaximal concentrations.1-4 Activation of agonist receptors coupled to members of the Gi family, such as adenosine 5′-diphosphate (ADP) P2Y12 receptors and epinephrine α2-adrenergic receptors, cannot stimulate platelet activation on their own but instead potentiate platelet activation in response to activation of receptors coupled to Gq.1,5 These include receptors for thrombin (PAR-1 and PAR-4), thromboxane A2 (TxA2; TP), and the ADP P2Y1 receptor. Conversely, strong Gq-coupled agonists such as thrombin can maximally activate platelets without the need for Gi signaling at high doses,1,5,6 whereas submaximal doses of thrombin or weaker agonists, such as TxA2 and ADP, require costimulation of a Gi pathway for robust platelet activation.2,3 The extent of platelet activation in vivo therefore depends on the exposure of individual platelets to variable concentrations of multiple agonists within local microenvironments.
As much as we have learned from in vitro studies, many questions remain about the coordination of platelet agonist signaling during hemostasis in vivo. For example, the concentrations of various agonists and how they change over time is difficult to precisely ascertain. Similarly, the spatial distributions of soluble platelet agonists within a developing hemostatic plug are likely to be nonuniform and highly dynamic. In this regard, we and others have sought to define the mechanisms by which platelet signaling pathways are regulated in time and space using a variety of intravital imaging and other in vivo approaches. We previously showed that agonist distribution is heavily influenced by local physical forces such as the geometry of the plasma-filled spaces within a platelet mass and its effects on solute transport and diffusion.7-9 These and other factors within the local microenvironment result in the formation of soluble platelet agonist gradients emanating from the site of injury into the hemostatic plug. Thrombin activity appears to be highest in the immediate vicinity of the injury site, where it promotes robust platelet activation, including granule secretion, as well as fibrin formation.10-14 We have referred to this area of highly activated platelets as the hemostatic plug core region.12 The core region is overlaid by a shell of minimally activated platelets extending further into the blood vessel lumen that is dependent on ADP P2Y12 receptor signaling.12 This core and shell nomenclature serves as a convenient description of the hierarchical organization of hemostatic plugs, reflecting a gradient of platelet activation extending from the site of injury. The contribution of TxA2 and epinephrine signaling to the hierarchical organization of hemostatic plugs has not been previously explored. It also remains unclear to what extent multiple platelet agonists overlap in time and space. In other words, does a single agonist dominate the platelet activation state within specific regions of a hemostatic plug, or do multiple agonist pathways need to be integrated to produce the variable degrees of platelet activation observed? Further, how do the relationships among different agonists change in the face of antiplatelet or anticoagulant therapy, and what are the consequences for hemostatic plug organization?
In the present study, we addressed these questions using a combination of pharmacologic and genetic approaches to examine how thrombin, TxA2, ADP/P2Y12, and epinephrine signaling, alone and in combination, contributes to the spatial organization of platelet activation within hemostatic plugs in vivo. We show for the first time that TxA2 signaling, like P2Y12 signaling, is critical and nonredundant with ADP for platelet accumulation in the outer shell of minimally activated platelets but not required for full platelet activation in the hemostatic plug core, where thrombin activity is highest. Combined inhibition of both TxA2 and P2Y12 signaling did not have an additive effect but instead was similar to inhibition of either pathway alone. In contrast, epinephrine signaling, which is ablated in platelets from Gz-knockout mice,5 was completely dispensable for the hemostatic response, even in the presence of a P2Y12 antagonist. Finally, inhibition of P2Y12 signaling in the context of attenuated thrombin activity demonstrated that thrombin signaling dominates platelet activation in the hemostatic plug core region. Taken together, these data provide novel insights into the way multiple platelet signaling pathways work together in time and space during the hemostatic response in vivo and help to account for the clinical impact of single and dual antiplatelet therapies.
Materials and methods
Mice and pharmacologic treatments
TP-deficient (C57BL/6 background) and Gz-deficient (C57Bl/6 background) mice were previously described.5,15 For pharmacologic studies, male C57Bl/6 mice 8 to 12 weeks of age were used (The Jackson Laboratory, Bar Harbor, ME). Mice were treated with aspirin once daily by oral gavage (1.25 mg/d) for 4 days. Inhibition of platelet function following this treatment protocol was demonstrated by a rightward shift in the dose–response curve to the Par-4 agonist peptide AYPGKF measured using platelet aggregation (supplemental Figure 1A). Cangrelor was used to inhibit P2Y12 signaling. Due to its short half-life (3-6 minutes),16 a bolus infusion of cangrelor (0.75 µg) was administered IV immediately before each laser injury made, as previously described.12 The effectiveness of this treatment protocol was demonstrated by complete inhibition of ADP-induced platelet aggregation (supplemental Figure 1B). The direct thrombin inhibitor bivalirudin was used to inhibit thrombin activity. It was administered as a bolus infusion immediately before the first injury (0.5, 1, or 4 µg/g body weight) followed by an additional bolus infusion (50% of the initial dose) every 20 minutes. Both cangrelor and bivalirudin were supplied by The Medicines Company (Parsippany, NJ). For laser injury studies, 5 to 10 injuries were made per mouse (the exact number in each mouse depended on available blood vessels). In addition, multiple thrombi generated in the same mice prior to drug infusion served as controls. Vehicle (normal saline) was infused prior to control injuries. The Institutional Animal Care and Use Committee of the University of Pennsylvania or Soochow University approved all animal studies.
