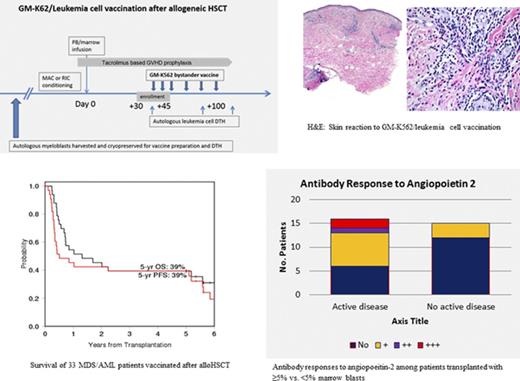
Key Points
GM-K562 admixed leukemia cell vaccination after allogeneic HSCT has biologic activity in MDS/AML.
Postvaccination antibody response to angiopoeitin-2 is associated with improved outcomes.
Abstract
We report a clinical trial testing vaccination of autologous myeloblasts admixed with granulocyte-macrophage colony-stimulating factor secreting K562 cells after allogeneic hematopoietic stem cell transplantation (HSCT). Patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) with ≥5% marrow blasts underwent myeloblast collection before HSCT. At approximately day +30, 6 vaccines composed of irradiated autologous myeloblasts mixed with GM-K562 were administered. Tacrolimus-based graft-versus-host disease (GVHD) prophylaxis was not tapered until vaccine completion (∼day 100). Thirty-three patients with AML (25) and MDS (8) enrolled, 16 (48%) had ≥5% marrow blasts at transplantation. The most common vaccine toxicity was injection site reactions. One patient developed severe eosinophilia and died of eosinophilic myocarditis. With a median follow-up of 67 months, cumulative incidence of grade 2-4 acute and chronic GVHD were 24% and 33%, respectively. Relapse and nonrelapse mortality were 48% and 9%, respectively. Progression-free survival (PFS) and overall survival (OS) at 5 years were 39% and 39%. Vaccinated patients who were transplanted with active disease (≥5% marrow blasts) had similar OS and PFS at 5 years compared with vaccinated patients transplanted with <5% marrow blasts (OS, 44% vs 35%, respectively, P = .81; PFS, 44% vs 35%, respectively, P = .34). Postvaccination antibody responses to angiopoietin-2 was associated with superior OS (hazard ratio [HR], 0.43; P = .031) and PFS (HR, 0.5; P = .036). Patients transplanted with active disease had more frequent angiopoeitin-2 antibody responses (62.5% vs 20%, P = .029) than those transplanted in remission. GM-K562/leukemia cell vaccination induces biologic activity, even in patients transplanted with active MDS/AML. This study is registered at www.clinicaltrials.gov as #NCT 00809250.
Introduction
Relapse is a major cause of treatment failure after allogeneic hematopoietic stem cell transplantation (HSCT). Cancer vaccinations represent a novel approach to stimulate tumor-specific immunity from the donor graft. Autologous leukemia vaccination strategies have biologic activity, but their efficacy is often limited by impaired host immunity from prior cytotoxic therapy and inability to overcome self-tolerance.1,2 Administration of leukemia vaccines after allogeneic transplantation could bypass these issues. The detection of donor immune responses specific to leukemia antigens such as PR1 and WT1 after allogeneic HSCT suggest that the graft-versus-leukemia effect can be enhanced by cancer vaccines.3,4 However, these are generally administered late after transplantation when the patient is off immune suppression.5-7 It is unknown whether vaccinations given within the first 100 days, while the patient is still on immune suppression, would stimulate leukemia-specific immunity, or exacerbate graft-versus-host disease (GVHD). Early vaccination after HSCT may be desirable because the lymphopenic milieu after conditioning fosters a surge of homeostatic cytokines, such as interleukin-7 (IL-7), IL-12, and IL-15, which promote T-cell activation and expansion.8-11 Regulatory T cells are also relatively deficient early after HSCT.12
Autologous tumor cell vaccines genetically engineered to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), collectively known as GVAX, have induced tumor-specific immune responses in patients with melanoma and non–small cell lung cancer.13,14 These responses have been correlated with enhanced antigen presentation by recruited dendritic cells and macrophages as well as improved coordinated cellular and humoral immunity by CD4+, CD8+ T lymphocytes, CD1d-restricted natural killer (NK) T (NKT) cells, and B lymphocytes.15,16 GVAX has also demonstrated biologic activity against myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) in 2 human clinical trials, including 1 after autologous HSCT.17,18 In murine transplant models, GVAX elicits potent leukemia-specific immunity when given 6 weeks after allogeneic transplantation.19
We previously demonstrated that tumor-specific immunity can be induced by vaccinating refractory MDS/AML patients with GVAX within the first 100 days of allogeneic HSCT.20 In this study, 9 of 10 patients who completed vaccinations had durable complete remissions, and correlative studies demonstrated coordinated NK-, T-, and B-cell responses after vaccination, with reduction in levels of the circulating soluble NKG2D ligands MICA and MICB, and robust antibody responses against angiogenic cytokines including angiopoietin 1 and 2, which were not present in nonvaccinated patients after HSCT.20,21
A disadvantage of this GVAX platform is that autologous leukemia cells were transduced with an adenoviral construct such that the tumor cell was the source of both antigen and GM-CSF production. This approach is hampered by the inconsistent transduction efficiency of autologous cells, resulting in highly variable production of GM-CSF from individual vaccine preparations. To render secretion of GM-CSF more reliable and less susceptible to variables associated with viral transduction, we created a GM-K562 cell line that is made from K562 cells stably transfected with a plasmid encoding GM-CSF that is amenable to large-scale manufacturing.
