Key Points
Patients with high-risk multiple myeloma have a median survival of <3 years.
Tandem autologous/allogeneic hematopoietic cell transplantation with bortezomib maintenance therapy improves survival in these patients.
Abstract
We evaluated tandem autologous/allogeneic hematopoietic cell transplantation followed by bortezomib maintenance therapy in a prospective phase 2 trial of treatment of high-risk multiple myeloma. The high-dose conditioning regimen for autologous hematopoietic cell transplantation consisted of melphalan 200 mg/m2. The nonmyeloablative conditioning regimen for the allogeneic transplant involved low-dose total body irradiation (2 Gy) with or without fludarabine (30 mg/m2 × 3 days). Among the 31 patients enrolled, 26 (84%) proceeded to HLA-matched allogeneic hematopoietic cell transplantation at a median of 61 (range, 41-168) days following the autologous transplant. Twenty-one patients (68%) started bortezomib (1.6 mg/m2 IV or 2.6 mg/m2 subcutaneously every 14 days for 9 months) at a median of 79 (range, 63-103) days after allogeneic transplantation. With a median follow-up of 51 (range, 16-86) months and based on intention to treat, the 2-year and 4-year progression-free survival and overall survival estimates among 24 newly diagnosed high-risk patients were 71% and 75%, and 52% and 61%, respectively. The 7 patients enrolled with relapsed or persistent disease had a 2-year and 4-year progression-free survival and overall survival rates of 14% and 43%, and 14% and 29%, respectively. These findings suggest that for patients with newly diagnosed high-risk multiple myeloma, bortezomib maintenance therapy after tandem autologous/allogeneic hematopoietic cell transplantation is safe and may prevent disease progression until full establishment of a graft-versus-myeloma effect. This benefit, however, does not extend to patients who enroll after unsuccessful prior therapy. This trial was registered at www.clinicaltrials.gov as #NCT00793572.
Introduction
Proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies have contributed to remarkable improvements in progression-free survival (PFS) and overall survival (OS) among patients with multiple myeloma (MM).1-5 Despite the efficacy of these agents, relapse remains inevitable for nearly all patients.6,7 Although patients with standard-risk myeloma can now anticipate a median survival of >7 years from diagnosis, a significant minority of patients continues to experience early relapse and death as a consequence of “high-risk” features.8 The definition of high risk has not been static, but includes at least 1 of the following findings at diagnosis: nonhyperdiploid immunoglobulin heavy chain translocation (specifically t[4;14], t[14;16], and t[14;20]), chromosome 17p deletion, 1p deletion, 1q gain, monosomal 13 by karyotype, complex karyotype, elevated serum lactate dehydrogenase (LDH) level, serum β2 microglobulin level ≥5.5 g/dL, plasmablastic morphology, or plasma cell leukemia. In addition, individuals with relapse after autologous hematopoietic cell transplantation (autoHCT), and those whose disease is treatment refractory, face early disease progression and death independent of other risk features.9 Although high-dose conditioning therapy followed by autoHCT remains a standard of care for all medically fit patients,10 this intervention does not ameliorate the differences in outcome predicted by risk stratification before autoHCT. In contrast, responses observed after allogeneic HCT (alloHCT) are not affected by certain high-risk features,11 illustrating differences in targets between high-dose autoHCT and the graft-versus-myeloma (GVM) effect associated with alloHCT.12 The potential for the GVM effect to overcome certain adverse prognostic features was identified in early alloHCT trials and has been further demonstrated by the ability of donor lymphocyte infusions to improve responses after alloHCT.13-16
To better exploit the GVM effect yet minimize the considerable risk of nonrelapse mortality (NRM) typically associated with high-intensity preparative regimens,17 studies over the past 2 decades have largely focused on reduced-intensity alloHCT. Some of these regimens have minimal tumor-cytotoxic activity, are essentially immunosuppressive and nonmyeloablative, and employ low-dose total body irradiation either alone or in combination with purine analogs.18 With reduced-intensity alloHCT, the expected NRM rate has decreased to ∼15% at 2 years after HCT.18,19 Because reduced-intensity, and in particular nonmyeloablative alloHCT, relies almost exclusively on the GVM effect to control the malignancy, it has been conducted mainly as part of a scheduled tandem transplant approach, whereby alloHCT is performed 2 to 6 months after cytoreductive autoHCT. In this way, the expected toxicity from high-dose autoHCT is temporally separated from complications related to the allograft procedure. Although the GVM effect provides a compelling theoretical rationale for alloHCT, prospective trials have led to conflicting outcomes.20-29 Despite the reduced-intensity conditioning, toxicity and NRM after alloHCT are considerably higher than after autoHCT, which has raised questions about the net-benefit of alloHCT for treatment of patients with MM.30,31
The administration of immunomodulatory agents to improve responses after alloHCT has had particular appeal as a means of enhancing the GVM effect. When the Hematologie Oncologie Volwassenen Nederland (HOVON) group explored lenalidomide maintenance therapy after alloHCT among 30 patients with newly diagnosed MM, however, almost half of the patients discontinued treatment with lenalidomide after only 2 cycles because of toxicity attributed to the exacerbation of acute graft-versus-host disease (GVHD).32 In contrast to immunomodulatory agents, proteasome inhibitors represent a mechanistically distinct class of antimyeloma agents that affect the function of T cells, which may have implications for GVHD after alloHCT.33,34 In murine models, for example, proteasome inhibitors selectively enhance apoptosis in allo-reactive T cells, which results in protection against acute GVHD without an impairment of the GVM effect.35 In the clinical setting, the proteasome inhibitor bortezomib has been safely administered after reduced-intensity alloHCT either immediately36 or as a late intervention (median of 20 months) after HCT.37 Recent findings have suggested that proteasome inhibitors may improve survival in high-risk patients.38,39 In the current study, we investigated whether a bortezomib-based maintenance regimen after tandem auto/alloHCT was safe and effective in preventing disease progression in patients with high-risk MM.