Response to vascular injury in mouse cremaster arterioles
Laser-induced injury in mouse cremaster muscle arterioles was performed essentially as described previously.12 The studies reported were performed using 1 of 2 similar microscopy systems housed either at the University of Pennsylvania or at Soochow University. Full details regarding both microscopy systems and image analysis are included in supplemental Methods. Mice were infused with fluorescently labeled antibodies against CD41 (MWReg30 F(ab)2, 0.12 µg/g body weight; BD Biosciences, San Jose, CA) and P-selectin (RB40.34, 0.2 µg/g body weight; BD Biosciences) via a jugular vein cannula. In some studies, mice were also infused with an antifibrin antibody (clone 59D8, 0.2 µg/g body weight; a generous gift from Rodney Camire (The Children’s Hospital of Philadelphia). Antibodies were labeled using Alexa Fluor monoclonal antibody labeling kits according to the manufacturer’s instructions (Invitrogen/Life Technologies).
Flow cytometric analysis of platelet activation
Mice were bled under isoflurane anesthesia from the retro-orbital plexus. 700 µL blood was collected in heparin, and platelet-rich plasma (PRP) was obtained as previously described.17 PRP was further diluted in Tyrode buffer containing 2 mM calcium to attain a platelet concentration of 5 × 108 platelets/mL. PRP was incubated with the indicated concentrations of Par-4 agonist peptide AYPGKF (Bachem, Torrance, CA) in the presence of saturating amounts of fluorophore-conjugated monoclonal antibodies (JON/A and CD62P; Emfret, Wurzburg, Germany) for 15 minutes at room temperature and analyzed on a FACSCanto II (BD Biosciences). The platelet population was gated based on forward/side scatter. Cyclooxygenase inhibition was achieved by incubating PRP with 1 mM aspirin for 30 minutes prior to activation. For activation studies in the presence of a P2Y12 inhibitor, 100 nM cangrelor was added just before the agonist addition. A separate aliquot of PRP with no inhibitors added served as a control group.
Statistics
All images were acquired and analyzed using Slidebook 6.0 imaging software (Intelligent Imaging Innovations, Denver, CO). Statistical analysis and graphs were produced using Prism 6.0 software (GraphPad). For cremaster arteriole injury studies, statistics were performed using the number of injuries as the n, as is typical for this assay. Data sets were compared using either the Mann-Whitney test or the Kruskal-Wallis test with the Dunn post-hoc test for multiple comparisons, unless otherwise noted.
Results
Role of TxA2 in the regulation of the hierarchical organization of hemostatic plugs
Prior studies have shown that either mechanical puncture or laser-induced injury in the mouse cremaster microcirculation produces a gradient of platelet activation at the site of injury.12 Platelets closest to the vessel wall are densely packed, fully activated, and P-selectin positive, forming a platelet plug core region that is overlaid by additional layers of loosely adherent P-selectin–negative platelets. The appearance of P-selectin in this case is used as a marker showing that α-granule secretion has occurred to differentiate fully activated platelets from less activated platelets. Here, we used the same experimental system to determine how TxA2, epinephrine, and thrombin signaling are integrated with P2Y12 signaling to regulate the development of this platelet activation gradient.
Because pharmacologic inhibition of COX-1 using aspirin has the potential to inhibit multiple COX-1 metabolites, we began our studies on the role of TxA2 signaling in the formation of platelet activation gradients by examining platelet accumulation and activation following laser-induced injury in TxA2 receptor–deficient (TP−/−) mice. We found that total platelet accumulation was attenuated in TP−/− mice compared with wild-type controls (Figure 1A-B). The median area under the CD41 vs time curve was reduced by ∼25% (Figure 1B; P = .0143), demonstrating the importance of TxA2 signaling for platelet accumulation following vascular injury. Remarkably, the P-selectin–positive area, which was used as a marker of the core region, was indistinguishable between wild-type and TP−/− mice (Figure 1C-D). Taken together, these results demonstrate that TxA2 signaling contributes primarily to platelet recruitment and retention in the outer shell of minimally activated platelets but is not required for the full activation of platelets in the hemostatic plug core region, where thrombin activity is highest.