Creation of GM-K562 cells allowed us to use a vaccine strategy using irradiated GM-K562 cells as a consistent local source of GM-CSF admixed with autologous tumor cells. This “bystander” vaccine bypasses the need for viral transduction and renders the vaccine preparation amenable to widespread use. Results on the safety and effectiveness of GM-K562 bystander vaccinations have been reported in chronic myeloid leukemia and AML, including after autologous transplantation.22-24
Investigators at our center have previously reported a trial using GM-K562 bystander vaccination after allogeneic HSCT for patient with chronic lymphoid leukemia (CLL).25 In this study, 18 patients received vaccinations with GM-K562 admixed with autologous CLL cells early after a reduced intensity conditioning (RIC) HSCT, with a 2-year progression-free survival (PFS) and overall survival (OS) of 82% and 88%, respectively. They further showed that the vaccination expanded CD8+ T cells that reacted against autologous tumor, but not against alloantigen bearing nonmalignant recipient cells, in contrast to the T cells from nonvaccinated CLL patients undergoing HSCT. Further analysis showed that 17% of the CD8+ T-cell clones isolated from 4 vaccinated patients solely reacted against CLL-associated antigens.
We now report the results of a prospective clinical trial investigating early postallogeneic HSCT vaccination using this admixed GM-K562/autologous leukemia cell vaccine in patients with advanced MDS or high-risk AML.
Methods
Study design
This was an open-label, phase 1 single-arm study. The primary objective was to assess the safety of vaccination. The secondary objectives were to assess the efficacy of vaccination as measured by the incidence of relapse, PFS, OS, and biologic activity. The study was originally planned to accrue 40 patients, but was halted after 33 patients when 1 patient developed a fatal hypereosinophilic syndrome related to the vaccine. The study received approval from the Dana-Farber/Harvard Cancer Center institutional review board and biosafety committees, the National Institutes of Health Recombinant DNA Advisory Committee, and the Food and Drug Administration. Informed written consent was obtained from all participants.
Patient population
This study was performed under 2 separate but linked institutional review board–approved protocols. The first protocol (NCT00809367) was a tissue collection/banking study to harvest myeloblasts from patients for potential future leukemia vaccine studies. Patients were eligible for myeloblast banking if they have advanced MDS (RA-EB1 or RA-EB2) or high-risk AML at diagnosis, or any AML in relapse or induction failure. After myeloblast harvest, patients may proceed directly to allogeneic HSCT or receive intervening cytoreductive therapy before HSCT at the discretion of their leukemia and/or transplant physician.
Patients with successful myeloblast harvest (≥6.0 × 105 blasts) on the previously mentioned banking study who went on to receive allogeneic myeloablative or RIC HSCT from a 9/10 or 10/10 HLA-matched related or unrelated donor are then enrolled on the current vaccine study between day +30 and day +45 after transplantation.
Additional criteria required to receive vaccination included absolute neutrophil count >500/μL, platelet count >10 000/μL, and absence of GVHD requiring systemic corticosteroid therapy. Enrollment to the vaccination trial occurred between January 2009 and March 2011.
Generation of GM-K562 cell line
GM-K562 was constructed in the Vector Core Laboratory of the Harvard Gene Therapy Initiative. The GM-K562 cell line was constructed by cotransfecting the parental cell line with 3.8 μg of a human GM-CSF expression vector (pUCMD.hGMCSFa) and 0.2 μg of a puromycin selection plasmid (pJ6Omega-puro). pUCMD.hGMCSFa was constructed by subcloning pMD.hGMCSF into pUC to remove the SV40 origin and sequenced junctions.
Transfected cells were selected with 2 μg/mL puromycin. Surviving cells were cloned by limiting dilution with puromycin selection. Individual clones were then screened for GM-CSF secretion using a human GM-CSF enzyme-linked immunosorbent assay (ELISA) kit (Endogen #EH-GMCSF). The highest expressing clone was subjected to a second round of limiting dilution cloning in puromycin-containing media to confirm consistent GM-CSF secretion and chosen for clinical production. These cells were transferred to the Harvard Gene Therapy Initiative Gene Vector Laboratory for production of a master cell bank (Lot 00502CK562).
Autologous myeloblast harvest, vaccine preparation, transplantation, and administration
Before HSCT, all patients underwent autologous tumor cell harvest from blood or bone marrow via phlebotomy, leukapheresis, or marrow aspiration on the previously mentioned banking study. The collection product was diluted in isotonic solution and mononuclear cells were enriched and isolated via Ficoll-Paque. A cytospin was made and the blast percentage was enumerated by a dedicated hematopathologist (O.P.). These cells were cryopreserved until the day of vaccine administration. Excess leukemia blasts, if available, were cryopreserved for autologous tumor cell delayed-type hypersensitivity (DTH) testing. Between the harvest and admission for HSCT, patients could receive additional therapy for MDS/AML at the discretion of the treating physician. Stem cell sources included bone marrow or granulocyte colony-stimulating factor mobilized peripheral blood. Choice of conditioning and GVHD prophylaxis regimens were at the discretion of the transplant physician.
On the day of vaccination, the vaccine dose was made by admixing autologous tumor cells (1 × 105 to 1.0 × 107 cells) with GM-K562 cells (1 × 107 cells). The mixture was irradiated at a dose of 10 000 cGy and resuspended in sterile saline. The irradiated vaccine dose was adjusted to a final volume of 1 mL in a 3-mL syringe and released for immediate injection. No more than 4 hours elapsed between thawing and vaccine administration.
Vaccination started between days +30 and +45 after HSCT. A series of 6 vaccinations was administered at 1-week intervals for the first 3 doses and then at 2-week intervals for the subsequent 3 doses. Vaccinations were administered half subcutaneously and half intradermally. Vaccinations were discontinued if there was disease relapse requiring immune suppression withdrawal or other therapeutic intervention, acute or chronic GVHD requiring systemic corticosteroids, or Common Toxicity Criteria grade 4 nonhematologic toxicity.
Vaccination site skin biopsies and DTH testing
Punch skin biopsies of vaccination sites were obtained 2 to 3 days after vaccines 1 and 5. Among patients who had extra blasts cryopreserved from their collection, separate injections of 1 × 106 irradiated autologous myeloblasts were administered intradermally for leukemia DTH testing concurrently with vaccines 1 and 5, and then 4 weeks after vaccine 6. Slides of skin biopsies of all vaccine and DTH sites were scored by a dermatopathologist (M.M.) who was blinded to the clinical results, as follows:
Grade 1: 2 to 3 lymphocytes, and, if present, eosinophils and neutrophils, in perivascular array with slight vessel involvement.
