Key Points
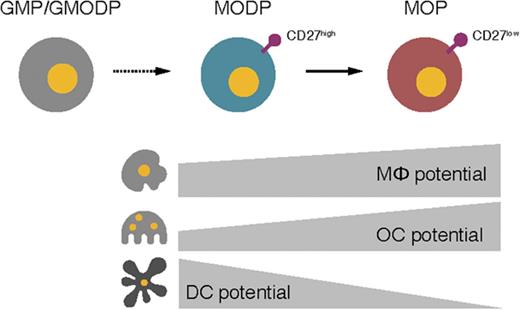
Under homeostatic conditions, MΦs, OCs, and DCs develop from a tripotent progenitor, the MODP.
In mouse bone marrow, we define a novel, bipotent MΦ/OC progenitor, the MOP, that lies downstream of the MODP.
Abstract
Monocytes/macrophages (MΦs), osteoclasts (OCs), and dendritic cells (DCs) are closely related cell types of high clinical significance, but the exact steps in their lineage commitment are unclear. In studies on MΦ and DC development, OC development is generally not addressed. Furthermore, findings on DC development are confusing, because monocytes can also differentiate into DC-like cells. To resolve these issues, we have examined the development of monocytes/MΦs, OCs, and DCs from common progenitors, using the homeostatic driver cytokines macrophage colony-stimulating factor, RANK ligand (L), and Flt3L. In mouse bone marrow, B220−CD11blow/−c-Kit+c-Fms+ cells could be dissected into a CD27+Flt3+ population that proved oligopotent for MΦ/OC/DC development (MODP) and a CD27low/−Flt3− population that proved bipotent for MΦ/OC development (MOP). Developmental potential and relationship of MODP and downstream MOP populations are demonstrated by differentiation cultures, functional analysis of MΦ/OC/DC offspring, and genome-wide messenger RNA expression analysis. A common DC progenitor (CDP) has been described as committed to plasmacytoid and conventional DC development. However, the human CDP proved identical to the MODP population, whereas the mouse CDP largely overlapped with the MODP population and was accordingly oligopotent for MΦ, OC, and DC development. The CX3CR1+ MΦ/DC progenitor (MDP) population described in the mouse generated MΦs and OCs but not DCs. Thus, monocytes/MΦs, OCs, and DCs share a common progenitor that gives rise to a bipotent MΦ/OC progenitor, but a dedicated DC progenitor is currently undefined. The definition of these progenitor populations may serve diagnostics and interventions in diseases with pathogenic activity of MΦs, OCs, or DCs.
Introduction
Monocytes/macrophages (MΦs), osteoclasts (OCs), and dendritic cells (DCs) are closely related myeloid cells that originate from the hematopoietic stem cell (HSC), with the exception of tissue-resident MΦs.1 MΦs and DCs are important phagocytes, antigen-presenting cells, and immune regulatory cells.2,3 OCs resorb bone in normal physiology and disease, often in close communication with immune cells.4 Understanding the molecular cues that guide MΦ, OC, and DC development is important for clinical diagnosis and therapy in infectious diseases, autoimmunity, and cancer. The exact steps in MΦ-, OC-, and DC-lineage commitment are unclear. Moreover, in the many studies on MΦ and DC development, OC development is generally not addressed.5-7
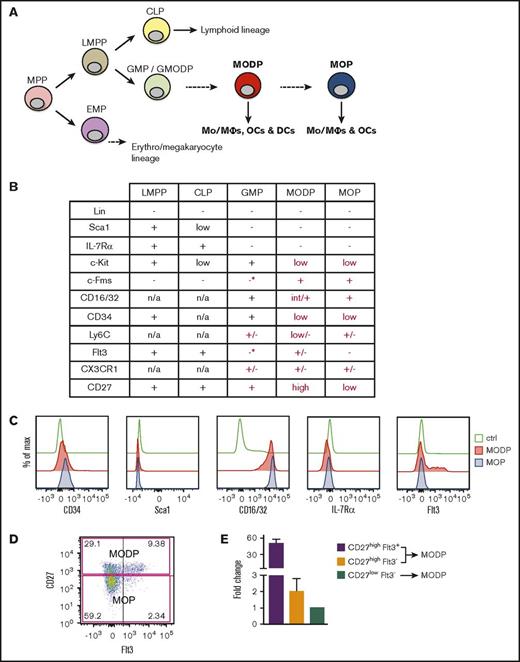
At the root of the hematopoietic tree, the self-renewing HSC yields the multipotent progenitor (MPP), which in turn gives rise to more lineage-restricted, oligopotent precursors. The classical model dictates that the MPP bifurcates into a common myeloid progenitor8,9 and a common lymphoid progenitor (CLP).9 However, recent data indicate that the MPP bifurcates into the EMP, a precursor with megakaryocyte/erythroid potential, and the LMPP, a precursor with combined myeloid and lymphoid potential10-12 (Figure 1A). The EMP gives rise, via more dedicated precursors, to eosinophilic and basophilic granulocytes (GRs), erythocytes, and megakaryocytes.13,14
Cell surface markers of MODP and MOP. (A) Hypothetical positioning of the MODP and MOP in the hematopoietic tree. (B) Overview of cell surface marker expression on LMPP, CLP, GMP, MODP, and MOP according to literature data (black) and according to our own flow cytometric analysis (red) (*marker present on <10% of the population). (C) Expression of indicated cell surface markers on MODP and MOP populations according to flow cytometry. (D) Phenotypic definition of CD27high MODP (upper quadrants) and CD27low MOP (lower quadrants) populations within B220−CD11blow/−c-Kit+c-Fms+ BM cells and their cell surface Flt3 expression according to flow cytometry. (E) Relative Flt3 mRNA expression, as determined by quantitative polymerase chain reaction in the indicated subsets of B220−CD11blow/−c-Kit+c-Fms+ BM cells. Data are representative of 2 experiments with n = 3. Error bars indicate standard deviations. Ctrl, control (unstained); max, maximum; n/a, not applicable.
Cell surface markers of MODP and MOP. (A) Hypothetical positioning of the MODP and MOP in the hematopoietic tree. (B) Overview of cell surface marker expression on LMPP, CLP, GMP, MODP, and MOP according to literature data (black) and according to our own flow cytometric analysis (red) (*marker present on <10% of the population). (C) Expression of indicated cell surface markers on MODP and MOP populations according to flow cytometry. (D) Phenotypic definition of CD27high MODP (upper quadrants) and CD27low MOP (lower quadrants) populations within B220−CD11blow/−c-Kit+c-Fms+ BM cells and their cell surface Flt3 expression according to flow cytometry. (E) Relative Flt3 mRNA expression, as determined by quantitative polymerase chain reaction in the indicated subsets of B220−CD11blow/−c-Kit+c-Fms+ BM cells. Data are representative of 2 experiments with n = 3. Error bars indicate standard deviations. Ctrl, control (unstained); max, maximum; n/a, not applicable.