Thromboxane receptor deficiency attenuates platelet accumulation in the outer shell region of hemostatic plugs in vivo. Laser-induced injury was performed in cremaster muscle arterioles of wild-type (blue) or TP−/− (red) mice as described in “Materials and methods.” (A-B) Total platelet accumulation following laser injury was measured by quantifying the CD41-positive area over time. (A) Graph showing the CD41-positive area over time (mean ± standard error of the mean [SEM]). (B) Graph showing the area under the CD41 vs time curve (AUC). Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. (C-D) α-Granule secretion following laser injury was used as a measure of platelet activation by quantifying the P-selectin–positive area over time. (C) Graph showing the P-selectin–positive area over time (mean ± SEM). (D) Graph showing the area under the P-selectin vs time curve. Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. Wild-type, n = 32 injuries in 4 mice; TP−/−, n = 39 injuries in 4 mice. NS, not significant; WT, wild-type.
Thromboxane receptor deficiency attenuates platelet accumulation in the outer shell region of hemostatic plugs in vivo. Laser-induced injury was performed in cremaster muscle arterioles of wild-type (blue) or TP−/− (red) mice as described in “Materials and methods.” (A-B) Total platelet accumulation following laser injury was measured by quantifying the CD41-positive area over time. (A) Graph showing the CD41-positive area over time (mean ± standard error of the mean [SEM]). (B) Graph showing the area under the CD41 vs time curve (AUC). Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. (C-D) α-Granule secretion following laser injury was used as a measure of platelet activation by quantifying the P-selectin–positive area over time. (C) Graph showing the P-selectin–positive area over time (mean ± SEM). (D) Graph showing the area under the P-selectin vs time curve. Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. Wild-type, n = 32 injuries in 4 mice; TP−/−, n = 39 injuries in 4 mice. NS, not significant; WT, wild-type.
Integration of TxA2 and P2Y12 signaling during hemostatic plug formation
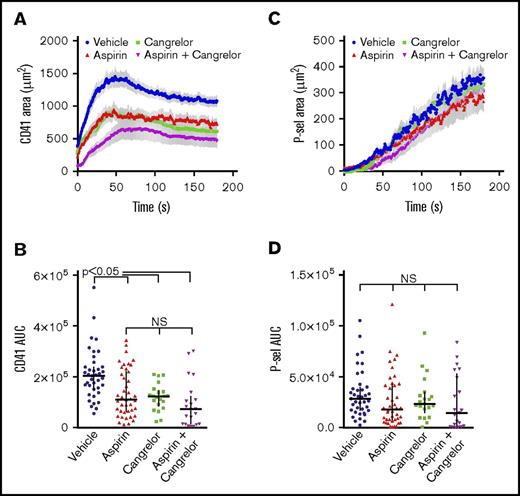
The above results demonstrate that TxA2 signaling is critical for platelet recruitment and/or retention in the outer shell region of hemostatic plugs, a finding very similar to the role of ADP/P2Y12 signaling observed previously.12 In the clinical setting, patients at risk of thrombosis are often prescribed dual antiplatelet therapy consisting of a P2Y12 antagonist plus aspirin, as clinical studies have demonstrated a benefit of this combination. Therefore, we also examined the impact of a P2Y12 antagonist coupled with aspirin on the spatiotemporal regulation of hemostatic plug formation in vivo. For this set of experiments, mice were treated with vehicle (water) or aspirin once daily via oral gavage (1.25 mg/d for 4 days). Inhibition of platelet function following this treatment protocol was demonstrated by a rightward shift in the dose–response curve to the Par-4 agonist peptide AYPGKF measured using platelet aggregation (supplemental Figure 1A). The P2Y12 antagonist (cangrelor) was administered IV immediately prior to each of the laser injuries, as described in “Materials and methods.” The photomicrographs in Figure 2 show the typical response to laser injury in mouse cremaster arterioles, including regions of differential platelet activation as described above. Either aspirin or cangrelor treatment alone resulted in a significant reduction in total platelet accumulation following laser-induced injury in cremaster arterioles (Figures 2 and 3A-B; P < .01). The degree of inhibition of platelet accumulation achieved by either aspirin or cangrelor alone was comparable. Compared with either antiplatelet agent on its own, dual antiplatelet therapy by administration of cangrelor plus aspirin resulted in a trend toward more substantial inhibition of platelet accumulation, but this was not statistically significant (Figures 2 and 3A-B). The combination of cangrelor and aspirin did not substantially attenuate formation of the hemostatic plug core region of P-selectin–positive platelets (Figures 2 and 3C-D), although these studies were not statistically powered to detect small differences among groups. Taken together, these data show that both P2Y12 signaling and TxA2 signaling are necessary for platelet recruitment in the outer shell layers of developing hemostatic plugs at a site of vascular injury, but neither of these signaling pathways is sufficient on its own.