Grade 2: 4 to 7 lymphocytes in perivascular array, and, if present, eosinophils and neutrophils, in superficial and deep array with slight to moderate vessel involvement.
Grade 3: 8 to 15 lymphocytes, and, if present, eosinophils and neutrophils, in perivascular superficial and deep and often interstitial array with moderate vessel involvement.
Grade 4: >15 lymphocytes, and, if present, eosinophils and neutrophils, in perivascular and interstitial array as well as superficial and deep involvement with moderate to severe vessel involvement.
Immunophenotyping and angiogenic cytokine antibody screening
Immunophenotyping of mononuclear cells and antibody responses were assessed on peripheral blood collected on the day of vaccine initiation, monthly during the vaccination period, and at 6, 9,12, and 18 months after vaccination. Regulatory T cells (Tregs) were defined as CD3+CD4+CD25medium-highCD127low; conventional CD4 T cells (Tcon) as CD3+CD4+CD25negative-lowCD127medium-high; NK cells as CD56+CD3−; NKT cells as CD56+CD3+; and B cells as CD3−CD19+. Screening of postvaccination antibody responses to a panel of angiogenic cytokines (L1-CAM, DEL-1, Ang1, Ang2, HGF, PDGF, VEGF-A, and PRGN) was performed by ELISA, as previous described.21
Statistical analyses
Patient characteristics were reported descriptively. OS was defined as time from transplant to death from any cause. PFS was defined as time from transplant to disease relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored for PFS at the time last known alive and progression-free. OS and PFS were estimated using the Kaplan-Meier method; the log-rank test was used for group comparisons. Cumulative incidences of nonrelapse death, relapse, and GVHD were constructed in the competing risks framework considering relapse, nonrelapse mortality (NRM), and death or relapse without developing GVHD, respectively, as competing events. The difference between cumulative incidences in the presence of a competing risk was tested using the Gray method.26 Univariable Cox regression analysis for OS and PFS was performed for variables listed in Table 1 and antibody responses to angiogenic cytokines. Penalized Cox model was explored for feature reduction and the Firth correction was used for multivariable models. Before modeling, the proportional hazards assumption and significance of interaction terms were examined. Immune reconstitution data and antibody response to angiogenic cytokines were also reported descriptively. All tests were 2-sided at a 0.05 level and multiple comparisons were not considered. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC) and R version 3.2.2 (the CRAN project, www.cran.r-project.org).
Baseline characteristics
| . | N . | % . |
|---|---|---|
| Age, median (range), y | 57 (20-70) | |
| Patient sex | ||
| M | 20 | 60.6 |
| F | 13 | 39.4 |
| Donor sex | ||
| M | 21 | 63.6 |
| F | 12 | 36.4 |
| Patient-donor sex | ||
| FF | 3 | 9.1 |
| FM | 10 | 30.3 |
| MF | 9 | 27.3 |
| MM | 11 | 33.3 |
| Disease | ||
| AML | 25 | 75.8 |
| MDS (RAEB) | 8 | 24.2 |
| Active disease at HSCT (BM ≥5% blasts) | 16 | 48.5 |
| Donor-HLA match (A, B, C, DRB1, DQB1) | ||
| 10/10 related | 14 | 42.4 |
| 10/10 unrelated | 10 | 30.3 |
| 9/10 unrelated | 9 | 27.3 |
| Cell source | ||
| BM | 1 | 3 |
| PBSC | 32 | 97 |
| Conditioning intensity* | ||
| MAC | 12 | 36.4 |
| RIC | 21 | 63.6 |
| GVHD prophylaxis | ||
| Tac/MTX | 16 | 48.5 |
| Tac/Sir | 5 | 15.2 |
| Tac/Sir/MTX | 12 | 36.4 |
| Disease Risk Index | ||
| Low | 2 | 6.1 |
| Intermediate | 11 | 33.3 |
| High | 18 | 54.6 |
| Very high | 2 | 6.06 |
| Patient-donor CMV serology | ||
| Any positive | 23 | 69.7 |
| . | N . | % . |
|---|---|---|
| Age, median (range), y | 57 (20-70) | |
| Patient sex | ||
| M | 20 | 60.6 |
| F | 13 | 39.4 |
| Donor sex | ||
| M | 21 | 63.6 |
| F | 12 | 36.4 |
| Patient-donor sex | ||
| FF | 3 | 9.1 |
| FM | 10 | 30.3 |
| MF | 9 | 27.3 |
| MM | 11 | 33.3 |
| Disease | ||
| AML | 25 | 75.8 |
| MDS (RAEB) | 8 | 24.2 |
| Active disease at HSCT (BM ≥5% blasts) | 16 | 48.5 |
| Donor-HLA match (A, B, C, DRB1, DQB1) | ||
| 10/10 related | 14 | 42.4 |
| 10/10 unrelated | 10 | 30.3 |
| 9/10 unrelated | 9 | 27.3 |
| Cell source | ||
| BM | 1 | 3 |
| PBSC | 32 | 97 |
| Conditioning intensity* | ||
| MAC | 12 | 36.4 |
| RIC | 21 | 63.6 |
| GVHD prophylaxis | ||
| Tac/MTX | 16 | 48.5 |
| Tac/Sir | 5 | 15.2 |
| Tac/Sir/MTX | 12 | 36.4 |
| Disease Risk Index | ||
| Low | 2 | 6.1 |
| Intermediate | 11 | 33.3 |
| High | 18 | 54.6 |
| Very high | 2 | 6.06 |
| Patient-donor CMV serology | ||
| Any positive | 23 | 69.7 |
BM, bone marrow; CMV, cytomegalovirus; F, female; M, male; MAC, myeloablative conditioning; MTX, methotrexate; PBSC, peripheral blood stem cell; RAEB, refractory anemia with excess blasts; Sir, sirolimus; Tac, tacrolimus.
RIC regimens included busulfan/fludarabine (20) and fludarabine /melphalan (1). Myeloablative regimens included cyclophosphamide/1200 cGy total body irradiation (11) and busulfan/cyclophosphamide (1).