In humans, it has been shown that neutrophilic GRs stem from the GR/MΦ progenitor (GMP) that lies downstream of the LMPP.14 We have recently shown that the human GMP has combined GR, MΦ, OC, and DC potential and is thus a GMODP. We have also identified downstream of the human GMODP a tripotent MΦ/OC/DC progenitor (MODP) that is devoid of GR potential15 (Figure 1A). In the mouse, combined MΦ and OC potential has been identified in a B220−CD11blow/−c-Kit+c-Fms+ bone marrow (BM) population.16-18 Originally, DC potential was claimed for this population on the basis of culture with granulocyte-macrophage colony-stimulating factor (GM-CSF).17 However, these conditions do not test homeostatic DC development from progenitors, as takes place in response to Flt3L, because GM-CSF promotes DC development from monocytes.19,20 We have found that B220−CD11blow/−c-Kit+c-Fms+ cells in mouse BM can be dissected into a CD27high subpopulation that can form MΦs, OCs, and DCs and a CD27low subpopulation that can form MΦ and OCs.21 In these cultures, DC development was driven with Flt3L. We hypothesize therefore, the existence of murine MODP and MOP populations that lie downstream of the GM(OD)P, as is suggested by our findings in humans (Figure 1A).
Using other markers, researchers have defined in mouse BM the MDP as a bipotent progenitor of monocytes/MΦs and DCs.22 The MDP was proposed to give rise to a common DC progenitor (CDP) that exclusively forms plasmacytoid DCs (pDCs) and conventional DCs (cDCs)23,24 and to a common monocyte progenitor (cMoP) that exclusively forms monocytes and MΦs.25,26 However, recent work suggests that under homeostatic conditions, the MDP can form only MΦs and not DCs, whereas the CDP can form DCs and MΦs.27 OC development was not addressed in these studies.
To clarify the origin of the OC and its developmental relationship to MΦs and DCs, we have investigated side-by-side MΦ, OC, and DC development from the candidate murine MODP and MOP populations21 and from the CDP and MDP populations. We have used the key homeostatic driver cytokines macrophage colony-stimulating factor (M-CSF), RANKL, and Flt3L and tested oligopotency at the clonal level. MΦ, OC, and DC progeny of the MODP and MOP were defined phenotypically and functionally by testing phagocytosis, bone resorption, antigen presentation, and T-cell priming ability, respectively. These data, together with gene expression profiling, confirmed without bias the existence of the MODP and MOP and their developmental relationship. They also revealed that the mouse23,24 and human28 CDP populations are similar, respectively identical to the MODP, and that the mouse MDP population22 is partially overlapping with the MOP and indeed devoid of DC potential.
Materials and methods
Mice
C57BL/6 wild-type (WT) mice, OT-I, and OT-II T-cell receptor (TCR) transgenic mice (Jackson) were used at 8-12 weeks of age. Experiments were approved by the institutional Experimental Animal Committee.
BM cell isolation
Mouse BM cells from tibiae, femurs, and ilia were treated with red blood cell lysis buffer. Pediatric human BM was obtained from healthy donors, as approved by the medical ethical committee of Leiden University Medical School (protocol P08.001) and with parental informed consent. BM cells were isolated by density gradient centrifugation on Ficoll and cryopreserved until use.
Flow cytometry
To isolate progenitor cells, we incubated BM cells with specific antibodies (supplemental Tables 1 and 2) in medium with serum and 0.01% deoxyribonuclease I (Sigma) for 45 minutes on ice. Dead cells were excluded by staining with 1 μg/mL propidium iodide or 0.15 μg/mL 6-diamidino-2-phenylindole (DAPI; both from Sigma). Cell sorting was performed on fluorescence-activated cell sorter (FACS) Aria or FACSAria Fusion (BD Biosciences). In vitro cultured cells were stained with diagnostic antibodies (supplemental Tables 1 and 2) in phosphate-buffered saline (PBS) with serum for 45 minutes on ice and analyzed on an LSRFortessa (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
In vitro differentiation of mouse progenitors into OCs, MΦs, or DCs
Sorted progenitor cells were seeded in 96-well plates (BD Falcon) (flat bottom for OCs, round bottom for MΦs and DCs) at 2000-3000 cells/well. For clonality assays, 1 (MΦ and DC cultures) or 5 precursor cells (OC cultures) were seeded per well. To induce OC differentiation, we cultured progenitor cells in α minimum essential medium (α-MEM) with 10% fetal calf serum (FCS) supplemented with 25 ng/mL recombinant mouse (rm) RANKL (R&D) and 25 ng/mL rm M-CSF (Peprotech). Bone chips were washed in PBS and incubated in α-MEM with 10% FCS for 30 minutes at 37°C prior to coculture. To induce MΦ differentiation, we cultured progenitor cells in RPMI-1640 with 10% FCS, supplemented with 20 ng/mL rm M-CSF. To induce DC differentiation, we cultured progenitor cells in RPMI-1640 with 10% FCS, supplemented with 200 ng/mL rm Flt3L (R&D) with or without 1-2 ng/mL rm M-CSF.
In vitro differentiation of human progenitors into OCs or MΦs
Human CDPs28 were sorted and cultured at 1000 cells per well in 96-well plates (BD Falcon) under OC or MΦ differentiation conditions, as has been previously described.15 For OC differentiation, cells were cultured in α-MEM with 10% FCS, 25 ng/mL recombinant human (rh) M-CSF, and 40 ng/mL rh RANKL. For MΦ differentiation, cells were cultured on a monolayer of OP9 cells in IMDM with 10% FCS, 25 ng/mL rh M-CSF, and 1000 U/mL rh interleukin-6 (IL-6). Growth factors were from R&D Systems.
Evaluation of OC formation
OC differentiation was assessed by tartrate-resistant acid phosphatase (TRAP) staining (Sigma-Aldrich) according to manufacturer’s instructions after 5 days of culture. Cells were washed with PBS, fixed with paraformaldehyde (Sigma) for 15 minutes, and washed with water. Staining was performed for 5-10 minutes, cells were washed, and nuclei were stained with 5% DAPI solution. DAPI+ TRAP+ OCs were analyzed with an AxioVision inverted microscope (Carl Zeiss Microscopy) with a CCD camera (Hamamatsu Photonics), combining fluorescence and phase contrast imaging. Images were processed with ZEN2012 software (Carl Zeiss Microscopy) and quantified in ImageJ (National Institutes of Health).
Functional analysis of progenitor-derived OCs
Bone resorption was determined as has been described.29 Briefly, bone chips were sonicated for 30 minutes in 10% NH4OH on ice to remove OCs, washed, and dried. Next, they were incubated for 10 minutes in a water-saturated (10%) alumn [KAl(SO4)2 × 12H2O] solution, washed with water, and dried. A droplet of Coomassie Brilliant Blue (CBB) solution was applied, and bone pieces were dried between pieces of filter paper. CBB-stained bone resorption pits were examined with semi-high-throughput microscopy, taking multiple images of each bone chip with a CCD camera (Axiocam HRc) fitted to a Zeiss Axiovert S 100 inverted microscope. Number and size of the resorption pits were determined with ImageJ software.