Dual antiplatelet treatment with aspirin and a P2Y12antagonist is similar to treatment with either drug alone. Representative photomicrographs showing the response to laser-induced injury in mouse cremaster arterioles 3 minutes after injury. All platelets are labeled with anti-CD41 (red), and degranulated platelets are labeled with anti–P-selectin (green; overlay of red and green is yellow). The fluorescence channels were binarized and are displayed overlaid on the bright-field channel. The response to injury is shown for mice treated with vehicle, aspirin alone, cangrelor alone, or aspirin and cangrelor. Scale bar, 10 µm. Arrows indicate the direction of blood flow.
Dual antiplatelet treatment with aspirin and a P2Y12antagonist is similar to treatment with either drug alone. Representative photomicrographs showing the response to laser-induced injury in mouse cremaster arterioles 3 minutes after injury. All platelets are labeled with anti-CD41 (red), and degranulated platelets are labeled with anti–P-selectin (green; overlay of red and green is yellow). The fluorescence channels were binarized and are displayed overlaid on the bright-field channel. The response to injury is shown for mice treated with vehicle, aspirin alone, cangrelor alone, or aspirin and cangrelor. Scale bar, 10 µm. Arrows indicate the direction of blood flow.
Dual antiplatelet treatment with aspirin and a P2Y12antagonist is similar to treatment with either drug alone. Laser-induced injury was performed in cremaster muscle arterioles of wild-type mice treated with vehicle (blue), aspirin alone (1.25 mg/d, red), cangrelor alone (0.75 µg prior to each injury, green), or aspirin plus cangrelor (magenta) as described in “Materials and methods.” (A) CD41-positive area over time (mean ± SEM). (B) Graph shows the area under the CD41 vs time curve (AUC). The line and error bars show the median and interquartile range. (C) P-selectin–positive area over time (mean ± SEM). (D) Area under the P-selectin vs time curve. The line and error bars show the median and interquartile range. Vehicle, n = 39 injuries in 8 mice; aspirin alone, n = 42 injuries in 9 mice; cangrelor alone, n = 21 injuries in 5 mice; aspirin plus cangrelor, n = 21 injuries in 5 mice.
Dual antiplatelet treatment with aspirin and a P2Y12antagonist is similar to treatment with either drug alone. Laser-induced injury was performed in cremaster muscle arterioles of wild-type mice treated with vehicle (blue), aspirin alone (1.25 mg/d, red), cangrelor alone (0.75 µg prior to each injury, green), or aspirin plus cangrelor (magenta) as described in “Materials and methods.” (A) CD41-positive area over time (mean ± SEM). (B) Graph shows the area under the CD41 vs time curve (AUC). The line and error bars show the median and interquartile range. (C) P-selectin–positive area over time (mean ± SEM). (D) Area under the P-selectin vs time curve. The line and error bars show the median and interquartile range. Vehicle, n = 39 injuries in 8 mice; aspirin alone, n = 42 injuries in 9 mice; cangrelor alone, n = 21 injuries in 5 mice; aspirin plus cangrelor, n = 21 injuries in 5 mice.
Role of epinephrine signaling in the regulation of the hierarchical organization of hemostatic plugs
Next, we sought to determine the role of epinephrine signaling in regulating platelet accumulation, activation, and fibrin deposition using mice lacking the α subunit of the heterotrimeric G protein, Gz. Gz, a Gi family member, is specifically coupled to epinephrine α2-adrenergic receptors in platelets and is entirely responsible for epinephrine-mediated platelet signaling.5 We found there was no difference in total platelet accumulation in Gz+/+ vs Gz−/− mice following laser injury in cremaster arterioles (Figure 4A-B). Similarly, no differences in P-selectin expression or fibrin accumulation were observed between Gz+/+ and Gz−/− mice (Figure 4C-F). These findings demonstrate that epinephrine signaling is dispensable for hemostatic plug formation in this experimental setting.
Epinephrine/Gzsignaling is dispensable for platelet recruitment and activation. Laser-induced injury was performed in cremaster muscle arterioles of Gz+/+ (blue, green) and Gz−/− (red, magenta) mice with vehicle (circles) or the P2Y12 antagonist cangrelor (triangles, 0.75 µg prior to each injury). (A-B) CD41-positive area over time (A; mean ± SEM) and area under the CD41 vs time curve (AUC) (B; median and interquartile range). (C-D) The P-selectin positive area over time (C; mean ± SEM), and area under the P-selectin vs time curve (D; median and interquartile range). (E-F) Fibrin accumulation following laser injury was used as a measure of thrombin activity by quantifying the fibrin positive area over time (E; mean ± SEM). (F) Graph showing the area under the fibrin vs time curve. The line and error bars show the median and interquartile range. Gz+/+ plus vehicle, n = 36 injuries in 7 mice; Gz−/− plus vehicle, n = 43 injuries in 8 mice; Gz+/+ plus cangrelor, n = 52 injuries in 7 mice; Gz−/− plus cangrelor, n = 65 injuries in 8 mice.