Results
Ninety patients underwent leukemia cell harvest on the companion banking study for this trial. Of these, 16 died before HSCT or did not receive HSCT, 33 enrolled on this trial and started vaccination, and 41 did not because of disease progression after HSCT (18), GVHD (12), insufficient cells harvested (7), death before day +30 (2), and patient refusal (2). Characteristics of the 33 patients who received vaccinations are shown on Table 1. Median age was 57 years (range, 20-70). Diseases included AML (25) and MDS (8). Twenty (61%) had high or very high Disease Risk Index. Twenty patients (61%) received additional chemotherapy between leukemia cell harvest and HSCT. At transplant admission, 16 patients (48%) had ≥5% marrow blasts (median, 10%; range, 5%-85%).
Nineteen (58%) patients were transplanted from unrelated donors. Twenty-four (73%) received a 10/10 donor-recipient HLA-matched graft. Graft source was predominantly granulocyte colony-stimulating factor mobilized blood (98%). Twenty-one (64%) received a RIC HSCT. GVHD prophylaxis included tacrolimus/methotrexate (16), tacrolimus/methotrexate/sirolimus (12), and tacrolimus/sirolimus (5).
The median time to vaccine initiation was day +35. Twenty-two (67%) patients completed all 6 vaccines. The remaining patients received 1 to 3 vaccines (6) or 4 to 5 vaccines (5). Reasons for vaccine cessation included disease relapse (4), GVHD (2), consent withdrawal (2), idiopathic pneumonia syndrome (1), severe eosinophilia (1), and physician decision (1).
Safety and tolerability
Vaccinations were generally well tolerated. Most patients developed mild, transient erythema, induration, and pruritus at the injection sites. These reactions tended to increase with the number of vaccines: erythema occurred in 46% and 73% of patients following the first and sixth vaccines, respectively. In all patients except 1, site reactions resolved spontaneously within 1 week. The exception was a patient who developed a persistent skin reaction after his fourth vaccine. He subsequently developed severe leukocytosis with hypereosinophilia and was found to have persistence of GM-K562 cells at the injection site; despite treatment steroids and nilotinib, he ultimately died from complications of eosinophil-laden thromboemboli and eosinophilic myocarditis.
Because of this event, the study investigators suspended the trial to conduct an extensive evaluation. A detailed investigation of the vaccine manufacturing procedures in this case did not reveal any atypical aspects. Rather, an unusual pattern of early immune reconstitution was evident in this patient that was characterized by high levels of circulating CCL17, elevated numbers of FoxP3+ Tregs, and impaired NK cell function. Comparison of Treg numbers in this patient relative to other vaccinated patients on this trial, and patients who received similar RIC HSCT with tacrolimus and sirolimus GVHD prophylaxis without vaccination, is shown in supplemental Figure 1.
There were no continued or additional safety concerns after a thorough investigation of this case, and the Food and Drug Administration lifted the investigational new drug hold to allow the study to reopen. However, after a delay of more than 1.5 years for this investigation, enthusiasm for this GM-K562 platform for vaccination had waned, and a competing randomized trial using the different GVAX platform was well under development. We therefore decided not to reopen the study.
GVHD
Vaccination was not associated with an increase in acute or chronic GVHD. The cumulative incidence of grade 2-3 acute GVHD was 24%. The cumulative incidence of chronic GVHD at 2 years was 33%. These numbers are comparable to previously reported GVHD incidence at our center.27
Clinical outcomes and survival
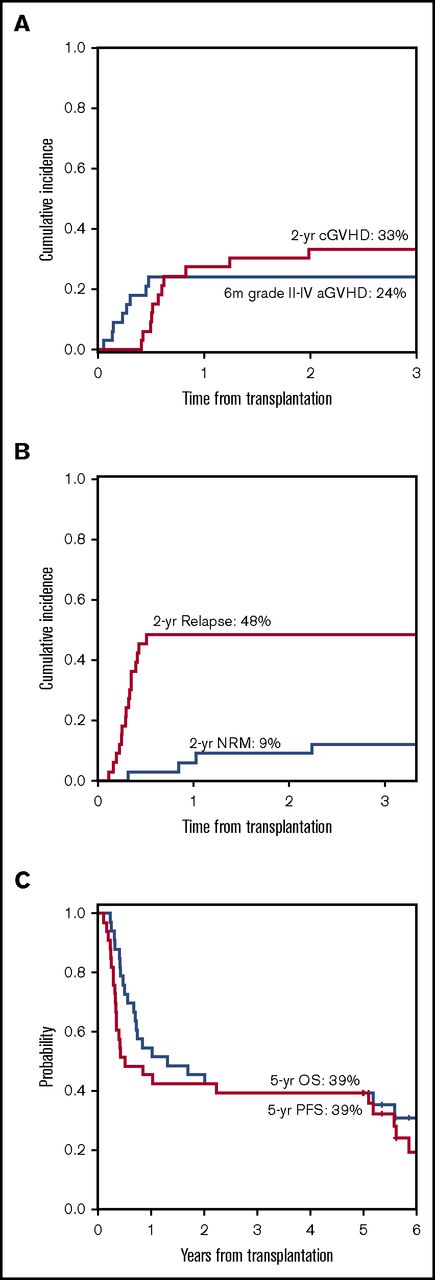
At the time of data lock on 16 May 2016, the median follow-up time among survivors was 67 months (range, 60-81). The summary of clinical outcomes is presented in Table 2. The cumulative incidence of relapse and NRM at 2 years after HSCT was 48% and 9%, respectively (Figure 1B). PFS and OS at 5 years after HSCT were 39% and 39%, respectively (Figure 1C). There was no statistical difference in PFS and OS between patients who completed all 6 vaccines vs those who did not. Although OS and PFS are not statistically different between patients transplanted with active disease and no active disease (44% vs 35%, respectively; P = .81 for OS, 44% vs 35%, respectively, for PFS, P = .34), the 5-year cumulative incidence of relapse was lower for patients who had ≥5% marrow blasts at transplant compared with patients transplanted in remission (38% vs 59%, P = .08), but NRM was higher (19% vs 6%, respectively, P = .07).