Methods
Study design
This prospective single-center, phase 2 trial was approved by the Fred Hutchinson Cancer Research Center Cancer Consortium Institutional Review Board, registered with the National Institutes of Health (NIH) Protocol Registration and Results System (www.clinicaltrials.gov, #NCT00793572), and all patients provided written informed consent. Patients with high-risk MM who had an HLA-genotypically identical sibling or a phenotypically matched relative, or a high likelihood of identifying an HLA-matched unrelated donor, were eligible to participate in this study. Additional eligibility criteria included age ≥18 years, and at least 1 high-risk feature independently predicting an OS of <50% at 2 years from diagnosis when treated with conventional therapy or high-dose therapy followed by autoHCT: t(4;14), t(14;16), del(17p) by fluorescence in situ hybridization; hypodiploidy; monosomy 13 or abnormal karyotype by conventional metaphase analysis (except isolated t[11;14] or constitutional cytogenetic abnormality); serum LDH concentration greater than twice the upper limit of normal; International Staging System (ISS) III disease; plasmablastic morphology (≥2%); relapsed disease after autoHCT; or relapsed or persistent disease (less than partial response) after at least 2 lines of conventional therapy. Patients were excluded if they were seropositive for HIV, pregnant, or unwilling to use contraception; if they had a concurrent nonhematological malignancy, active infection, significant cardiac, pulmonary, or hepatic dysfunction, uncontrolled hypertension, or ≥grade 2 peripheral neuropathy.
Separate eligibility criteria were applied before proceeding to bortezomib maintenance therapy, which was initiated between 60 and 120 days after alloHCT with a planned cumulative treatment period of 9 months (1.6 mg/m2 IV or 2.6 mg/m2 subcutaneously every 14 days; subcutaneous doses split between days 1 and 4 of each cycle). Respective inclusion criteria required a platelet count >50 000/μL, absolute neutrophil count >1000/μL, serum transaminase concentrations <3 times upper limit of normal, confirmed postmenopausal status, or agreement to use effective contraception. Patients were excluded if they had acute or chronic GVHD requiring treatment with >1 mg/kg per day of prednisone, grade 2 or higher peripheral neuropathy, uncontrolled infection, significant cardiac disease, progressive myeloma, or a total serum bilirubin concentration >1.5 times upper limit of normal, or if they had received other investigational therapy.
At the conclusion of the study, the clinical trial data were analyzed by D.J.G., B.E.S., M.F., and M.M. All authors had access to the primary data.
Preparative regimen and immunosuppression after transplant
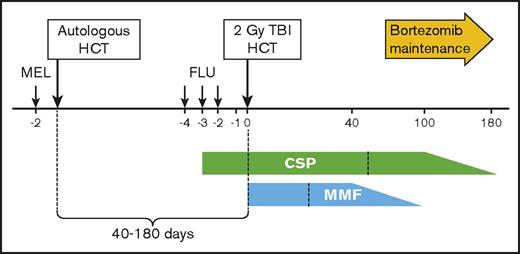
Eligible patients received high-dose melphalan (200 mg/m2 IV) 2 days before autoHCT using peripheral blood stem cells mobilized with growth factor alone or in combination with chemotherapy. At least 2.5 × 106 CD34+ cells per kilogram were required to proceed to autoHCT. Upon recovery from high-dose melphalan, between 40 and 180 days after autoHCT, patients proceeded to nonmyeloablative alloHCT. The preparative regimen for the allograft consisted of total body irradiation at 2 Gy on the day of HCT. Recipients of unrelated grafts were also given fludarabine (30 mg/m2 on days −4, −3, and −2) before infusion of stem cells. The treatment schema and the posttransplant immunosuppression schedule are summarized in Figure 1.
Treatment schema. Melphalan (MEL) was administered at a dose of 200 mg/m2 IV 2 days before the autograft using peripheral blood stem cells. Upon recovery from high-dose MEL, between 40 and 180 days after the autograft, patients proceeded to the nonmyeloablative allograft. The preparative regimen for the allograft consisted of total body irradiation (TBI) at 2 Gy on the day of transplant (HCT). Recipients of unrelated grafts were also given fludarabine (FLU) 30 mg/m2 on 3 consecutive days. Administration of cyclosporine (CSP) began at 5.0 mg/kg orally twice daily on days −3 through +100 (or +56 if related donor), and doses were then tapered to day 180. Mycophenolate mofetil (MMF) was given 15 mg/kg orally thrice daily until day +27 and twice daily thereafter only for unrelated donor recipients, with tapering of doses beginning on day +40 and treatment scheduled to end on day +96. Recipients of grafts from related donors received MMF at 15 mg/kg orally twice daily until day +27.