Functional analysis of progenitor-derived MΦs
Phagocytic capacity of MΦs was evaluated by coculture for 3 h with 6- to 10-µm fluorescent microbeads (Life Technologies or SPHERO) at 1:2 ratio (MΦ:beads). Phagocytosing mouse MΦs were identified with a CD11b+F4/80+FITC+ phenotype and visualized with fluorescence and phase-contrast microscopy. Phagocytosing human MΦs were visualized with phase-contrast microscopy (Nikon Eclipse TS100).
Functional analysis of progenitor-derived DCs
T-cell priming capacity of DCs was assessed in cocultures with Vβ5+CD8+ OT-I and Vβ5+CD4+ OT-II T cells. DCs were loaded with 0.25 mg/mL ovalbumin (OVA) protein (Sigma-Aldrich) and stimulated with 1 μg/mL lipopolysaccharide (LPS) (Sigma) for 5 h at 37°C. T cells were labeled with 0.5 μM carboxyfluorescein succinimidyl ester (CFSE; BD Biosciences) in medium for 10 minutes at room temperature. Next, a 1:1 mix of CFSE-labeled OT-I and OT-II T cells was added to DCs at a 1:10 ratio (DC:T), and cells were cocultured for 3 days. T-cell proliferative response was determined with flow cytometry according to CFSE dilution. Data were analyzed with FlowJo software.
mRNA deep sequencing
Per sample, 20 000-100 000 progenitors pooled from 2 mice were dissolved in Trizol reagent (Ambion). Messenger RNA (mRNA) were isolated, and deep sequencing was performed on triplicate samples using a HiSeq200 (Illumina). Sequence reads were aligned with TopHat software to the Ensembl gene set Mus musculus GRCm38_77. Reads were counted using htseq-count software. Hierarchical clustering was done in R and uses 1-correlation as the distance function. Reads for protein-coding genes were normalized and analyzed for differential expression using R package limma. Principal component analysis (PCA) was performed, and a heat map was generated using Qlucore Omics Explorer software, considering significant differential gene expression with P < .001. Gene ontology (GO) analyses were performed using Ingenuity Pathway Analysis (IPA), Cytoscape,30 and Gene Set Enrichment Analysis (GSEA).31 The statistical criteria used for Biological Networks Gene Ontology (BiNGO) analysis were P value cutoff of .001, false discovery rate q value cutoff of 0, Jaccard coefficient cutoff of 0.25, and combined constant of 0.5. Preranked GSEA was also performed on the differentially expressed genes using 1000 permutations and a weighted statistic to assess statistical significance. The curated gene sets used for analysis are available at the Molecular Signature Database of Broad Institute (GSE97380).
Statistical analysis
Statistical evaluation was performed in GraphPad Prism 6 (GraphPad Software, Inc.). Paired Student t test or 1-way analysis of variance was used to calculate significant differences.
Results
Cell surface markers of MODP and MOP
In our study on OC development in a mouse model of chronic immune activation,21 we followed Arai et al,16 who described OC potential in CD11blow/−c-Kit+c-Fms+ mouse BM cells. We examined this population for CD27 expression, because we found a CD27-dependent high bone mass phenotype in the CD70 transgenic mice that we studied.21 In this way, we obtained preliminary evidence for the existence of CD27high MODP and CD27low MOP populations among B220−CD11blow/−c-Kit+c-Fms+ BM cells21 (supplemental Figure 1A). Both MODP and MOP populations lacked Sca1 and IL-7Rα (Figure 1C; supplemental Figure 1B), distinguishing them from the LMPP/CLP9,10 (Figure 1B). The MOP lacked Flt3, both at the protein (Figure 1D) and mRNA levels (Figure 1E). The MODP contained Flt3+ and Flt3− cells (Figure 1D) but overall had higher Flt3 mRNA levels than did the MOP (Figure 1E). To relate the MODP and MOP to the GMP, we used the original markers Sca1, c-Kit, CD34, and CD16/328,9 (Figure 1B; supplemental Figure 1C), in combination with CD27, c-Fms, and Flt3. This revealed that Flt3 and c-Fms (M-CSF receptor/CD115) were absent from >90% of cells in the GMP population (Figure 1B; supplemental Figure 1D). To assess OC development from the GMP, it was purified and cultured by flow cytometry with the OC differentiation factor RANKL32,33 and with M-CSF. We found that the GMP population could give rise to OCs (supplemental Figure 1E). This result, together with our findings in humans,15 supports the concept that the GMP population contains a progenitor with combined GR, MΦ, OC, and DC potential that lies upstream of the MODP. We further hypothesized that the MOP lies downstream of the MODP (Figure 1A).
Validation of developmental potential of MODP and MOP: formation of functional OCs
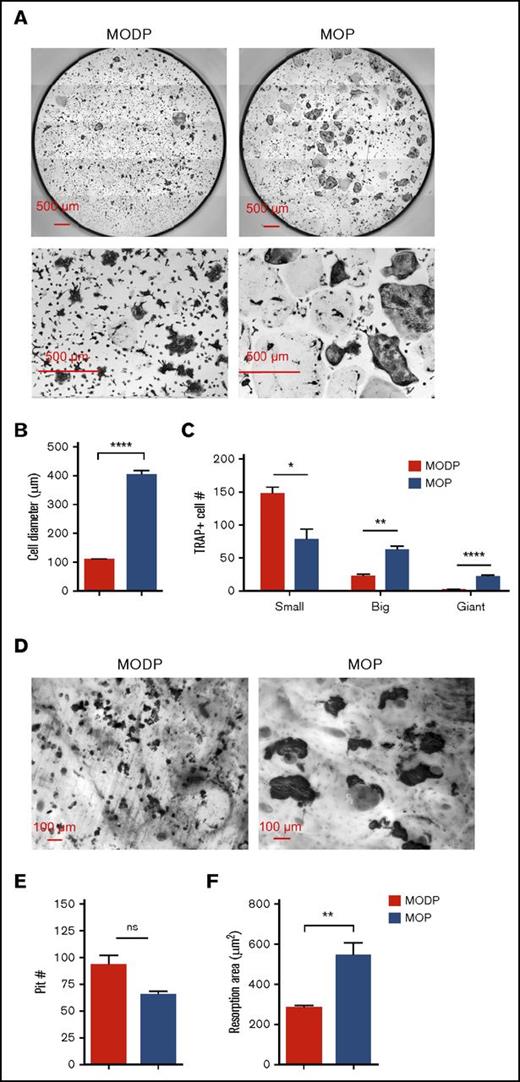
Next, we set out to validate the developmental potential of the MODP and MOP, defining for the first time MΦ and DC offspring by both phenotype and function. To assess OC development, we MODP and MOP purified and cultured by flow cytometry with RANKL and M-CSF. After 6 days, OC formation was diagnosed microscopically and quantified after TRAP staining of the cells. In both MODP- and MOP-derived OC cultures, TRAP+ OCs were detected (Figure 2A). The MODP-derived OCs were smaller in size than were the MOP-derived OCs (Figure 2B). During OC differentiation, single cells fuse to form multinucleated, mature OCs.32,33 At the time point of analysis, MODP-derived OCs had a less mature phenotype, with the majority of TRAP+ cells being mononucleated, whereas MOP-derived OCs included more multinucleated cells (Figure 2A,C). On day 4, the MOP-derived culture contained 3 populations: 1) CD11b− F4/80−; 2) CD11b+ F4/80+; and 3) CD11b+ F4/80− (supplemental Figure 2A). Cells in population 1 were small according to forward scatter, c-Kit+/−Ly6C+/−RANK−, qualifying them as progenitors. Cells in population 2 were medium-sized, c-Kit−Ly6C−RANK+F4/80+ cells, qualifying them as MΦs. Cells in population 2 were large, c-Kit−Ly6C−F4/80−RANKhigh, qualifying them as OCs (supplemental Figure 2B-C). In these cultures, OC fusion reproducibly occurred on the night of day 4. These results argue that the OCs differentiated directly from the MOP rather than from (CD11b+Ly6C+, F4/80+, or both) monocytes/MΦs, as reportedly occurs under inflammatory conditions.34 Functionality of OCs was assessed by a bone-resorption assay.29 Both progenitor populations gave rise to bone-resorbing OCs (Figure 2D-E). However, MOP-derived OCs created larger resorption areas than did MODP-derived OCs (Figure 2D,F), in agreement with their larger size. Thus, MOPs more efficiently generate mature, functional OCs than do MODPs.