Summary of clinical outcomes
| . | From HSCT . | From first VAX* . |
|---|---|---|
| Follow-up time among survivors | ||
| Median (range), mo | 67 (60-81) | |
| 5-y OS (95% CI) | 39% (23-55) | 39% (23, 55) |
| 5-y PFS (95% CI) | 39% (23-55) | 39% (23-55) |
| 2-y cumulative incidence of relapse (95% CI) | 48% (30-64) | |
| 2-y cumulative incidence of NRM (95% CI) | 9% (2.2-22) | |
| 180-d cumulative incidence of grade 2-4 aGVHD (95% CI) | 24% (11-40) | |
| 2-y cumulative incidence of cGVHD relapse (95% CI) | 33% (17-50) |
| . | From HSCT . | From first VAX* . |
|---|---|---|
| Follow-up time among survivors | ||
| Median (range), mo | 67 (60-81) | |
| 5-y OS (95% CI) | 39% (23-55) | 39% (23, 55) |
| 5-y PFS (95% CI) | 39% (23-55) | 39% (23-55) |
| 2-y cumulative incidence of relapse (95% CI) | 48% (30-64) | |
| 2-y cumulative incidence of NRM (95% CI) | 9% (2.2-22) | |
| 180-d cumulative incidence of grade 2-4 aGVHD (95% CI) | 24% (11-40) | |
| 2-y cumulative incidence of cGVHD relapse (95% CI) | 33% (17-50) |
VAX, vaccination.
Median time from HSCT day 0 to VAX 1 = 35 d.
Clinical outcomes after HSCT and vaccination. (A) Grade 2-3 acute and chronic GVHD, (B) NRM and relapse, and (C) PFS and OS. aGVHD, acute GVHD; cGVHD, chronic GVHD.
Clinical outcomes after HSCT and vaccination. (A) Grade 2-3 acute and chronic GVHD, (B) NRM and relapse, and (C) PFS and OS. aGVHD, acute GVHD; cGVHD, chronic GVHD.
Pathologic responses at vaccination sites
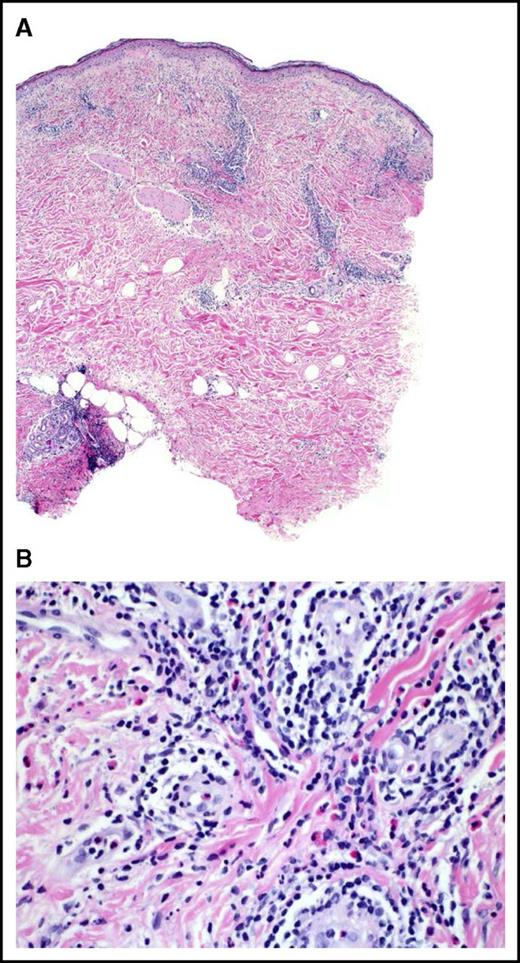
Vaccine site skin biopsies were available from 30 patients after vaccine 1 and 18 after vaccine 5. Pathologic examination of skin biopsies often demonstrated an influx of eosinophils, dendritic cells, macrophages, neutrophils, and lymphocytes, with frequent perivascular and perineural distribution (Figure 2). Pathologic skin reactions for vaccine 1 sites were grade 1 or higher in 29 (97%) patients, with 11 (37%) being moderate to severe (grade 3-4). After vaccine 5, grade 1 or higher vaccine site reaction was observed in all 18 patients, with 12 (67%) scored as grade 3-4.
Representative histopathologic response at vaccination site. (A) Hematoxylin and eosin (H&E) 4×: a fully developed reaction to vaccination. Note the prominent infiltration of lymphocytes at all levels of the skin, including the subcutaneous fat. (B) H&E 40×: higher magnification reveals numerous perivascular lymphocytes and eosinophils with perineural infiltration. The endothelial cells of the venules are swollen. There are scattered mononuclear histiocytes present in the infiltrate.
Representative histopathologic response at vaccination site. (A) Hematoxylin and eosin (H&E) 4×: a fully developed reaction to vaccination. Note the prominent infiltration of lymphocytes at all levels of the skin, including the subcutaneous fat. (B) H&E 40×: higher magnification reveals numerous perivascular lymphocytes and eosinophils with perineural infiltration. The endothelial cells of the venules are swollen. There are scattered mononuclear histiocytes present in the infiltrate.
Presence of grade 3-4 vaccine site reactions was not associated with improvement in OS or PFS, when compared with patients who had grade 0-2 reactions.
Pathologic responses at leukemia DTH sites
Autologous leukemia cell DTH testing was performed in 14 patients at vaccine 1 (DTH1), 11 patients at vaccine 5 (DTH2), and 4 patients after vaccine 6. Unlike vaccine site reactions, clinically evident DTH reactions were uncommon, being observed in 2 patients only. One patient had erythema and induration of 0.5 cm; a second patient had a 3-cm area of erythema.
Skin biopsies of DTH sites were available and reviewed for 14 patients at DTH1 and 8 patients at DTH2. Histopathologic skin reactions grade ≥1 were observed in 11 of 14 patients (81%) at DTH1, with 3 (21%) having mild to moderate (grade 2-3) reactions. At DTH2 sites, 7 of 8 patients (88%) had grade ≥1 reaction and 5 of 8 patients (63%) had grade 2-3 reactions.