Treatment schema. Melphalan (MEL) was administered at a dose of 200 mg/m2 IV 2 days before the autograft using peripheral blood stem cells. Upon recovery from high-dose MEL, between 40 and 180 days after the autograft, patients proceeded to the nonmyeloablative allograft. The preparative regimen for the allograft consisted of total body irradiation (TBI) at 2 Gy on the day of transplant (HCT). Recipients of unrelated grafts were also given fludarabine (FLU) 30 mg/m2 on 3 consecutive days. Administration of cyclosporine (CSP) began at 5.0 mg/kg orally twice daily on days −3 through +100 (or +56 if related donor), and doses were then tapered to day 180. Mycophenolate mofetil (MMF) was given 15 mg/kg orally thrice daily until day +27 and twice daily thereafter only for unrelated donor recipients, with tapering of doses beginning on day +40 and treatment scheduled to end on day +96. Recipients of grafts from related donors received MMF at 15 mg/kg orally twice daily until day +27.
Patients were evaluated before beginning each bortezomib treatment cycle (every 14 days) to identify any treatment-related toxicity, which was graded based on National Cancer Institute Common Terminology Criteria for Adverse Events v3.0. Treatment was held for grade 4 neutropenia (<500/μL) or thrombocytopenia (<25 000/μL) and resumed after a 1 dose-level reduction following count recovery. For grade 2 or higher nonhematologic toxicity, bortezomib was withheld until the toxicity resolved to less than grade 2, and the treatment was resumed after a 1 dose-level reduction. Neuropathy was monitored before each treatment cycle. If grade 1 neurologic toxicity with pain or grade 2 toxicity without pain was present, the bortezomib dose was reduced by 1 dose level. Administration of bortezomib was withheld if patients had grade 3 neurologic toxicity or grade 2 toxicity with pain. In this case, treatment with bortezomib was reinstated only if the toxicity resolved. If grade 4 neurologic toxicity was present, bortezomib therapy was permanently discontinued.
Grading of acute GVHD was performed according to established criteria.40,41 Patients were monitored for acute GVHD through day 100 or until discharge from the study center. Chronic GVHD monitoring was performed by the patient’s primary hematologist/oncologist and through annual visits to the Long-Term Follow-Up Clinic at the Fred Hutchinson Cancer Research Center.
Statistical analysis
The primary endpoint of this phase 2 study was the 2-year PFS (from the time of autoHCT; intention-to-treat analysis). In newly diagnosed patients with high-risk features as defined above, autoHCT alone had resulted in PFS of <50% at 2 years in most historical series.42,43 Therefore, for purposes of the present study, an observed 2-year PFS of >50% for newly diagnosed patients was considered potentially efficacious and deemed a benchmark for success. With a sample size of 40 patients having a true PFS rate of 60% at 2 years, the probability of observing a 2-year PFS of at least 50% was 93%. For patients who had failed autoHCT or at least 2 lines of conventional therapy, PFS and OS were expected to be inferior to patients with newly diagnosed disease; a 2-year OS > 40% for the former group would have been deemed a success. Therefore, final analysis for the 2 groups was prespecified to be performed separately. The study was closed to accrual after enrolling 31 patients because the accrual rate declined to the point where it was no longer reasonable to expect completion of enrollment as originally planned. Enthusiasm for offering alloHCT to patients with MM had decreased following the publication of randomized trial data, which suggested that no net benefit was associated with alloHCT compared with a second autoHCT in patients with mainly standard-risk MM.27 Safety stopping rules were planned for early NRM, severe GVHD, and bortezomib toxicity, and the study was monitored by a Data Safety Monitoring Board. OS and PFS were estimated by the Kaplan-Meier method. Cumulative incidence frequencies of relapse, NRM, and GVHD were estimated in the competing risks setting according to methods previously described.44
Results
Patient characteristics
Thirty-one patients were enrolled between 2009 and 2014. Patients had a median age of 49 (range, 33-69) years. Twenty-four patients were newly diagnosed and included 17 who met established criteria for high-risk cytogenetics (del17p, n = 12; t[4;14], n = 2; t[14;16], n = 2; t[14;20], n = 1) and 7 patients who had high-risk disease according to ISS III disease (serum β2 microglobulin level >5.5 g/dL) at diagnosis (Table 1). Seven patients were enrolled due to relapsed disease after previous autoHCT (n = 6) or induction treatment failure (n = 1). These patients had received a median of 3 (range, 1-6) prior treatment regimens. At the time of diagnosis, ISS staging was as follows: stage III, n = 22; stage II, n = 4; and stage I, n = 5. To distinguish the newly diagnosed patients from those with recurrent or refractory disease, the former will hereafter be described as the “newly diagnosed” group and the latter as the “failed prior therapy” group.
