Key Points
Two types of fetal liver stromal cell populations are identified: mesenchymal cells and prehepatic cells.
The prehepatic stromal cell population exhibits a unique capacity to support the production of megakaryocytes from human and mouse HSCs.
Abstract
The fetal liver is the site of a major expansion of the hematopoietic stem cell (HSC) pool and is also a privileged organ to study megakaryocyte progenitor differentiation. We identified in the mouse fetal liver at day 13.5 a discrete stromal cell population harboring a CD45−TER119−CD31−CD51+VCAM-1+PDGFRα− (V+P−) phenotype that lacked colony-forming unit fibroblast activity and harbored an hepatocyte progenitor signature. This previously undescribed V+P− population efficiently supported megakaryocyte production from mouse bone marrow HSC and human peripheral blood HSC-myeloid progenitors cultured in the presence of limited cytokine concentrations. Megakaryocytes obtained in V+P− cocultures were polyploid, positive for CD41/CD42c, and efficiently produced proplatelets. Megakaryocyte production appeared to be mediated by an expansion of the progenitor compartment through HSC–stromal cell contact. In conclusion, the fetal liver contains a unique cellular microenvironment that could represent a platform for the discovery of regulators of megakaryopoiesis.
Introduction
The proliferation and differentiation of hematopoietic stem cells (HSCs) are regulated by a microenvironment combining cellular and extracellular components, such as extracellular matrices, growth factors, and other biomolecules, which collectively exert their influence on HSC maintenance and differentiation. A particular microenvironment that regulates the self-renewal and the maintenance of HSCs is also referred as the stem cell niche, a concept first proposed by R. Schofield.1 The cellular elements constituting the niche were first identified among the fibroblastic cells that form colonies in the appropriate conditions (colony-forming unit fibroblast [CFU-F]).2 CFU-F initiating cells and their progeny are also referred to as mesenchymal stem cells or stromal precursor cells.
While significant progress in understanding the mechanisms involved in the maintenance of a self-renewing HSC has been achieved, very few studies have focused on the identification of the microenvironment regulating the commitment toward a given lineage, particularly the megakaryocytic lineage. This question is of great interest when considering our limited ability to reproduce in culture the megakaryopoiesis and thrombopoiesis efficiencies of the native environment. Reports considering the role of bone marrow stromal cells are conflicting with respect to their capacity to support megakaryopoiesis. Some studies suggest that contact with stromal cell precursors negatively controls megakaryocytic differentiation of the human hematopoietic cell line K5623,4 or human CD34 progenitors,5,6 whereas other studies suggest that stromal cells support or enhance megakaryopoiesis.7-9 This apparent contradiction may reside in differences in experimental design and in the complexity of the processes involved in the generation of megakaryocytes (MKs) from HSCs. Indeed, MKs are generated from HSCs through multiple steps of committed MK progenitors, including a bipotent megakaryocytic erythroid progenitor (MEP), leading to the production of a unipotent MK precursor, which will then mature into large polyploid MKs that will extend proplatelets in the circulation. How, where and which stromal precursor cells intervene in this complex but well-orchestrated process is still subject to questions.
Stromal cells produce a number of hematopoietic cytokines and other soluble factors regulating megakaryopoiesis.10 The major cytokine regulating megakaryopoiesis, thrombopoietin (TPO), stimulates the production of MKs, but not the final maturation: proplatelet production.11 This highlights the fact that the factors or cellular elements controlling the different steps of megakaryopoiesis are bound to be different from the commitment of HSCs toward the MK lineage and during the maturation of MK progenitors and precursors. Similarly, it is likely that different stages of MK expansion and maturation are regulated by distinct cellular microenvironments, and different hematopoietic tissues can be considered to explore this question.