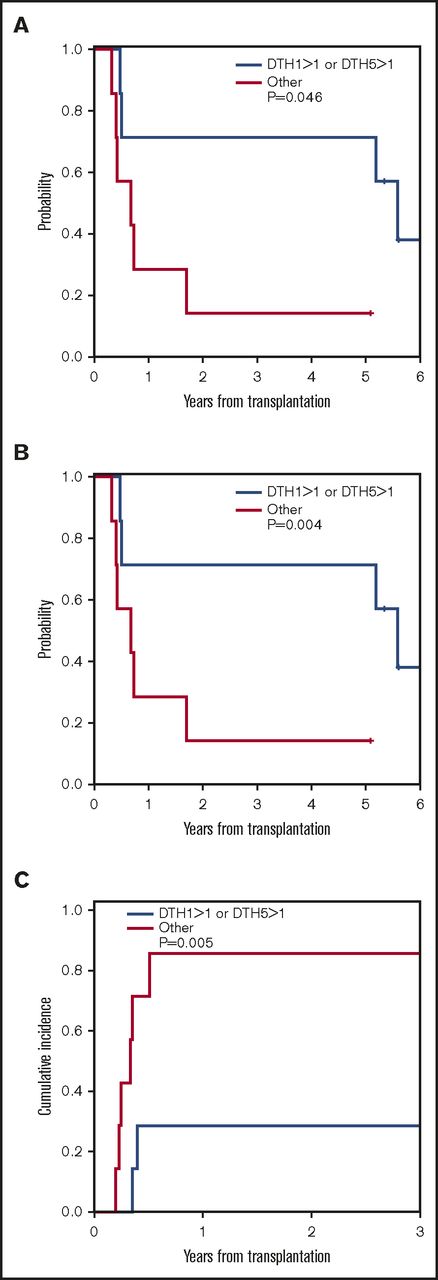
Presence of histopathologic DTH reaction at DTH1 was associated with a trend toward lower 3-year relapse (55% vs 67%, P = .07) and improved 5-year PFS (45% vs 33%, P = .09). When we included patients who had robust (grade >1) DTH responses at DTH1 or DTH2 only, there was a lower incidence of relapse and improved PFS and OS compared with those who had grade 1 or no DTH responses at either of these 2 time points: 3-year relapse 29% vs 86%, P = .005; 5-year PFS, 71% vs 14%, P = .004; 5-year OS, 71% vs 14% P = .046, respectively (Figure 3 A-C). These results suggest that robust pathologic DTH responsiveness after vaccination is associated with lower relapse after vaccination.
Correlation of robust (>grade 1) autologous leukemia cell DTH responses during vaccine 1 or vaccine 5. (A) OS; (B) PFS; (C) relapse.
Correlation of robust (>grade 1) autologous leukemia cell DTH responses during vaccine 1 or vaccine 5. (A) OS; (B) PFS; (C) relapse.
Recovery of T, B, and NK cells after vaccination
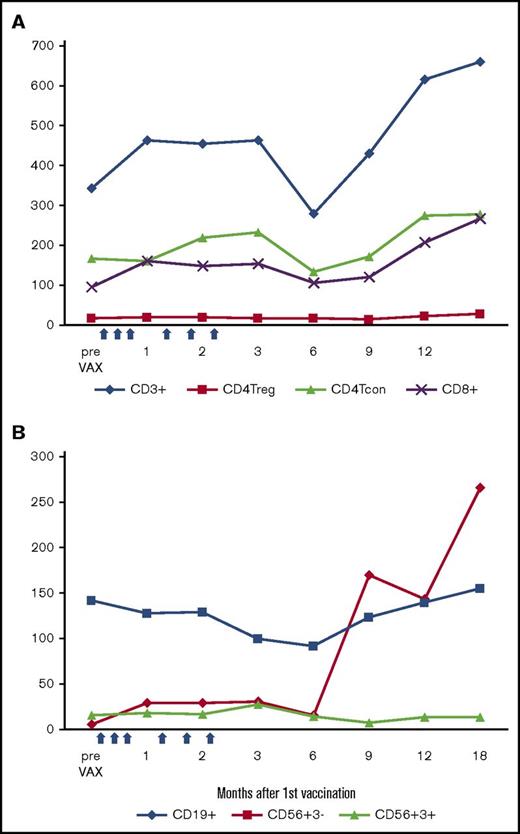
Vaccination did not affect T-, B-, and NK-cell recovery after transplant. As shown in Figure 4A and supplemental Table 1, during the first 2 months after starting vaccination, CD3, CD4 Tcon, and CD8 T-cell counts increased significantly compared with the prevaccination levels at about day +30 after HSCT. At 6 months after vaccine initiation (∼7-7.5 months after HSCT), the CD3 and CD4 counts briefly returned to the prevaccination levels and then gradually increased. NK cells (CD56+CD3−) recover rapidly after transplant and did not change during vaccination (Figure 4B). NK cell numbers declined slightly after vaccinations, but rebounded to double the pre-HSCT levels at 9 months. CD56+CD3+ (NKT) cells recovered slowly and did not change notably during vaccination. B-cell recovery occurred rapidly starting after 6 months after HSCT.
Immune reconstitution after autologous myeloblast/GM-K562 vaccination. (A) Absolute CD3, CD4 Treg, CD4 Tcon, and CD8 cell counts. (B) Absolute B, NK, and NKT cell counts. Dots indicate median values at each time point; blue arrows indicate 6 vaccinations.
Immune reconstitution after autologous myeloblast/GM-K562 vaccination. (A) Absolute CD3, CD4 Treg, CD4 Tcon, and CD8 cell counts. (B) Absolute B, NK, and NKT cell counts. Dots indicate median values at each time point; blue arrows indicate 6 vaccinations.