Phenotype and functionality of MODP- and MOP-derived OCs. Sorted MODPs and MOPs were plated at 3000 cells/well with or without bovine bone chips and cultured in the presence of RANKL and M-CSF for 6 days. (A) CCD microscopic images (bottom row: digital zoom in) of TRAP- and DAPI-stained OC cultures. (B-C) Quantitative analysis of MODP- and MOP-derived OCs. Fused cells with multiple nuclei were counted as 1 cell. (B) OC diameter presented as the mean value of 80 (MODP derived) or 100 (MOP derived) TRAP+ OCs. (C) Total TRAP+ OC yield per well in absolute number (#), with OCs subdivided according to nuclei count. Small: 3-10 nuclei; big: 10-20 nuclei; giant: >20 nuclei. Data represent pooled data from 2 experiments (n = 4). Error bars indicate standard error of the mean. (D) CCD microscopic images of CBB-stained OCs on bovine bone chips. (E-F) Quantification by ImageJ capture and manual counting of CBB-stained pits on bone chips from MODP- derived OC cultures (n = 6) and MOP-derived OC cultures (n = 5). In each graph, all pits were counted and measured. Resorption area was calculated as total pit size divided by the number of pits.29 Error bars indicate standard error of the mean. *P < .05; **P < .01; ****P < .0001. ns, not significant.
Phenotype and functionality of MODP- and MOP-derived OCs. Sorted MODPs and MOPs were plated at 3000 cells/well with or without bovine bone chips and cultured in the presence of RANKL and M-CSF for 6 days. (A) CCD microscopic images (bottom row: digital zoom in) of TRAP- and DAPI-stained OC cultures. (B-C) Quantitative analysis of MODP- and MOP-derived OCs. Fused cells with multiple nuclei were counted as 1 cell. (B) OC diameter presented as the mean value of 80 (MODP derived) or 100 (MOP derived) TRAP+ OCs. (C) Total TRAP+ OC yield per well in absolute number (#), with OCs subdivided according to nuclei count. Small: 3-10 nuclei; big: 10-20 nuclei; giant: >20 nuclei. Data represent pooled data from 2 experiments (n = 4). Error bars indicate standard error of the mean. (D) CCD microscopic images of CBB-stained OCs on bovine bone chips. (E-F) Quantification by ImageJ capture and manual counting of CBB-stained pits on bone chips from MODP- derived OC cultures (n = 6) and MOP-derived OC cultures (n = 5). In each graph, all pits were counted and measured. Resorption area was calculated as total pit size divided by the number of pits.29 Error bars indicate standard error of the mean. *P < .05; **P < .01; ****P < .0001. ns, not significant.
Validation of developmental potential of MODP and MOP: formation of functional MΦs
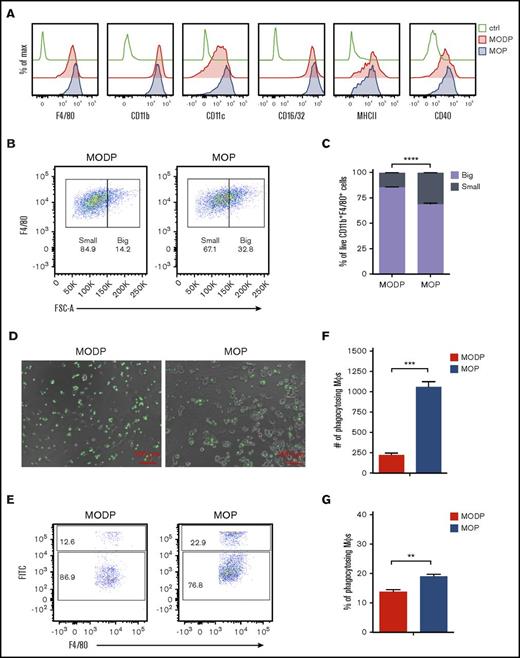
To assess MΦ development, we purified and cultured MODP and MOP by flow cytometry with M-CSF. After 6 days, cells were analyzed for surface phenotype and function. Live cells from MODP- and MOP-derived cultures uniformly expressed the MΦ markers F4/80, CD11b, CD11c, CD16/32, major histocompatibility complex II (MHCII), and CD40 at comparable levels (Figure 3A). Notably, MOP-derived cultures contained significantly more big MΦs than did MODP-derived cultures, as diagnosed by forward scatter of CD11b+F4/80+ cells (Figure 3B-C). MΦs formed in these cultures displayed phagocytosis of fluorescent beads, as diagnosed by microscopy (Figure 3D) and flow cytometry (Figure 3E-G). MOP-derived cultures contained significantly more phagocytosing MΦs than did MODP-derived cultures (Figure 3F-G). Thus, MOPs more efficiently generate mature, functional MΦs than do MODPs.