Patient characteristics
| Patient (PIN) . | Age/sex . | Main “high-risk” feature at diagnosis . | Additional risk feature(s) at diagnosis . | ISS stage at diagnosis . | R-ISS stage at diagnosis . | Number of regimens before autoHCT* . | Days from autoHCT to alloHCT . | Donor type . | HCT-CI . | Bortezomib initiated . | Bortezomib completed . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/M | ISS III | — | III | II | 3 | NA | NA | 2 | NA | NA |
| 2 | 63/M | t(14;20) | Δ 13 by cyto | I | I | 3 | 61 | MUD | 2 | Y | Y |
| 3 | 42/M | del 17p, t(4;14) | 1q+, Δ 13 by cyto | III | III | 1 | 48 | MRD | 3 | Y | Y |
| 4 | 48/F | del 17p | — | III | III | 2 | 87 | MUD | 4 | N | NA |
| 5 | 47/M | del 17p | — | II | II | 3 | 106 | MRD | 1 | Y | N |
| 6 | 47/M | del 17p | — | I | II | 4 | 53 | MRD | 2 | Y | N |
| 7 | 48/F | del 17p, t(4;14) | — | III | III | 3 | 65 | MRD | 2 | Y | Y |
| 8 | 41/F | ISS III | — | III | II | 2 | 58 | MRD | 2 | Y | N |
| 9 | 46/F | ISS III | Δ 13 by cyto | III | III | 2 | 57 | MUD | 3 | Y | N |
| 10 | 44/M | del 17p, t(4;14) | — | III | III | 1 | 60 | MRD | 2 | Y | Y |
| 11 | 59/F | del 17p | — | I | II | 1 | 168 | MRD | 3 | Y | Y |
| 12 | 52/F | ISS III | Δ 13 by cyto | III | III | 2 | 76 | MRD | 1 | Y | N |
| 13 | 56/F | ISS III | Complex karyotype | III | II | 2 | 56 | MUD | 5 | N | NA |
| 14 | 52/M | del 17p | — | III | III | 3 | 111 | MRD | 2 | N | NA |
| 15 | 69/M | t(4;14), ISS III | — | III | III | 1 | 101 | MUD | 3 | N | NA |
| 16 | 62/M | t(14;16) | — | III | III | 2 | 61 | MRD | 0 | Y | Y |
| 17 | 46/M | t(4;14) | — | I | II | 3 | 59 | MUD | 2 | Y | N |
| 18 | 43/F | del 17p | Δ 13 by cyto | III | III | 4 | 107 | MRD | 5 | Y | Y |
| 19 | 32/F | del 17p | Δ 13 by cyto | III | III | 2 | 51 | MRD | 2 | Y | Y |
| 20 | 63/M | del 17p | — | III | III | 2 | 58 | MRD | 3 | Y | N |
| 21 | 46/F | t(14;16) | — | II | II | 4 | 58 | MRD | 1 | Y | Y |
| 22 | 49/F | ISS III | — | III | III | 5 | NA | NA | 3 | N | NA |
| 23 | 63/M | del 17p, t(4;14) | — | III | III | 1 | NA | NA | 5 | N | NA |
| 24 | 57/F | ISS III | — | III | II | 2 | NA | NA | 5 | N | NA |
| 25 | 68/M | Failed prior autoHCT | Δ 13 by cyto | I | I | 6 | NA | NA | 5 | N | NA |
| 26 | 50/F | Failed 2 lines of therapy | Δ 13 by cyto | II | II | 4 | 47 | MRD | 1 | Y | Y |
| 27 | 55/M | Failed prior autoHCT, del 17p | — | III | III | 2 | 66 | MRD | 1 | Y | N |
| 28 | 62/M | Failed prior autoHCT, t(4;14) | Δ 13 by cyto | III | III | 6 | 121 | MUD | 5 | N | NA |
| 29 | 55/M | Failed prior autoHCT, del 17p | — | III | III | 3 | 108 | MUD | 5 | Y | N |
| 30 | 57/F | Failed prior autoHCT | Δ 13 by cyto | III | II | 4 | 59 | MUD | 0 | Y | N |
| 31 | 43/M | Failed prior autoHCT, del 17p | — | II | II | 5 | 41 | MRD | 2 | Y | N |
| Patient (PIN) . | Age/sex . | Main “high-risk” feature at diagnosis . | Additional risk feature(s) at diagnosis . | ISS stage at diagnosis . | R-ISS stage at diagnosis . | Number of regimens before autoHCT* . | Days from autoHCT to alloHCT . | Donor type . | HCT-CI . | Bortezomib initiated . | Bortezomib completed . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/M | ISS III | — | III | II | 3 | NA | NA | 2 | NA | NA |
| 2 | 63/M | t(14;20) | Δ 13 by cyto | I | I | 3 | 61 | MUD | 2 | Y | Y |
| 3 | 42/M | del 17p, t(4;14) | 1q+, Δ 13 by cyto | III | III | 1 | 48 | MRD | 3 | Y | Y |
| 4 | 48/F | del 17p | — | III | III | 2 | 87 | MUD | 4 | N | NA |
| 5 | 47/M | del 17p | — | II | II | 3 | 106 | MRD | 1 | Y | N |
| 6 | 47/M | del 17p | — | I | II | 4 | 53 | MRD | 2 | Y | N |
| 7 | 48/F | del 17p, t(4;14) | — | III | III | 3 | 65 | MRD | 2 | Y | Y |
| 8 | 41/F | ISS III | — | III | II | 2 | 58 | MRD | 2 | Y | N |
| 9 | 46/F | ISS III | Δ 13 by cyto | III | III | 2 | 57 | MUD | 3 | Y | N |
| 10 | 44/M | del 17p, t(4;14) | — | III | III | 1 | 60 | MRD | 2 | Y | Y |
| 11 | 59/F | del 17p | — | I | II | 1 | 168 | MRD | 3 | Y | Y |
| 12 | 52/F | ISS III | Δ 13 by cyto | III | III | 2 | 76 | MRD | 1 | Y | N |
| 13 | 56/F | ISS III | Complex karyotype | III | II | 2 | 56 | MUD | 5 | N | NA |
| 14 | 52/M | del 17p | — | III | III | 3 | 111 | MRD | 2 | N | NA |
| 15 | 69/M | t(4;14), ISS III | — | III | III | 1 | 101 | MUD | 3 | N | NA |
| 16 | 62/M | t(14;16) | — | III | III | 2 | 61 | MRD | 0 | Y | Y |