Antibody responses to angiogenic cytokines after vaccination
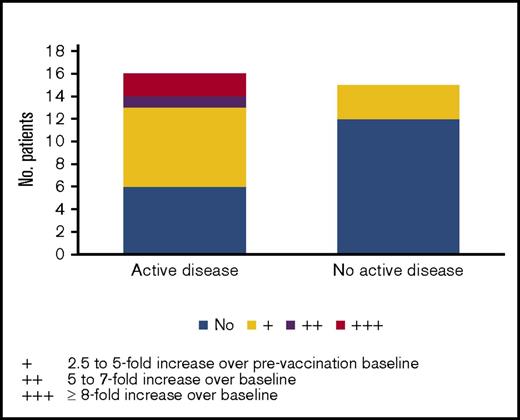
Analyses of sera from patients after vaccination demonstrated robust antibody responses against a panel of 8 angiogenic cytokines previously shown to be associated with the graft-versus-leukemia response after GVAX reactions.21 These include L1CAM, DEL-1, angiopoietin-1, angiopoietin-2, VEGF-A, progranulin, platelet-derived growth-BB, and hepatocyte growth factor. As shown on Table 3, robust (≥2+) peak antibody responses against these proteins were observed. When antibody responses to each of these proteins were included in Cox models as a time-dependent variable in the presence of active disease, response to angiopoietin-2 was significantly associated with OS (hazard ratio, 0.43; 95% confidence interval [CI], 0.18-0.85, P = .031) and PFS (hazard ratio, 0.5; 95% CI, 0.24-0.89, P = .036). We then examined the association between disease status and response. As shown in Figure 5, patients with active disease at HSCT were more likely to mount responses to angiopoietin-2 than those without active disease (62.5% vs 20%, respectively, P = .029, Fisher’s exact test). Angiopoietin-2 responses were also stronger among patients with active disease. Most responses developed immediately after vaccination through 7 months after transplantation.
Semiquantitative ELISA analysis of antibody response to angiogenic cytokines in vaccinated patients
| Patient . | L1 . | DEL-1 . | Ang1 . | Ang2 . | HGF . | PDGF . | VEGF-A . | PGRN . | Total VAX given . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | ++ | 6 | ||||
| 2 | 6 | ||||||||
| 3 | + | + | +++ | + | + | +++ | 4 | ||
| 4 | 2 | ||||||||
| 5 | 2 | ||||||||
| 6 | + | ++ | + | + | ++ | + | 6 | ||
| 7 | + | ++ | +++ | + | ++ | + | + | 6 | |
| 8 | + | + | 6 | ||||||
| 9 | + | 3 | |||||||
| 10 | + | 3 | |||||||
| 11 | + | + | ++ | + | ++ | ++ | + | 6 | |
| 12 | + | + | 6 | ||||||
| 13 | 6 | ||||||||
| 14 | ++ | + | + | + | + | 6 | |||
| 15 | 2 | ||||||||
| 16 | 6 | ||||||||
| 17 | +++ | + | +++ | 1 | |||||
| 18 | + | 6 | |||||||
| 19 | ++ | + | + | ++ | 6 | ||||
| 20 | ++ | + | + | ++ | 6 | ||||
| 21 | 6 | ||||||||
| 22 | + | +++ | + | +++ | + | + | 5 | ||
| 23 | 2 | ||||||||
| 24 | +++ | +++ | +++ | 6 | |||||
| 25 | + | + | 6 | ||||||
| 26 | 4 | ||||||||
| 27 | 2 | ||||||||
| 28 | + | + | + | + | 6 | ||||
| 29 | 6 | ||||||||
| 30 | + | + | ++ | + | + | 6 | |||
| 31 | + | + | + | + | 6 |
| Patient . | L1 . | DEL-1 . | Ang1 . | Ang2 . | HGF . | PDGF . | VEGF-A . | PGRN . | Total VAX given . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | ++ | 6 | ||||
| 2 | 6 | ||||||||
| 3 | + | + | +++ | + | + | +++ | 4 | ||
| 4 | 2 | ||||||||
| 5 | 2 | ||||||||
| 6 | + | ++ | + | + | ++ | + | 6 | ||
| 7 | + | ++ | +++ | + | ++ | + | + | 6 | |
| 8 | + | + | 6 | ||||||
| 9 | + | 3 | |||||||
| 10 | + | 3 | |||||||
| 11 | + | + | ++ | + | ++ | ++ | + | 6 | |
| 12 | + | + | 6 | ||||||
| 13 | 6 | ||||||||
| 14 | ++ | + | + | + | + | 6 | |||
| 15 | 2 | ||||||||
| 16 | 6 | ||||||||
| 17 | +++ | + | +++ | 1 | |||||
| 18 | + | 6 | |||||||
| 19 | ++ | + | + | ++ | 6 | ||||
| 20 | ++ | + | + | ++ | 6 | ||||
| 21 | 6 | ||||||||
| 22 | + | +++ | + | +++ | + | + | 5 | ||
| 23 | 2 | ||||||||
| 24 | +++ | +++ | +++ | 6 | |||||
| 25 | + | + | 6 | ||||||
| 26 | 4 | ||||||||
| 27 | 2 | ||||||||
| 28 | + | + | + | + | 6 | ||||
| 29 | 6 | ||||||||
| 30 | + | + | ++ | + | + | 6 | |||
| 31 | + | + | + | + | 6 |
Absorbence of ELISA measured at 450 nm.
, Optical density (OD) of posttreated sample is 1-2 times higher than pretreated, ++, OD is 2-3 times higher; +++, OD is >3 times higher.
Peak antibody responses to angiopoeitin-2 after vaccination among patients transplanted with active disease (≥5% marrow blasts) vs no active disease (<5% marrow blasts).
Peak antibody responses to angiopoeitin-2 after vaccination among patients transplanted with active disease (≥5% marrow blasts) vs no active disease (<5% marrow blasts).
Discussion
This trial investigated a leukemia vaccination strategy with irradiated autologous tumor cells admixed with GM-K562 early after allogeneic HSCT for patients with advanced MDS/AML. Our results demonstrate that, despite concurrent immune suppressive therapy, vaccination elicited biologic activity. Patients who underwent HSCT having ≥5% marrow blasts and received vaccinations had compatible PFS compared with vaccinated patients transplanted with <5% marrow blasts, suggesting that the vaccine may have benefited some of these higher risk patients. Concordant with this, we observed patients with active disease at HSCT were more likely to mount antibody responses to angiopoietin-2 after vaccination compared with those transplanted without active disease. We also documented pathologic DTH responses to autologous leukemia cells after vaccination that were sustained or increased from vaccines 1 to 5 were associated with significant improvement in relapse and PFS. Another interesting observation is that eosinophilic infiltration, a hallmark of GVAX reactions in previous studies,14,28 was a common feature at vaccination sites.