Epinephrine/Gzsignaling is dispensable for platelet recruitment and activation. Laser-induced injury was performed in cremaster muscle arterioles of Gz+/+ (blue, green) and Gz−/− (red, magenta) mice with vehicle (circles) or the P2Y12 antagonist cangrelor (triangles, 0.75 µg prior to each injury). (A-B) CD41-positive area over time (A; mean ± SEM) and area under the CD41 vs time curve (AUC) (B; median and interquartile range). (C-D) The P-selectin positive area over time (C; mean ± SEM), and area under the P-selectin vs time curve (D; median and interquartile range). (E-F) Fibrin accumulation following laser injury was used as a measure of thrombin activity by quantifying the fibrin positive area over time (E; mean ± SEM). (F) Graph showing the area under the fibrin vs time curve. The line and error bars show the median and interquartile range. Gz+/+ plus vehicle, n = 36 injuries in 7 mice; Gz−/− plus vehicle, n = 43 injuries in 8 mice; Gz+/+ plus cangrelor, n = 52 injuries in 7 mice; Gz−/− plus cangrelor, n = 65 injuries in 8 mice.
The ADP P2Y12 receptor coupled to Gi2 is critical for platelet accumulation in the outer shell region of hemostatic plugs following vascular injury, but it appears to be dispensable for full platelet activation and formation of the core region. Because Gz is the only other abundant Gi family member present in platelets,18,19 we hypothesized that Gz signaling may provide an alternative Gi signal in the absence of P2Y12 activation to support residual platelet accumulation and activation. To test this hypothesis, we examined laser-induced thrombus formation in Gz+/+ and Gz−/− mice treated with cangrelor. As already shown, P2Y12 antagonism alone attenuated total platelet accumulation in wild-type (Gz+/+) mice but had no effect on α-granule secretion or fibrin deposition (Figure 4). The results were similar in Gz−/− mice (Figure 4). Inhibition of P2Y12 significantly attenuated total platelet accumulation in Gz−/− mice to an extent similar to that observed in Gz+/+ mice (Figure 4A-B). Further, P2Y12 inhibition in Gz−/− mice had no effect on full platelet activation and α-granule secretion or fibrin deposition (Figure 4C-F). These results demonstrate that Gz signaling does not compensate for the loss of Gi2 signaling during P2Y12 receptor inhibition. Thus, the residual platelet accumulation and formation of a normal core region appear to occur independently of these 2 major Gi signaling pathways.
Integration of P2Y12 and thrombin signaling during the hemostatic response in vivo
In vitro studies have demonstrated that positive feedback via ADP/P2Y12/Gi signaling is required for maximal platelet activation in response to submaximal concentrations of thrombin, but this requirement is negated at high thrombin concentrations.6,20 We confirmed these findings, showing that both aspirin and, to a greater extent, cangrelor treatment of mouse platelets results in a right shift in the PAR-4 agonist peptide dose–response curve, as measured by flow cytometric analysis of JON/A binding (αIIbβ3 integrin activation) or P-selectin expression (α granule secretion; supplemental Figure 2). The studies reported here and elsewhere examining the effect of P2Y12 inhibition on hemostatic plug formation in vivo would therefore suggest that thrombin activity in the core region is sufficiently high such that it does not require Gi signaling for maximal platelet activation. However, we also hypothesized that P2Y12/Gi signaling may become important for core formation in settings where thrombin generation or activity are reduced, such as during anticoagulant therapy. To test this hypothesis, we examined the hierarchical organization of hemostatic plugs in mice treated with a direct thrombin inhibitor (bivalirudin) with or without a P2Y12 antagonist (cangrelor). Because maximal inhibition of thrombin activity completely abolishes core formation, we first performed a bivalirudin dose–response study to find the dose of this thrombin inhibitor that results in an intermediate reduction in P-selectin expression and core region formation. We found that bivalirudin dose-dependently attenuates platelet accumulation and activation in addition to fibrin accumulation in this experimental system (supplemental Figure 3). The lowest dose used, 0.5 mg/kg, resulted in a 64% reduction in platelet accumulation, 66% reduction in P-selectin expression, and 57% reduction in fibrin formation compared with vehicle-treated controls (P < .01 vs vehicle; supplemental Figure 3). This dose was selected to examine the additional effect of P2Y12 antagonism.