| 17 | 46/M | t(4;14) | — | I | II | 3 | 59 | MUD | 2 | Y | N |
| 18 | 43/F | del 17p | Δ 13 by cyto | III | III | 4 | 107 | MRD | 5 | Y | Y |
| 19 | 32/F | del 17p | Δ 13 by cyto | III | III | 2 | 51 | MRD | 2 | Y | Y |
| 20 | 63/M | del 17p | — | III | III | 2 | 58 | MRD | 3 | Y | N |
| 21 | 46/F | t(14;16) | — | II | II | 4 | 58 | MRD | 1 | Y | Y |
| 22 | 49/F | ISS III | — | III | III | 5 | NA | NA | 3 | N | NA |
| 23 | 63/M | del 17p, t(4;14) | — | III | III | 1 | NA | NA | 5 | N | NA |
| 24 | 57/F | ISS III | — | III | II | 2 | NA | NA | 5 | N | NA |
| 25 | 68/M | Failed prior autoHCT | Δ 13 by cyto | I | I | 6 | NA | NA | 5 | N | NA |
| 26 | 50/F | Failed 2 lines of therapy | Δ 13 by cyto | II | II | 4 | 47 | MRD | 1 | Y | Y |
| 27 | 55/M | Failed prior autoHCT, del 17p | — | III | III | 2 | 66 | MRD | 1 | Y | N |
| 28 | 62/M | Failed prior autoHCT, t(4;14) | Δ 13 by cyto | III | III | 6 | 121 | MUD | 5 | N | NA |
| 29 | 55/M | Failed prior autoHCT, del 17p | — | III | III | 3 | 108 | MUD | 5 | Y | N |
| 30 | 57/F | Failed prior autoHCT | Δ 13 by cyto | III | II | 4 | 59 | MUD | 0 | Y | N |
| 31 | 43/M | Failed prior autoHCT, del 17p | — | II | II | 5 | 41 | MRD | 2 | Y | N |
Patients 1-24 were newly diagnosed and eligible for the study because of high-risk features as outlined in “Methods.” Patients 25-31 had failed prior autoHCT or >2 lines of therapy and were therefore eligible for the study.
—, additional risk features were not present; Δ 13 by cyto, deletion chromosome 13 by conventional metaphase cytogenetic analysis; F, female; HCT-CI, hematopoietic cell transplant comorbidity index; M, male; PIN, patient identification number.
A change of “regimen” was defined as the addition of at least 1 new, previously not used drug for systemic myeloma-directed therapy (radiation therapy was also considered a “regimen”). Thirty-one of 31 patients (100%) received bortezomib prior to enrollment, and 25/31 patients (80%) received an immunomodulatory agent (either lenalidomide or thalidomide) before autoHCT. Sixteen patients received chemomobilization therapy, and 10 patients received external-beam radiation therapy, each counted as a separate regimen.
Adherence to treatment plan
Among the 24 newly diagnosed patients, 20 proceeded to alloHCT after autoHCT. Four patients did not proceed to alloHCT for the following reasons: patient choice (n = 1), multiorgan failure (n = 1), congestive heart failure (n = 1), and progressive disease (PD) (n = 1). Among the 7 patients who had failed prior therapy, 6 patients proceeded to alloHCT after autoHCT and 1 patient died early after autoHCT as a consequence of sepsis and multiorgan failure. At the time of autoHCT, 7 patients were in complete remission (CR), 11 in partial remission, 6 had stable disease, and 7 had PD. Among the 26 patients who proceeded to alloHCT at a median of 61 days (range, 41-168) after autoHCT, 17 had HLA-matched related donors and 9 had HLA-matched unrelated donors (Table 1).
Five patients received the tandem auto/alloHCT but did not proceed to bortezomib maintenance therapy for the following reasons: PD (n = 5), neuropathy (n = 1), or unspecified (n = 1). Among the 21 patients who started bortezomib maintenance therapy, 10 completed the full 9-month course of treatment.
Toxicity of bortezomib maintenance therapy
Bortezomib was initially administered IV (n = 14). When evidence emerged that bortezomib may have reduced toxicity with preserved efficacy when administered subcutaneously, the route of administration was changed, which affected the last 4 patients treated.45 During the maintenance phase, 190 doses of bortezomib were administered to 21 patients. On 27 occasions, the dose was held, and on 13 occasions, the dose was reduced due to infection (n = 7), neuropathy (n = 7), gastrointestinal symptoms (n = 7), GVHD (n = 3), thrombocytopenia (n = 1), congestive heart failure (n = 1), deep venous thrombosis (n = 1), or hypotension (n = 2). Eleven patients did not complete the planned 9 months of bortezomib maintenance therapy. Reasons for premature withdrawal included PD (n = 7), patient choice (n = 1), severe headache (n = 1), diarrhea requiring hospitalization (n = 1), and liver function abnormalities (n = 1).