Megakaryopoiesis mainly occurs in the bone marrow in adults but is also observed during embryogenesis. In the embryo, megakaryopoiesis proceeds following colonization of the fetal liver by HSCs originating in the aorta-gonad-mesonephros and possibly also by MK progenitors already present in the yolk sac.12 Large mature MKs are observed in the fetal liver from around 13 days of development in the mouse13 (Manuela Tavian, INSERM UMR S949, oral communication, 16 November 2015). The fetal liver therefore represents an attractive tissue to study the microenvironment supporting the different stages of megakaryopoiesis.
In this study, we characterized and isolated different stromal cell populations from mouse fetal liver with different functional properties. We found that a particular population with a hepatocyte progenitor signature supported efficient expansion of MK-committed progenitors able to produce fully mature MKs.
Materials and methods
Isolation of fetal liver stromal cells
Pregnant females from timed breeding protocol were killed using CO2 inhalation followed by cervical dislocation. Fetuses were harvested, and the fetal liver was dissected under a binocular microscope. Fetal liver cell suspensions were obtained after digestion with 3 mg/mL collagenase I (Worthington Biochemical, Freehold, NJ) for 10 min at 37°C, dilution with PBS-2% newborn serum, and filtration through a 70 μm cell strainer (BD Biosciences, San Jose, CA). Fetal liver hematopoietic cells were depleted after labeling with biotinylated TER119 and anti-mouse CD45 antibodies using sheep anti-rat antibodies Dynabeads (Life Technologies AS, Oslo, Norway). The nonmagnetic fraction is referred to as the Hem− fraction and represents between 10% and 20% of the number of input cells. Hem− cells were labeled with allophycocyanin-Cy7–conjugated streptavidin, allophycocyanin-conjugated anti-CD140a, fluorescein isothiocyanate–conjugated anti-CD31, phycoerythrin-conjugated anti-CD51, and phycoerythrin-Cy7–conjugated anti-CD106 antibodies. Acquisition of data for analysis was performed on a BD LSRII or a Fortessa-X20 flow cytometer (BD Biosciences), and cell sorting was performed on a BD Aria I or a BD Aria II flow cytometer (BD Biosciences). Flow cytometry data analyses were conducted using BD FACSDiva software (BD Biosciences). Stromal cells were sorted as Hem−CD31−CD51+VCAM-1+PDGFRα− (V+P−), Hem−CD31−CD51+VCAM-1−PDGFRα+ (V−P+), and Hem−CD31−CD51+VCAM-1+PDGFRα+ (V+P+) as described in Figure 1A. A detailed list of antibodies used is provided in supplemental Table 1.
Isolation and characterization of different stromal cell populations from mouse fetal livers. Fetal liver cell suspensions obtained following enzymatic digestion of dissected E13.5 fetal liver were subjected to fluorescence-activated cell sorting analysis and CFU-F assays. (A) Fluorescence-activated cell sorting analysis before (i) and after (ii) magnetic depletion of CD45/TER119 hematopoietic cells, and the gating strategy used for the sorting of the different cell populations tested for CFU-F content (iii-iv). Dot plots and histograms are representative figures with the mean ± standard error of the mean (SEM) from 27 independent experiments. (B) Sorted cell populations were plated in α-minimal essential medium 20% fetal calf serum, and CFU-F frequencies were determined in limiting dilution assays using L-Calc software. Values represent mean ± SEM from 3 independent experiments. (C) Hem−CD51+VCAM-1+PDGFRα− (V+P−) cells that failed to produce CFU-F were sorted and plated in EGM-2. (i) Phase contrast images of the adherent layers constituted of large spread cells. Scale bar represents 40 μm. (ii) Expression of E-cadherin (E-Cadh), c-met, epithelial cell adhesion molecule (EpCAM), and Dlk-1 for the V+P− cell population. Shown is a representative histogram from 3 independent experiments. All histogram markers are based upon fluorescence-minus-one controls. (iii) Expression of Hnf4a, Alb, Afp, Krt18, c-met, G6pc, and GAPDH transcripts in sorted cells from the fetal liver and total adult liver. Figures on the left side of the panel indicate amplicon size. FL, fetal liver; MW, molecular weight; Neg, negative.