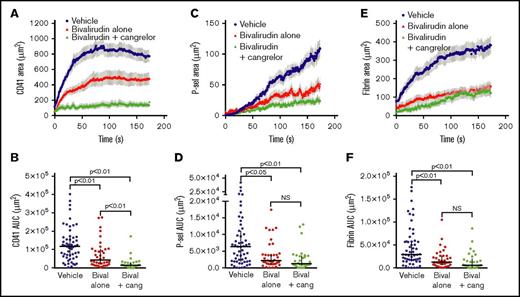
The addition of cangrelor to mice treated with 0.5 mg/kg bivalirudin resulted in an 89% reduction in total platelet accumulation compared with vehicle-treated mice (P < .01; Figure 5A-B). This decrease in platelet accumulation was significantly more than that achieved by this dose of bivalirudin alone (P < .01, bivalirudin alone vs bivalirudin + cangrelor; Figure 5A-B). In contrast, the attenuation of P-selectin expression observed in mice treated with bivalirudin and cangrelor was not significantly different than that observed following treatment with bivalirudin alone (Figure 5C-D). Similarly, dual administration of cangrelor and bivalirudin had no greater effect on fibrin formation than treatment with bivalirudin alone (Figure 5E-F). Taken together, these results demonstrate that while P2Y12 signaling is essential in the hemostatic plug shell region, it does not appear to play a major role in hemostatic plug core formation, even when thrombin activity is reduced.
P2Y12signaling does not contribute to thrombin mediated platelet activation in the core region. Laser-induced injury was performed in cremaster muscle arterioles of wild-type mice treated with vehicle (blue), bivalirudin alone (0.5 µg/g, red), or bivalirudin plus cangrelor (0.75 µg prior to each injury, green) as described in “Materials and methods.” (A-B) Total platelet accumulation over time (A; mean ± SEM) and area under the CD41 vs time curve (AUC) (B; line and error bars show the median and interquartile range). (C-D) The P-selectin–positive area over time (C; mean ± SEM) and area under the P-selectin vs time curve (D; line and error bars show the median and interquartile range). (E-F) Fibrin accumulation over time (E; mean ± SEM) and the area under the fibrin vs time curve (F; median and interquartile range). Vehicle, n = 55 injuries in 12 mice; bivalirudin alone, n = 38 injuries in 6 mice; bivalirudin plus cangrelor, n = 29 injuries in 7 mice. The vehicle and bivalirudin alone groups shown are the same as those included in supplemental Figure 3.
P2Y12signaling does not contribute to thrombin mediated platelet activation in the core region. Laser-induced injury was performed in cremaster muscle arterioles of wild-type mice treated with vehicle (blue), bivalirudin alone (0.5 µg/g, red), or bivalirudin plus cangrelor (0.75 µg prior to each injury, green) as described in “Materials and methods.” (A-B) Total platelet accumulation over time (A; mean ± SEM) and area under the CD41 vs time curve (AUC) (B; line and error bars show the median and interquartile range). (C-D) The P-selectin–positive area over time (C; mean ± SEM) and area under the P-selectin vs time curve (D; line and error bars show the median and interquartile range). (E-F) Fibrin accumulation over time (E; mean ± SEM) and the area under the fibrin vs time curve (F; median and interquartile range). Vehicle, n = 55 injuries in 12 mice; bivalirudin alone, n = 38 injuries in 6 mice; bivalirudin plus cangrelor, n = 29 injuries in 7 mice. The vehicle and bivalirudin alone groups shown are the same as those included in supplemental Figure 3.
Discussion
A model of the hemostatic response has recently emerged in which the development of gradients of platelet agonists shaped by physical forces present within the evolving platelet plug result in a gradient of platelet activation emanating from the injury site.7,8,14,21-27 Prior studies demonstrated the importance of thrombin and ADP/P2Y12 signaling in establishing this platelet activation gradient.12 Here, we have focused on the contribution of 2 additional soluble platelet agonists, TxA2 and epinephrine, whose role in the spatiotemporal regulation of platelet activation during the hemostatic response has not been previously reported. Further, we examined the contribution of the P2Y12 signaling pathway to platelet activation by each of the other major soluble agonists to determine the role of this clinically important positive feedback mechanism in the spatiotemporal regulation of platelet activation. We found that either pharmacologic inhibition or genetic deletion of TxA2 signaling results in loss of the outer layers of minimally activated platelets from hemostatic plugs, without significantly impacting the hemostatic plug core region of fully activated platelets. This effect is very similar to what was previously reported for inhibition of P2Y12 signaling alone.12 The combined inhibition of TxA2/TP and ADP/P2Y12 signaling did not result in a significant additive effect, suggesting that these 2 signaling pathways act in concert to promote platelet recruitment and retention in the outer shell layers of the platelet plug. In contrast, epinephrine signaling was completely dispensable in this experimental setting, even when the system was stressed by additionally inhibiting P2Y12 signaling. Finally, the inhibition of P2Y12 signaling in combination with a submaximal dose of thrombin inhibitor resulted in an additive effect on attenuation of platelet accumulation, but not on full platelet activation and formation of a stable core region.