GVHD and NRM
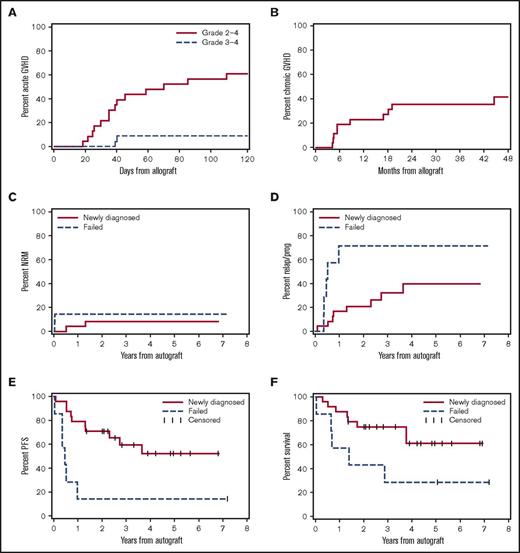
Among the 26 patients who proceeded to alloHCT, the rates of acute GVHD grade 2 to 4 and 3 to 4 were 60% and 10%, respectively (Figure 2A). With median and minimum follow-up of 51 months and 16 months, respectively, the rate of chronic GVHD according to NIH criteria was 35% at 2 years (Figure 2B). We found no indication that the risk or manifestations of GVHD were modified by bortezomib maintenance therapy. The 2-year cumulative risks of NRM for newly diagnosed patients (n = 24) and those who had failed prior therapy (n = 7) were 8% and 14%, respectively (Figure 2C).
Major outcomes after HCT for all patients enrolled. Cumulative incidence frequencies of acute GVHD grades 2 to 4 and 3 to 4 (A), and of chronic GVHD according to NIH criteria (B). Cumulative incidence frequencies of NRM (C) and myeloma relapse or progression (D). Kaplan-Meier estimates of PFS (E) and OS (F). Panels C-F are organized according to patients treated for newly diagnosed high-risk myeloma (n = 24; solid lines) or failed prior therapy (n = 7; dashed lines) as further described in “Methods.” Outcomes shown in panels C-F are based on intention to treat and counted from the time of autoHCT.
Major outcomes after HCT for all patients enrolled. Cumulative incidence frequencies of acute GVHD grades 2 to 4 and 3 to 4 (A), and of chronic GVHD according to NIH criteria (B). Cumulative incidence frequencies of NRM (C) and myeloma relapse or progression (D). Kaplan-Meier estimates of PFS (E) and OS (F). Panels C-F are organized according to patients treated for newly diagnosed high-risk myeloma (n = 24; solid lines) or failed prior therapy (n = 7; dashed lines) as further described in “Methods.” Outcomes shown in panels C-F are based on intention to treat and counted from the time of autoHCT.
Relapse risk and survival
With a median follow-up of 51 (range, 16-86) months and based on intention to treat, the 2-year and 4-year cumulative risks of relapse or progression among newly diagnosed patients with high-risk features who proceeded to tandem auto/alloHCT (n = 24) were 21% and 40%, respectively. In contrast, patients transplanted because they had failed prior therapy (n = 7) had 2-year and 4-year cumulative risks of relapse and progression of 71% and 71%, respectively (Figure 2D). The 2-year and 4-year PFS and OS estimates among newly diagnosed patients with high-risk features who proceeded to tandem auto/alloHCT were 71% and 52% and 75% and 61%, respectively. Patients transplanted because they had failed prior therapy had a 2-year and 4-year PFS and OS of only 14% and 14% and 43% and 29%, respectively (Figure 2E-F).
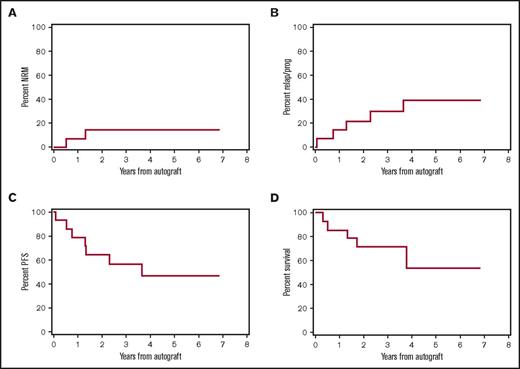
Of the 24 newly diagnosed patients enrolled, 14 were stage III according to the Revised ISS (R-ISS).46 Outcomes for the R-ISS III patients are shown in Figure 3, although this analysis was not prespecified because the R-ISS did not exist at the conception of our study. The 2-year and 4-year point estimates for this group were as follows: OS, 71% and 54%; PFS, 64% and 47%; relapse and progression, 21% and 39%; and NRM, 14% and 14%.
Major outcomes after HCT for patients with newly diagnosed stage 3 disease according to the R-ISS, n = 14. Cumulative incidence frequencies of NRM (A) and myeloma relapse or progression (B). Kaplan-Meier estimates of PFS (C) and OS (D). All outcomes shown are based on intention to treat and counted from the time of autoHCT.
Major outcomes after HCT for patients with newly diagnosed stage 3 disease according to the R-ISS, n = 14. Cumulative incidence frequencies of NRM (A) and myeloma relapse or progression (B). Kaplan-Meier estimates of PFS (C) and OS (D). All outcomes shown are based on intention to treat and counted from the time of autoHCT.