Isolation and characterization of different stromal cell populations from mouse fetal livers. Fetal liver cell suspensions obtained following enzymatic digestion of dissected E13.5 fetal liver were subjected to fluorescence-activated cell sorting analysis and CFU-F assays. (A) Fluorescence-activated cell sorting analysis before (i) and after (ii) magnetic depletion of CD45/TER119 hematopoietic cells, and the gating strategy used for the sorting of the different cell populations tested for CFU-F content (iii-iv). Dot plots and histograms are representative figures with the mean ± standard error of the mean (SEM) from 27 independent experiments. (B) Sorted cell populations were plated in α-minimal essential medium 20% fetal calf serum, and CFU-F frequencies were determined in limiting dilution assays using L-Calc software. Values represent mean ± SEM from 3 independent experiments. (C) Hem−CD51+VCAM-1+PDGFRα− (V+P−) cells that failed to produce CFU-F were sorted and plated in EGM-2. (i) Phase contrast images of the adherent layers constituted of large spread cells. Scale bar represents 40 μm. (ii) Expression of E-cadherin (E-Cadh), c-met, epithelial cell adhesion molecule (EpCAM), and Dlk-1 for the V+P− cell population. Shown is a representative histogram from 3 independent experiments. All histogram markers are based upon fluorescence-minus-one controls. (iii) Expression of Hnf4a, Alb, Afp, Krt18, c-met, G6pc, and GAPDH transcripts in sorted cells from the fetal liver and total adult liver. Figures on the left side of the panel indicate amplicon size. FL, fetal liver; MW, molecular weight; Neg, negative.
Establishment of cocultures with stromal cells and mouse HSC or MK progenitors
The sorted stromal cell populations were cultured in 24-well plates in α-minimal essential medium supplemented with 20% fetal calf serum (Hyclone, South Logan, UT) or with EGM-2 BulletKit (catalog no. CC-3162; Lonza, Walkersville, MD) as indicated. Confluent layers were gently washed and hematopoietic cells seeded onto the stromal layers in Stemspan (STEMCELL Technologies, Vancouver, Canada) supplemented with a low cytokine cocktail (7.5 ng/mL recombinant mouse [rm] stem cell factor, 5 ng/mL rmFlt3L, 10 ng/mL rmTPO, and 1 ng/mL recombinant human interleukin-6 (R&D Systems, Minneapolis, MN) previously determined to be suboptimal for stimulating the proliferation of single Lin−Sca-1+c-kit+ cells plated in the absence of stromal cells (data not shown). Cultures were maintained in 5% CO2, 5% O2, humidified air at 37°C for 3 to 13 days as indicated. For noncontact coculture experiments, HSCs were seeded into a Transwell insert with a 0.3-μm-pore polyester membrane.
Analyses of MKs produced in cocultures
For simultaneous analysis of the phenotype and ploidy, Hoechst 33342 (BD Biosciences) was added to the coculture medium at a final concentration of 1 μg/mL, and the culture was maintained at 37°C for 2 hours. Then, nonadherent cells were harvested, washed, and labeled with anti-CD41 and anti-CD42c antibodies. Cells were washed twice and resuspended in PBS-2% newborn serum supplemented with 2.5 μg/mL 7-aminoactinomycin D (Sigma, St Louis, MO) for dead cell exclusion. MKs were identified as cellular events expressing both CD41 and CD42c antigens, among the viable cellular events and the large cellular events of high ploidy as presented in supplemental Figure 1. These large cellular events permeable to 7-aminoactinomycin D are observed only in cultures where large cells (MKs) are visible (supplemental Figure 1Ai-ii). These events were sorted, and the observation of Giemsa-stained cells confirmed that the culture contained only large, mature MKs (supplemental Figure 1Bi-iii). Therefore, these large events involve mature MKs and were consequently included in the analysis.