Phenotype and functionality of MODP- and MOP-derived MΦs. Sorted MODPs and MOPs were plated at 3000 cells/well and cultured in the presence of M-CSF for 6 days. (A) Flow cytometric detection of indicated markers on MODP- and MOP-derived cells. (B) Flow cytometry plots showing the size of MODP- and MOP-derived MΦs, defined by F4/80+ phenotype (gated on DAPI−CD11b+ cells) according to forward scatter (FSC). (C) Frequency of small and big MΦs (CD11b+F4/80+ cells) in MODP- and MOP-derived cultures. (D-G) To evaluate phagocytosis capacity, we cocultured cells with FITC+ microbeads. (D) Microscopic illustration of cells containing green fluorescent beads in MODP- and MOP-derived MΦ cultures. (E) Flow cytometry plots depicting the percentage of FITC+F4/80+CD11b+ cells in MODP- and MOP-derived MΦ cultures. (F-G) Quantification by flow cytometry of FITC+F4/80+CD11b+ cells as indicated by number (#) and frequency (%). Data are representative of 3 experiments with n = 3. Error bars indicate standard error of the mean. **P < .01; ***P < .005; ****P < .0001.
Phenotype and functionality of MODP- and MOP-derived MΦs. Sorted MODPs and MOPs were plated at 3000 cells/well and cultured in the presence of M-CSF for 6 days. (A) Flow cytometric detection of indicated markers on MODP- and MOP-derived cells. (B) Flow cytometry plots showing the size of MODP- and MOP-derived MΦs, defined by F4/80+ phenotype (gated on DAPI−CD11b+ cells) according to forward scatter (FSC). (C) Frequency of small and big MΦs (CD11b+F4/80+ cells) in MODP- and MOP-derived cultures. (D-G) To evaluate phagocytosis capacity, we cocultured cells with FITC+ microbeads. (D) Microscopic illustration of cells containing green fluorescent beads in MODP- and MOP-derived MΦ cultures. (E) Flow cytometry plots depicting the percentage of FITC+F4/80+CD11b+ cells in MODP- and MOP-derived MΦ cultures. (F-G) Quantification by flow cytometry of FITC+F4/80+CD11b+ cells as indicated by number (#) and frequency (%). Data are representative of 3 experiments with n = 3. Error bars indicate standard error of the mean. **P < .01; ***P < .005; ****P < .0001.
Validation of developmental potential of MODP and MOP: formation of functional DCs
To assess DC development, we cultured the progenitor populations with Flt3L.19 Under these conditions, the MOP did not generate any offspring (Figure 4A). The MODP generated both pDCs (MHCII+B220+CD11c+CD11b+) and cDCs (MHCII+B220−CD11c+CD11b+) (Figure 4B). The cDC population contained about 20% XCR1+ cells, the subset that is known for its antigen cross-presentation ability.35 DC output from the murine MODP was similar upon culture with Flt3L alone or in combination with low-dose M-CSF (supplemental Figure 3A-B), whereas DC output from the human MODP was increased by the combination.15 Strikingly, the murine MODP preferentially generated F4/80+CD11b+ MΦs when cultured with Flt3L and high-dose M-CSF (supplemental Figure 3C). So the dose of M-CSF was important for the developmental fate of the MODP offspring.
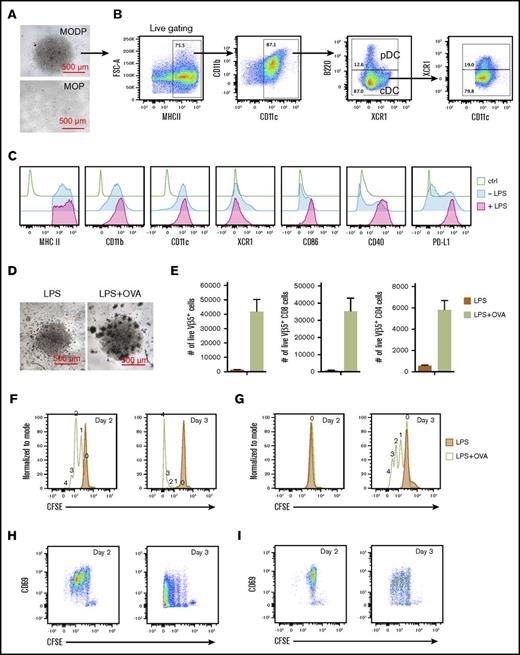
Phenotype and functionality of MODP-derived DCs. (A-C) Sorted MODPs and MOPs were plated at 3000 cells/well and cultured in the presence of Flt3L and 1 ng/mL M-CSF for 8 days. (A) Microscopic illustration of MODP- and MOP-derived DC cultures at day 8. (B) FACS plots depicting the percentages of cDCs and pDCs within MHCII+CD11c+CD11b+ cells in MODP-derived DC cultures. (C) Detection of indicated markers on MODP-derived DCs after incubation with or without LPS for 16 h. (D-H) LPS-stimulated DCs were loaded with OVA or not and cocultured with CFSE-labeled, Vβ5+ CD8+ OT-I and CD4+ OT-II T cells. (D) Microscopic illustration of cocultures at day 3. (E) Quantification of T-cell expansion, expressed as absolute number (#) of live Vβ5+, CD8+, or CD4+ T cells per well (n = 2). (F-G) Histograms depicting the proliferation of CD8+ (F) and CD4+ (G) T-cell coculture, as detected by CFSE dilution. (H-I) Plots depicting CD69 expression on dividing CD8+ (H) and CD4+ (I) T cells. Data are representative of 3 experiments.
Phenotype and functionality of MODP-derived DCs. (A-C) Sorted MODPs and MOPs were plated at 3000 cells/well and cultured in the presence of Flt3L and 1 ng/mL M-CSF for 8 days. (A) Microscopic illustration of MODP- and MOP-derived DC cultures at day 8. (B) FACS plots depicting the percentages of cDCs and pDCs within MHCII+CD11c+CD11b+ cells in MODP-derived DC cultures. (C) Detection of indicated markers on MODP-derived DCs after incubation with or without LPS for 16 h. (D-H) LPS-stimulated DCs were loaded with OVA or not and cocultured with CFSE-labeled, Vβ5+ CD8+ OT-I and CD4+ OT-II T cells. (D) Microscopic illustration of cocultures at day 3. (E) Quantification of T-cell expansion, expressed as absolute number (#) of live Vβ5+, CD8+, or CD4+ T cells per well (n = 2). (F-G) Histograms depicting the proliferation of CD8+ (F) and CD4+ (G) T-cell coculture, as detected by CFSE dilution. (H-I) Plots depicting CD69 expression on dividing CD8+ (H) and CD4+ (I) T cells. Data are representative of 3 experiments.