In additional post hoc analysis, outcomes were analyzed according to remission status at the time of alloHCT (n = 20): 8 patients were in CR, 8 in partial remission, 3 had stable disease, and 1 had PD. For patients transplanted in CR, 2-year and 4-year OS and PFS estimates were 67% and 50% and 44% and 33%, respectively. For patients transplanted not in CR, 2-year and 4-year OS and PFS estimates were 70% and 55% and 59% and 50%, respectively.
No patient in this study was given donor lymphocyte infusion for relapse. If patients did not have active GVHD at the time of relapse, immunosuppression was usually withdrawn sequentially under close observation for signs of GVHD.
Discussion
High-dose chemotherapy followed by autoHCT remains a standard of care in the management of patients with MM, although the role of reduced-intensity alloHCT remains a subject of debate.47 Clinical trials evaluating sequential auto/alloHCT conducted over the past 2 decades have yielded discordant findings that likely reflect differences in patient eligibility criteria, conditioning regimens, and the length of follow-up. Among the 6 prospective randomized trials performed to compare either single or tandem autoHCT with tandem auto/alloHCT,20-29 2 studies showed that tandem auto/alloHCT was associated with an improvement in both PFS and OS,22,24 whereas the remaining trials did not find statistically significant differences in PFS and OS between the 2 groups. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 trial comparing tandem autoHCT with tandem auto/alloHCT after “biological randomization” has been the largest such study performed to date.27 Among standard-risk patients enrolled in this trial (n = 625), the 3-year PFS and OS were 46% vs 43% (P = .67), and 80% vs 77% (P = .19) for the tandem autoHCT vs tandem auto/alloHCT groups, respectively. Similarly, among the high-risk patients (n = 85) defined by elevated serum β2-microglobulin levels and chromosome 13 deletion by conventional cytogenetic analysis, there was no statistically significant difference in 3-year PFS and OS between the tandem autoHCT and the tandem auto/alloHCT group: 33% vs 40% (P = .74) and 67% vs 59% (P = .46), respectively. As expected, the NRM risk at 3 years was higher in the group that included alloHCT (11% vs 4%). A trend toward improved PFS at 6 months (P = .012) was observed in patients who developed chronic GVHD, which was interpreted to suggest the presence of an immunological GVM effect after alloHCT. Overall, however, the nonmyeloablative alloHCT was unable to provide benefit to all patients compared with a second melphalan-based autoHCT. A possibly important limitation of this study was that enrollment was allowed even after investigators knew whether an HLA-identical sibling donor had been identified, which might have introduced an important referral bias as shown by the higher percentage of patients with high-risk disease “biologically assigned” to the auto/alloHCT group.
In the current report, we describe the outcome of patients with high-risk MM after tandem auto/alloHCT followed by bortezomib maintenance therapy. With a median follow-up of >4 years, our findings suggest a significant benefit associated with maintenance therapy after alloHCT. Among the 31 high-risk patients enrolled on our study, 17 patients met enrollment criteria based on high-risk cytogenetic features at diagnosis. In addition, when retroactively applied, 14 of the newly diagnosed patients (58%) met R-ISS stage III criteria at diagnosis. Based on these considerations, patients on our study would be expected to have a median OS of 2 to 3 years. However, the OS estimate for newly diagnosed high-risk patients was 75% at 2 years and 61% at 4 years; thus, the median OS has not yet been reached.
The HOVON group recently reported the possibility that bortezomib might partially abrogate certain high-risk cytogenetic features. They that found that a regimen incorporating bortezomib into induction and maintenance therapy after tandem autoHCT resulted in a statistically significant improvement in PFS and OS among patients with del(17p) disease.38 Our findings are consistent with these results, despite the shorter period of bortezomib maintenance therapy.
The findings presented here are encouraging when viewed in the context of current treatment options for patients with high-risk MM. Although significant advances have been made in MM management, patients with high-risk features continue to face early relapse and death. Autologous stem cell transplant remains a standard of care for all patients, including those with high-risk features; however, the benefit to this population is limited. A recent report from the University of Texas MD Anderson Cancer Center reported a PFS of only 10.3 months and OS of 32.4 months following autoHCT in patients with high-risk MM.48 Similarly, in a recent review of 11 clinical trials assessing the efficacy of alloHCT in MM, the median PFS ranged from 5.2 to 36.8 months and OS from 13 to 63 months.49 No study, however, had prospectively evaluated the role of maintenance bortezomib therapy following reduced-intensity alloHCT.
Although mechanisms by which bortezomib maintenance therapy may lead to sustained remissions after reduced-intensity alloHCT are unknown, it is tempting to postulate that the proteasome inhibitor affords ongoing disease control early after the allograft, thus acting as a temporizing agent before a potent GVM effect is fully established. Clinical studies at our institution have demonstrated that nonmyeloablative alloHCT from HLA-identical sibling donors for the treatment of MM was most effective when performed in patients with low tumor burden.18 The ongoing phase 3 BMT CTN 1302 trial incorporates bortezomib into the preparative regimen, followed by randomization to either placebo or ongoing maintenance therapy with the oral proteasome inhibitor, ixazomib (NCT02440464). Although this study will address some questions about the role of proteasome inhibitor-based maintenance therapy after reduced-intensity alloHCT for patients with newly diagnosed high-risk MM, the encouraging results from our study should motivate a confirmatory prospective randomized trial.