This study provides further evidence that cancer vaccination early after allogeneic HSCT is feasible. There are several potential advantages to administering cancer vaccines early after transplant. First, this period may be ideal because the lymphopenic state after the preparative regimen fosters an abundance of homeostatic cytokines, which promote the expansion of dendritic cells, NK cells, and T cells. Second, early vaccination is especially important after RIC HSCT in which early disease relapse is common. Finally, early vaccination may allow patients to mount vaccine responses before they develop GVHD, a condition that is itself severely immune suppressive. This is relevant because murine transplant models have demonstrated that tumor vaccine responses are abolished when the vaccine is administered to animals with active GVHD.19
Considering that 48% of patients were not in remission at HSCT, we would have expected few to achieve sustained remission. In this context, our 5-year PFS survival of 44% among patients transplanted with active disease appears encouraging. However, we acknowledge that there is a selection bias here because only patients whose disease remitted after HSCT would have been eligible to start vaccination. To indirectly compare this result with our institutional data, we identified 65 unvaccinated patients who underwent first allogeneic HSCT between 2007 and 2012 with advanced MDS or relapsed, refractory, or high-risk AML who engrafted without GVHD requiring systemic therapy, and were alive and relapse/progression-free and GVHD-free at day +35. When compared with this contemporaneous cohort of active MDS/AML patients transplanted without vaccinations who most closely resembled the vaccinated cohort with regard to transplant and disease characteristics, there was a statistically significant improvement in 5-year PFS (44% vs 20%, P = .007) and 5-year OS (44% vs 26%, P = .04) in favor of vaccination. Again, these results must be interpreted with caution because the study population is small and selected, and less than one-half of the patients who had cell harvest on the companion myeloblast banking protocol proceeded to HSCT and started vaccinations on this trial. Nonetheless, our results are intriguing as several of long-term survivors had large burden of leukemia at HSCT admission, including a refractory AML patient who had >90% circulating blasts.
Findings of this trial are also consistent with our previously published study testing adenovirus-transduced GVAX after allogeneic HSCT.20 In that study, responding patients demonstrated decrease in circulating levels of the NKG2D ligands MICA and MICB, and analyses of postvaccination sera revealed a broad and potent humoral response, with 6 of 9 long-term survivors developing antibodies to multiple angiogenic cytokines. Immunologic analyses of samples from patients on our study in an unbiased fashion through screening postimmunization sera against tumor-derived complementary DNA expression libraries and protein microarrays have similarly revealed high levels of postvaccination antibodies to angiogenic cytokines. In addition, potent antibody responses against angiopoetin-2 were most apparent in patients transplanted with active disease and were associated with superior PFS and OS. The question of why angiogenic cytokine antibodies would be associated with antileukemic response is under investigation, but we speculate that autologous myeloblasts and/or bone marrow stromal elements within the vaccine inoculum may express angiogenic cytokines. The finding of enhanced antibody responses in patients who were transplanted with active disease is intriguing. It is possible that the higher burden of myeloblasts at the time of conditioning may enhance tumor antigen availability to antigen-presenting cells early after transplantation and thus augment the response to the vaccination.
One patient on this study had persistent GM-K562 at the vaccine site and died of eosinophilic myocarditis resulting from proliferation and release of high levels of GM-CSF. Our trial was terminated early over this concern. An extensive investigation into this event confirmed that all laboratory procedures were followed and that the vaccine dose was correctly irradiated before administration. The patient was on tacrolimus and sirolimus as GVHD prophylaxis during the vaccination and did not have any GVHD. It is conceivable that some GM-K562 cells in the vaccine survived the high-dose radiation and were not rejected because he was on 2 potent immune suppressive agents early after HSCT. The unusual immune reconstitution in this patient characterized by alterations in innate and adaptive responses may also have contributed to the persistence of the GM-K562 cells. Although no other cases of persistent GM-K562 cells or prolonged eosinophilia were observed in this or our previously published study of GM-K562 admixed CLL vaccine,25 this event raises concern over the safety of irradiated K562 cells as vaccines in severely immune compromised patients after allogeneic HSCT. In this context, other approaches using highly immunogenic scaffolds as vaccine implants have been developed. These biodegradable scaffolds release fixed concentrations of GM-CSF, Toll-like receptor agonist, and tumor cell lysate over a prolonged period to create a controlled immunogenic environment to induce tumor immunity in vivo. This new approach generates effective tumor immunity in preclinical models but does not require the use of viable tumor cells or K562.29-31
In summary, we have demonstrated that GM-CSF–secreting leukemia cell vaccination using irradiated GM-K562 admixed with autologous myeloblasts early after transplantation induces biologic activity in patients transplanted with active MDS/AML. The results of this study should spur future investigation of GM-CSF–secreting and/or other cancer vaccines early after allogeneic HSCT.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (grants R01 CA183559, R01 CA183560, and P01 CA142106), the John B. Fisher Fund, and the Ted and Eileen Pasquarello Research Fund.
Authorship
Contribution: V.T.H. designed and wrote the research protocol, performed the research, analyzed the data, and wrote the paper; H.T.K. provided the statistical design of the research, analyzed the data, interpreted the data, and participated in writing the paper; N.B. gathered research data and assisted in the writing the paper; M.M. reviewed the dermatopathology slides and performed the pathologic grading of vaccine and delayed-type hypersensitivity responses; O.P. reviewed hematopathology slides to enumerate myeloblasts needed for vaccine production; M.P. performed the postvaccination antibody studies; H.D. processed the cells to generate the vaccine; N.C.S. performed immune reconstitution studies comparing the patient with unexpected death with other vaccinated and nonvaccinated patients; C.R. performed immunophenotyping analyses on all patients on this trial; J.R. supervised the laboratory staff that made the vaccine and performed immune reconstitution studies; G.D. supervised and performed immune-correlative studies; and C.C., E.P.A., J.K., J.H.A., and R.J.S. assisted in the design of the research, enrolled patients to the study and took direct care of patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vincent T. Ho, Dana-Farber Cancer Institute, 450 Brookline Ave, D-2030, Boston, MA 02215; e-mail: vincent_ho@dfci.harvard.edu.