Next, we determined whether MODP-derived DCs were functional in terms of antigen cross-presentation and T-cell priming. Upon incubation with LPS, MODP-derived DCs displayed increased levels of MHCII, CD40, CD86, and PD-L1, in agreement with their activation36 (Figure 4C). DCs were stimulated with LPS and loaded with OVA protein and next cultured with Vβ5+ TCR transgenic OVA-specific CD8+ OT-I and CD4+ OT-II T cells. The T cells had been labeled with CFSE to follow their proliferative response. At day 3 after coculture, OVA-dependent T-cell proliferation was microscopically evident (Figure 4D) and quantified by flow cytometry for both CD8+ (OT-I) and CD4+ (OT-II) T cells (Figure 4E). CD8+ T cells started to divide by day 2 and had undergone 4 divisions by day 3 (Figure 4F), whereas CD4+ T cells remained undivided by day 2 and started to divide on day 3 (Figure 4G). Expression of early activation marker CD69 was seen on CD8+ and CD4+ T cells prior to entering the cell cycle and was gradually lost when cells had undergone cell division (Figure 4H-I).
In conclusion, the MOP is devoid of Flt3L-dependent DC potential, and the MODP generates DCs that can cross-present antigens and induce MHC class I– and MHC class II–restricted antigen-specific T-cell responses.
Validation of developmental potential of MODP and MOP: transcriptome analysis
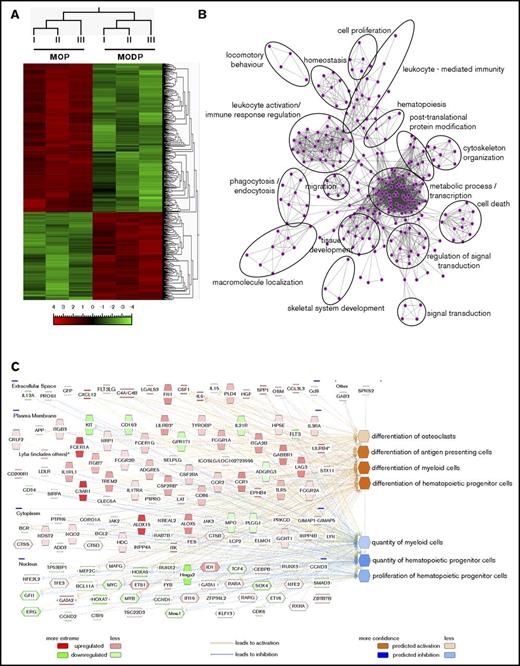
To further define the MODP and MOP and their developmental relationship, we performed mRNA deep sequencing (RNAseq). PCA showed that the MOP samples clustered together, as did the MODP samples (Figure 5A; supplemental Figure 4A), confirming the crucial role of the CD27 marker in discriminating MODP and MOP among B220−CD11blow/−c-Kit+c-Fms+ BM cells. Using a statistical criterion of P < .001 and a log2-fold differential expression of >4 or ≤4, 724 protein-coding genes were differentially expressed between the MOP and MODP (GSE97380; Figure 5A; supplemental Figure 4B). BiNGO analysis categorized the differentially expressed genes in many biological processes, including “leukocyte activation,” “leukocyte-mediated immunity,” “phagocytosis,” and “skeletal system development” (Figure 5B). IPA identified as upregulated in the MOP, among others, proteins mapping to the functional categories of “differentiation of OCs,” “differentiation of antigen presenting cells,” and “differentiation of myeloid cells” (Figure 5C; supplemental Table 3). These categories and the molecules contained within them support our findings that the MOP is more committed to OC and MΦ formation than is the MODP. Upregulated in the MODP were proteins mapping to the functional categories “proliferation of hematopoietic progenitor cells” and “quantity of hematopoietic progenitor cells,” supporting the idea that the MODP lies upstream of the MOP. Moreover, consistent with IPA, GSEA showed that the MODP is enriched in gene signatures related to “hematopoiesis stem cell and progenitor cells” and “early progenitor cells” in comparison with the MOP (supplemental Figure 4C). Taken together, the RNAseq data support the definition of MODP and MOP as determined in in vitro differentiation cultures and associated functional assays of cell offspring.
Transcriptome analysis of MODP and MOP. MODPs and MOPs were sorted from BM of 6 mice. Cell samples of 2 mice each were pooled to generate 3 biological replicates for RNAseq. Sequence reads were analyzed, as is indicated in the Materials and Methods section. (A) Hierarchical clustering and heat map of the 724 differentially expressed genes (P < .001; log2-fold change >4 or ≤4) from the 3 different samples (I, II, III). Upregulated and downregulated genes are ordered separately according to the degree of fold change expression (from “high” to “low”). The scale bar denotes log2 value. (B) GO network, generated by BiNGO. Nodes represent gene sets, and edges represent mutual overlap. Thus, highly redundant gene sets are grouped together as clusters, highlighted by the black circles. (C) GO analysis with IPA of the differentially expressed genes. Molecules with higher mRNA expression in the MOP are depicted in shades of red, whereas molecules with lower mRNA expression are in shades of green (log2 ratios). The molecules depicted clusters in the listed functional categories of genes, and their subcellular localization is also shown (extracellular space, plasma membrane, cytoplasm, and nucleus).
Transcriptome analysis of MODP and MOP. MODPs and MOPs were sorted from BM of 6 mice. Cell samples of 2 mice each were pooled to generate 3 biological replicates for RNAseq. Sequence reads were analyzed, as is indicated in the Materials and Methods section. (A) Hierarchical clustering and heat map of the 724 differentially expressed genes (P < .001; log2-fold change >4 or ≤4) from the 3 different samples (I, II, III). Upregulated and downregulated genes are ordered separately according to the degree of fold change expression (from “high” to “low”). The scale bar denotes log2 value. (B) GO network, generated by BiNGO. Nodes represent gene sets, and edges represent mutual overlap. Thus, highly redundant gene sets are grouped together as clusters, highlighted by the black circles. (C) GO analysis with IPA of the differentially expressed genes. Molecules with higher mRNA expression in the MOP are depicted in shades of red, whereas molecules with lower mRNA expression are in shades of green (log2 ratios). The molecules depicted clusters in the listed functional categories of genes, and their subcellular localization is also shown (extracellular space, plasma membrane, cytoplasm, and nucleus).