The finding that inhibition of either TxA2 signaling or P2Y12 signaling had a similar effect on platelet accumulation in the shell region demonstrates that neither of these signaling pathways is sufficient by itself to support platelet recruitment and retention during hemostatic plug formation in vivo. Further, the lack of an additive effect of combined TxA2 and P2Y12 inhibition suggests that these signaling pathways are dependent on each other to promote the level of platelet activation necessary for accumulation of platelets in the outer layers of a hemostatic plug. From a mechanistic point of view, this is consistent with the findings of Kunapuli and colleagues that stimulation of Gq signaling downstream of the TP receptor in platelets absolutely requires costimulation of a Gi-coupled signaling pathway, such as that provided by P2Y12, for robust platelet aggregation in vitro.2 More recent work from the Bergmeier laboratory has elucidated the mechanism by which Gq signaling works together with Gi signaling (via P2Y12 receptors) to regulate the small GTPase Rap1b28 via coordination of CalDAG-GEF1 and RASA3 activity.28-31 The results of the current study suggest that in the outer layers of a hemostatic plug, TxA2 signaling provides a Gq signaling component that is unable by itself to overcome inhibition of Rap1b activation by RASA3. Additional Gi signaling downstream of P2Y12 is necessary to sufficiently activate Rap1b and αIIbβ3 to mediate platelet accumulation.
Our results showing that robust platelet activation in the hemostatic plug core is preserved in the setting of dual antiplatelet therapy have a number of implications regarding thrombin formation and activity. First, they suggest that thrombin dominates platelet activation in the core region with apparently minimal contribution from ADP and TxA2. Further supporting this conclusion is the finding that P2Y12 antagonism has minimal or no effect on core formation, even in the presence of a thrombin inhibitor. Second, our findings suggest that TxA2 and P2Y12 signaling do not significantly contribute to thrombin generation in this setting and that the loss of the outer platelet layers during dual antiplatelet treatment does not impair local thrombin activity. Third, studies have shown that high concentrations of thrombin can induce maximal platelet activation independent of P2Y12 signaling.1,6,29 By extension, the results of the current study suggest that the thrombin concentration in the core region is near the top of its dose–response curve for platelet activation and is spatially distributed as a steep concentration gradient. This is consistent with our previous findings using a thrombin biosensor.14 Conversely, the lack of full platelet activation in the shell region shows that neither TxA2 nor ADP has reached concentrations that are at the top of the respective dose–response curve. It should also be noted that inhibition of TxA2 and P2Y12 signaling, either alone or in combination, did not result in complete inhibition of platelet accumulation in the shell region. The residual P-selectin–negative population may be the result of submaximal platelet activation by thrombin as the thrombin gradient rapidly declines or other weak platelet-signaling mechanisms (eg, glycoprotein Ib/von Willebrand factor interactions).
The lack of an effect of Gz deficiency on platelet accumulation or activation demonstrates that epinephrine signaling is not required for the hemostatic response, at least in the experimental setting employed here. It is possible that epinephrine might be an important alternative to P2Y12/Gi signaling in settings of systemic catecholamine release, such as sympathetic nervous system stimulation. Our findings are consistent with previous reports showing a normal bleeding time in Gz-deficient mice.5 A mild bleeding diathesis has been reported in human patients with reduced platelet α2-AR expression, although it was unclear whether the impaired platelet response to epinephrine was the causal factor.32 Pozgajova et al33 reported a highly variable effect of α2-AR deficiency on tail bleeding time in mice and a mild effect on thrombus stability in 2 separate thrombosis models, further suggesting that the importance of platelet epinephrine signaling is context dependent.
Finally, the current studies were performed in the microvasculature following small penetrating injuries that resulted in minimal bleeding. Based on prior studies comparing effects of P2Y12 antagonists in the micro- and macrovasculature,22 it is likely that the results obtained in the microvasculature model used here will translate in at least a general way to larger vessels and more substantial vascular injuries. However, it is also possible that differences in vessel architecture and hemodynamic variables result in a hemostatic response adapted for the macrovasculature that differs in interesting ways from the response observed in small arterioles. Future studies examining hierarchical organization of the hemostatic response in a variety of settings are clearly warranted to sort these issues out. It should also be noted that due to the variability inherent in the microvasculature injury model used here, the studies presented are not statistically powered to detect subtle differences among experimental groups. Therefore, while the data show that TxA2 and ADP signaling predominate in the shell region and that thrombin is primarily responsible for platelet activation in the core region, we cannot rule out minor contributions of any of these signaling pathways to platelet activation throughout the hemostatic plug.