Our finding that individuals with relapsed or treatment-refractory MM have profoundly shorter rates of OS and PFS after auto/alloHCT followed by bortezomib maintenance therapy than those observed in patients with newly diagnosed high-risk disease is consistent with prior reports suggesting distinct biological characteristics of the disease in the 2 populations.50 Mainly because of the procedural risks, some groups have suggested tandem auto/alloHCT should be reserved as the last resort of salvage therapies, an approach that has likely reinforced the notion that alloHCT is ineffective as consolidation or salvage treatment of patients with MM.
In the present study, 84% of patients receiving autoHCT proceeded to alloHCT, and 68% ultimately started bortezomib maintenance therapy. Although 43% of study enrollees completed the planned 9 months of bortezomib treatment, disease progression was the reason for stopping bortezomib early in the majority of treated patients. Although the protocol was eventually amended to further reduce the toxicity risk by administering bortezomib subcutaneously instead of IV, no patient had to stop treatment early because of peripheral neuropathy. It is reasonable to assume that subcutaneous administration of bortezomib will further improve tolerability and therefore result in better adherence to maintenance therapy after alloHCT.
Based on current maintenance strategies following autoHCT, the treatment duration with bortezomib in our trial was relatively short, and the IV route of administration for the initial cohort of 14 patients might have contributed to early treatment discontinuation. In addition, dose reductions and early discontinuation of bortezomib may have reduced the benefit of this maintenance strategy. In future studies, subcutaneous bortezomib administration and a longer treatment period may reduce toxicity yet enhance efficacy associated with this maintenance strategy.
Our study has several limitations. First, because of the relatively small sample size, findings will require confirmation in larger and ideally multi-institutional prospective trials. Even though enthusiasm for alloHCT in patients with MM decreased because of discouraging results from randomized trials conducted primarily in patients with standard-risk disease (contributing to early closure of our study), more recent findings including those presented here should motivate further evaluation of the approach in patients with high-risk disease.
Second, single-center data could be more susceptible to bias. For example, exclusion of patients with active GVHD (requiring prednisone at doses ≥1 mg/kg per day) from starting bortezomib maintenance therapy could have resulted in more favorable outcomes. Reasons for exclusion of such patients were data from preclinical studies51 showing that delayed administration of bortezomib was associated with increased GVHD-dependent gastrointestinal toxicity. Incidence and severity of acute and chronic GVHD in our study population, however, were similar to those reported in previous publications of HLA-matched nonmyeloablative transplantation.18,24 It is therefore unlikely that excluding patients with active GVHD at the time eligibility for bortezomib maintenance therapy was determined introduced a clinically important selection bias.
Third, only 67% of patients with newly diagnosed disease started bortezomib maintenance therapy after the allograft and 56% of those completed the intended 9 months of treatment. The minimum duration of maintenance therapy needed to confer a benefit in this setting is unknown. It is conceivable, though, that even a truncated course of bortezomib could have prevented early progression after transplant and served as a “bridge” to establishment of a GVM effect.
In summary, the role of alloHCT in the management of MM remains a subject of ongoing debate, because randomized trials have generated conflicting results as to whether relapse prevention through a GVM effect clearly outweighs the significant risks of the procedure. Interpretation has been further complicated by an absence of studies integrating recently approved novel therapies into allograft regimens. Unfortunately, one-quarter of myeloma patients still expect a median survival of only 2 years by virtue of high-risk features, including adverse cytogenetics, elevated LDH concentrations, or ISS stage III disease. The findings of our phase 2 bortezomib maintenance trial help to shed some light on the role of alloHCT in the management of high-risk disease. Our results suggest that alloHCT followed by bortezomib maintenance may help to overcome the poor prognosis associated with high-risk features, but this benefit is limited to patients receiving an allograft early in the course of the disease.
Acknowledgments
The authors thank Helen Crawford, Andrea McCool, and Bonnie Larson for assistance with manuscript preparation. The authors are also grateful to the physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff caring for their patients and to the patients who participated in this study.
The authors are grateful for research funding from the National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL108307) (M.M.) and National Cancer Institute (grants CA078902, CA151682, CA205248, and CA015704) (R.S.). Additional support was provided by Millennium Pharmaceuticals, Inc/Takeda Oncology (M.M.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or its subsidiary Institutes and Centers.
Authorship
Contribution: D.J.G. analyzed and interpreted the data and drafted the manuscript; D.G.M. assisted with the study design and edited the manuscript; B.E.S. performed the statistical analysis; B.M.S. assisted with study design and edited the manuscript; L.A.H. assisted with the study design and data interpretation and edited the manuscript; P.S.B. assisted with data interpretation and edited the manuscript; P.J.M. assisted with data interpretation and edited the manuscript; M.F. oversaw the cytogenetic risk categorization and edited the manuscript; G.E.G. assisted with data interpretation and edited the manuscript; M.E.B. was responsible for data collection and entry and edited the manuscript; R.S. assisted with data interpretation and edited the manuscript; and M.M. designed and conducted the study, analyzed and interpreted data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Mielcarek, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, Mail Stop D1-100, P.O. Box 19024, Seattle, WA 98109; e-mail: mmielcar@fhcrc.org.