Comparison of CDP and MDP with MODP and MOP: surface phenotype
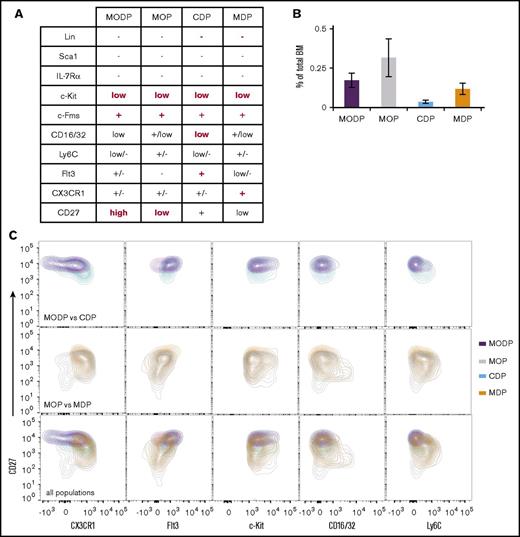
Fogg et al described a murine MDP that is bipotent for Mo/MΦ and DC formation,22 but others described a murine CDP that is restricted to cDC and pDC formation23,24 (Figure 6A). It was not investigated in these studies whether MDP or CDP can generate OCs. We here set out to relate the phenotype and developmental potential of the MDP and CDP to the MODP and MOP as defined by us. All 4 progenitor populations were very rare, representing less than 0.3% of total BM, with the CDP being least abundant and the MOP being most abundant (Figure 6B). The 4 progenitor populations were analyzed for all cell surface markers used by other investigators6 and by ourselves in 1 staining mixture (Figure 6A; supplemental Figure 5A-D). A detailed side-by-side examination of the surface markers revealed that the MODP and CDP overlapped significantly regarding CX3CR1, Flt3, c-Kit, CD27, CD16/32, Ly6C, and CD27 expression and so did the MOP and MDP (Figure 6C). Overlay of all populations highlighted the MOP’s and MDP’s lower Flt3 and higher CD16/32 and Ly6C levels in comparison with the MODP and CDP. Thus, the cell surface markers used to define the CDP and MODP select similar, but not identical, cell populations and so do the cell surface markers used to define the MDP and MOP.
Surface phenotype of CDP and MDP compared with MODP and MOP. (A) Overview of cell surface marker expression on MODP, MOP, CDP, and MDP, according to our own flow cytometric analysis and literature data.6 Indicated in red are the markers used for definition and flow cytometric purification. (B) MODP, MOP, CDP, and MDP populations as a percentage of total live BM cells (mean ± SD; n = 3). (C) Contour plots showing CX3CR1, Flt3, c-Kit, CD16/32, or Ly6C and CD27 expression on MODP versus CDP (top), MOP versus MDP (middle), or all 4 progenitor populations (bottom) in 1 plot. Data are representative of 3 experiments.
Surface phenotype of CDP and MDP compared with MODP and MOP. (A) Overview of cell surface marker expression on MODP, MOP, CDP, and MDP, according to our own flow cytometric analysis and literature data.6 Indicated in red are the markers used for definition and flow cytometric purification. (B) MODP, MOP, CDP, and MDP populations as a percentage of total live BM cells (mean ± SD; n = 3). (C) Contour plots showing CX3CR1, Flt3, c-Kit, CD16/32, or Ly6C and CD27 expression on MODP versus CDP (top), MOP versus MDP (middle), or all 4 progenitor populations (bottom) in 1 plot. Data are representative of 3 experiments.
Comparison of CDP and MDP with MODP and MOP: differentiation potential
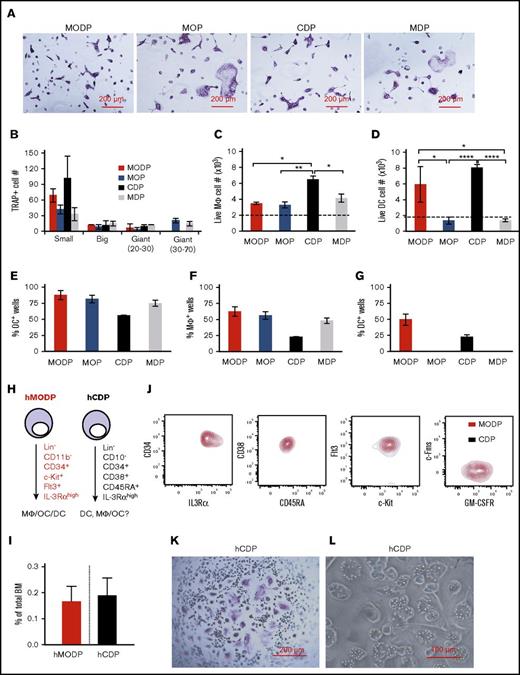
To further clarify the relationship between these cell populations, we sorted them (supplemental Figure 5) and cultured them side by side under conditions for OC, MΦ, or DC development. Interestingly, the CDP and MDP yielded OCs, just as the MODP and MOP did, a property that had not previously been described22-24 (Figure 7A). As with the MOP, the MDP was more committed to OC formation than was the MODP and CDP: its offspring was enriched in big and giant multinucleated OCs, whereas MODP and CDP mostly yielded small OCs with fewer nuclei (Figure 7A-B). All populations gave rise to MΦs: most of the live MHCII+ cells (supplemental Figure 6A) derived from the MΦ differentiation cultures expressed the MΦ markers CD11b and F4/80 (Figure 7C). However, only the MODP and CDP gave rise to DCs, including cDCs and pDCs (supplemental Figures 6B and 7D).
Differentiation potential of murine CDP and MDP compared with MODP and MOP and redefinition of the human CDP. (A-D) Bulk cultures. Sorted MODP, MOP, CDP, and MDP populations were cultured at 2000 cells per well under OC differentiation conditions for 5-6 days (A-B) and under MΦ (C) or DC (D) differentiation conditions for 7-8 days. (A) Light microscopic images showing TRAP+ multinuclear OCs. (B) Total TRAP+ OC yield per well, with OCs subdivided in small (3-10 nuclei), big (10-20 nuclei), giant (20-30 nuclei), and giant (30-70 nuclei). (C) MΦ yield in numbers of live MHCII+ progeny expressing CD11b and F4/80. (D) DC yield in numbers of total live cDC (MHCII+B220−CD11c+) and pDC (MHCII+B220+CD11c+) output. Data are representative of 3 experiments. Error bars indicate standard deviations. (E-G) Clonality assays. Sorted MODP, MOP, CDP, and MDP were cultured at 5 cells per well under OC differentiation conditions (E) or at 1 cell per well under MΦ (F) or DC (G) differentiation conditions. After 5 to 12 days, cultures were analyzed. Histograms depict percentages of wells with a colony out of 48 wells seeded. Data are derived from 2 experiments. Error bars indicate standard deviations. (H-I) Redefinition of the human (h)CDP. (H) Representation of the differentiation potential and cell surface markers of the hMODP15 and the hCDP.28 (I) Flow cytometric detection of indicated markers on hMODP (red) versus hCDP (black) within the same sample. Data are representative of 3 different donors. (J) Frequency of hMODPs and hCDPs among live BM cells (mean ± SD; n = 3). (K-L) Sorted hCDPs were cultured at 1000 cells per well under OC differentiation conditions for 8-10 days and under MΦ differentiation conditions for 14 days. (K) Light microscopic images showing TRAP+ multinuclear OCs. (L) Light microscopic images showing bead phagocytosing MΦs. Data are representative of 3 experiments. *P < .05; **P < .01; ****P < .0001.