In conclusion, the studies reported here show for the first time how thrombin, P2Y12, and TxA2 signaling are coordinated during development of a hierarchical organization of hemostatic plugs in vivo. Taken together with results of prior studies describing the development of soluble agonist gradients during hemostatic plug formation, we propose an updated model of platelet signaling during the hemostatic response. Although thrombin, TxA2, and ADP are all likely present within the developing hemostatic plug core region, Gq-mediated thrombin signaling dominates resulting in robust platelet activation, including granule secretion and stable adhesion. However, thrombin distribution is spatially restricted due to hindered transport as the platelet mass consolidates.7-9 Meanwhile, TxA2 and ADP released from platelets activated by thrombin in the core region submaximally stimulate platelets in the shell region via TP and P2Y12 receptors, respectively, with coordinated Gq and Gi pathway signaling resulting in accumulation of loosely adherent, minimally activated platelets that do not secrete their α-granules. The requirement of both TxA2 and P2Y12 signaling for platelet accumulation sheds new light on the mechanisms of action of pharmacologic agents that target these signaling pathways. The finding that inhibition of either or both of these pathways strips away the outer shell of minimally activated platelets from hemostatic plugs while largely sparing the core region of fully activated platelets helps explain their relatively low bleeding risk in vivo, even when used in combination.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors gratefully acknowledge research funding from the National Heart, Lung and Blood Institute of the National Institutes of Health (grants P01-HL040387, P01-HL120846, and UM1-HL120877) (T.J.S. and L.F.B.), The Medicines Company (T.J.S. and L.F.B.), the Natural Science Foundation of China (grants 81370373, 91439112, and 81620108001 [L.Z.] and grants 31300781 and 81670134 [C.T.]), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (L.Z.). The intravital microscopy system at the University of Pennsylvania was partially funded by the National Center for Research Resources (shared instrument grant S10-RR26716-1).
Authorship
Contribution: J.S. and S.S. conducted experiments, analyzed data, and edited the manuscript; J.W., J.T., S.G., C.N.M., and C.T. conducted experiments and provided laboratory support; Y.Y. provided critical reagents, analyzed and interpreted data, and edited the manuscript; L.F.B. analyzed and interpreted data and edited the manuscript; and L.Z. and T.J.S. designed and supervised experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: L.F.B. and T.J.S. received research funding from The Medicines Company. The remaining authors declare no competing financial interests.
Correspondence: Timothy J. Stalker, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: tstalker@pennmedicine.upenn.edu; and Li Zhu, Cyrus Tang Hematology Center, Soochow University, 199 Renai Rd, Building 703, Room 09, Suzhou 215123, China; e-mail: zhul@suda.edu.cn.
References
Author notes
J.S. and S.S. contributed equally to this study.


![Figure 1. Thromboxane receptor deficiency attenuates platelet accumulation in the outer shell region of hemostatic plugs in vivo. Laser-induced injury was performed in cremaster muscle arterioles of wild-type (blue) or TP−/− (red) mice as described in “Materials and methods.” (A-B) Total platelet accumulation following laser injury was measured by quantifying the CD41-positive area over time. (A) Graph showing the CD41-positive area over time (mean ± standard error of the mean [SEM]). (B) Graph showing the area under the CD41 vs time curve (AUC). Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. (C-D) α-Granule secretion following laser injury was used as a measure of platelet activation by quantifying the P-selectin–positive area over time. (C) Graph showing the P-selectin–positive area over time (mean ± SEM). (D) Graph showing the area under the P-selectin vs time curve. Data points represent AUCs of individual hemostatic plugs. The line and error bars show the median and interquartile range. Wild-type, n = 32 injuries in 4 mice; TP−/−, n = 39 injuries in 4 mice. NS, not significant; WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/27/10.1182_bloodadvances.2017009498/3/m_advances009498f1.jpeg?Expires=1767724723&Signature=VZlw~J5LwMzvPlpa6W8wUVkrJ1FQJ3Jfc7I7D8hTtPfS6xAFXS4UOko8xP8KJV5BC~tnjbVaEFNRfJjWQZtQ2cs-erVPSzim9Br~ickmHw5R6kunYLZtQ19Qe6Ga55vIW~vYTbueo0ThSecY1BELB0dhhVAUzVOPnGK~J4rcyyXjO18u1nCk0WFU~6y8HFmjFXzA7xs8bujCJnGGHMqiXst4kdXgoiUkOtA-HXaIzfRasYQoEZtEJzZUHtIPWnliuzCma~ZZnpXtY4sF6-kwssWFGrROz52mN2rbZ-aDEULc9vZGme~v57DQvPQAdxLOJF2pI9rR0tBWW~sCkylI6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)