Differentiation potential of murine CDP and MDP compared with MODP and MOP and redefinition of the human CDP. (A-D) Bulk cultures. Sorted MODP, MOP, CDP, and MDP populations were cultured at 2000 cells per well under OC differentiation conditions for 5-6 days (A-B) and under MΦ (C) or DC (D) differentiation conditions for 7-8 days. (A) Light microscopic images showing TRAP+ multinuclear OCs. (B) Total TRAP+ OC yield per well, with OCs subdivided in small (3-10 nuclei), big (10-20 nuclei), giant (20-30 nuclei), and giant (30-70 nuclei). (C) MΦ yield in numbers of live MHCII+ progeny expressing CD11b and F4/80. (D) DC yield in numbers of total live cDC (MHCII+B220−CD11c+) and pDC (MHCII+B220+CD11c+) output. Data are representative of 3 experiments. Error bars indicate standard deviations. (E-G) Clonality assays. Sorted MODP, MOP, CDP, and MDP were cultured at 5 cells per well under OC differentiation conditions (E) or at 1 cell per well under MΦ (F) or DC (G) differentiation conditions. After 5 to 12 days, cultures were analyzed. Histograms depict percentages of wells with a colony out of 48 wells seeded. Data are derived from 2 experiments. Error bars indicate standard deviations. (H-I) Redefinition of the human (h)CDP. (H) Representation of the differentiation potential and cell surface markers of the hMODP15 and the hCDP.28 (I) Flow cytometric detection of indicated markers on hMODP (red) versus hCDP (black) within the same sample. Data are representative of 3 different donors. (J) Frequency of hMODPs and hCDPs among live BM cells (mean ± SD; n = 3). (K-L) Sorted hCDPs were cultured at 1000 cells per well under OC differentiation conditions for 8-10 days and under MΦ differentiation conditions for 14 days. (K) Light microscopic images showing TRAP+ multinuclear OCs. (L) Light microscopic images showing bead phagocytosing MΦs. Data are representative of 3 experiments. *P < .05; **P < .01; ****P < .0001.
Next, we performed clonal assays to validate the oligopotency of the MODP, MOP, CDP, and MDP for OC, MΦ, and DC formation. Concordant with the bulk culture results, all 4 progenitor populations generated OCs and MΦs (Figure 7E-F; supplemental Figure 6C), whereas only the MODP and CDP yielded DCs (Figure 7G). We conclude that the cell surface markers of MODP and CDP enrich BM cells for oligopotent MΦ/OC/DC progenitors, whereas the cell surface markers of the MOP and MDP enrich BM cells for bipotent MΦ/OC progenitors.
The human CDP is identical to the MODP
We recently described the human (h)MODP15 and noted that its cell surface phenotype was similar to that of the hCDP that was described shortly before that28 (Figure 7H). Both progenitor populations are very rare, representing about 0.2% of total BM (Figure 7I). To determine the relationship between these populations, we stained human BM cells simultaneously for all the characteristic markers used in both studies and gated them accordingly (supplemental Figure 6D-E). The resulting overlay plots revealed that the hCDP and the hMODP are identical (Figure 7J). As was expected from this analysis, the hCDP yielded OCs (Figure 7K) and MΦs (Figure 7L) under the relevant differentiation conditions, described by Xiao et al.15 Thus, the hCDP is in fact the hMODP.
Discussion
Suda et al originally described MΦ and OC potential of CD11blow/negc-Kit+c-Fms+ mouse BM cells and showed that this population is devoid of B-cell and GR potential.16 We and others specified that OC potential resides in the B220− fraction of the CD11blow/negc-Kit+c-Fms+ BM cell population,18,21 and we demonstrated the DC potential of this fraction using Flt3L21 instead of GM-CSF as used by Miyamoto et al.17 In the current study, we demonstrate that CD27highFlt3+/low and CD27lowFlt3− subsets of this population harbor a tripotent MODP and a bipotent MOP, respectively. We here validate the developmental relationship between the MODP and MOP by transcriptomics using mRNA deep sequencing. The gene set “differentiation of OCs” was enriched in the MOP (Figure 6C; supplemental Table 3). This set includes TYROBP (= DAP12), INPP4B, and OSTM1, which are important regulators of OC genesis in mouse and human hematopoiesis.37-39 As other examples, we found that the transcription factors SPI1 (= PU.1), GATA2, and LIMD1 were upregulated, in agreement with their proven role in mouse OC development.40-42 Gfi-1, which contributes to DC development,43 was downregulated. Altogether, the gene set offers a host of novel markers and potential mediators which are valid for both mouse and human MΦ, OC, and DC development. For example, it allowed us to use the IL-3Rα chain as a diagnostic marker to identify the human MODP.15
The mouse MDP was defined as a Lin−CD16/32+CD34+c-Kit+c-Fms+CX3CR1+ BM population.22 Both in this study and in another,44 these cells were found to form monocytes/MΦs and DCs. However, DC development was driven in these studies with GM-CSF, which promotes DC development from monocytes.19,20 In agreement with others,20,27 we found that the MDP is devoid of DC potential when stimulated with Flt3L. OC potential was not previously tested for the MDP population. We here show that the markers used to define the MDP population select a very similar population as the markers used to define the MOP and prove that both populations yield MΦs and OCs, but not DCs, under homeostatic cytokine conditions.
Furthermore, we have found that the markers used to define the murine CDP selected for a population that is very similar, to the murine MODP, as defined in this study. We also found that the markers used to define the human CDP selected for a population that is identical to the human MODP as defined previously.15 OC potential of the CDP population has been missed in earlier studies,23-28 because it has not been tested. MΦ potential has in fact been observed for the mouse CDP, both in vitro and in vivo.27 Our data provide a coherent view of the developmental decisions made in MΦ, OC, and DC development from a common MODP. The MODP is the most common downstream DC progenitor currently known and yields a MOP with restricted MΦ/OC potential. The existence of the MOP indicates that MΦs and OCs are more closely related to each other than to DCs. The definition of these progenitor populations is expected to aid clinical diagnostics and targeted interventions in diseases with pathogenic activity of MΦs, OCs, or DCs.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank A. Lankester (Leiden University Medical Center) for providing bone marrow samples, T. de Vries (Academic Centre for Dentistry, Amsterdam, The Netherlands), D. van de Zwan, F. van Diepen, M. v. Baalen, A. Pfauth, and E. Mul for expert assistance and T. Ahrends for data analysis and making the visual abstract.
This work was supported by the Landsteiner Foundation for Blood Transfusion Research (grant 1355), awarded to Y.X. and J.B. and by a fellowship from the Foundation Alfonso Martín Escudero (J.P.).
Authorship
Contribution: Y.X. designed and supervised research, performed experiments, analyzed data, and wrote the paper; J.P. and J.G. performed experiments, analyzed data, and contributed to writing the paper; L.W. and L.v.H. performed experiments; I.d.R. analyzed data; and J.B. supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yanling Xiao or Jannie Borst, Division of Tumor Biology and Immunology, The Netherlands Cancer Institute–Antoni van Leeuwenhoek, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: y.xiao@nki.nl or j.borst@nki.nl.
References
Author notes
J.P. and J.G. contributed equally to this study.