Results
Isolation and characterization of different stromal cell populations from mouse fetal livers
A flow cytometry sorting strategy was devised to identify functionally distinct stromal cell populations in embryonic day 13.5 (E13.5) mouse fetal liver cell suspensions (Figure 1A). Preliminary experiments showed that CFU-Fs were restricted to the CD45−TER119−CD31−CD51+ fraction (data not shown). To further characterize the stromal cell population identified by CD51 expression, we performed magnetic depletion of the hematopoietic fraction (Hem+) of the cell suspension, which represented 80.4% of the viable cells (Figure 1Ai-ii). Following screening of a panel of antibodies, further subdivision of the Hem−CD31−CD51+population relied on differential expression of VCAM-1 and PDGFRα. Four discrete subpopulations of cells could be resolved: Hem−CD31−CD51+-VCAM-1−PDGFRα− (V−P−), Hem−CD31−CD51+-VCAM-1−PDGFRα+(V−P+), Hem−CD31−CD51+-VCAM+PDGFRα+(V+P+), and Hem−CD31−CD51+-VCAM-1+PDGFRα− (V+P−) (Figure 1Aiii-iv). Following sorting, these 4 subpopulations were evaluated for CFU-F activity, which was observed for all subpopulations except V+P− cells (Figure 1B). However, this population formed adherent layers when grown in EGM-2 medium and displayed a distinct morphology (Figure 1Ci), indicating a unique property of this cell population. Further flow cytometry and reverse transcription polymerase chain reaction analyses demonstrated the expression of Hnf4a, Alb, Afp, Krt18, epithelial cell adhesion molecule, E-cadherin, c-met, and Dlk-1 (Figure 1Cii-iii) and the absence of expression of G6pc, suggesting that this population contains hepatic progenitors, but not mature hepatocytes.14,15 A detailed phenotypic characterization by flow cytometry of the stromal cell populations is provided in supplemental Figure 2. In agreement with this, the number of V+P− cells per liver was significantly increased in livers from E16.5 embryos, a developmental stage at which the hepatic development is fully underway16-18 (supplemental Figure 3). Moreover, V+P− cells were absent or barely detected in the rest of the embryo at E13.5 or in livers from adult or newborn mice (supplemental Figure 4).
V+P− stromal cells are unable to support long-term repopulating HSCs
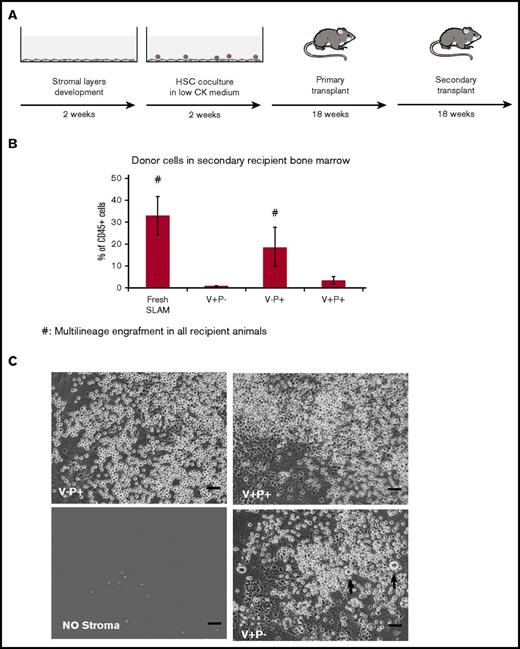
We evaluated the capacity of fetal liver–derived stromal cells to support HSC maintenance. Stromal cells were cocultured with 20 HSCs isolated from adult bone marrow of PTPRC (CD45.1+) mice as Lin−Sca-1+c-kit+CD48−CD150+ cells (SLAM) and serially transplanted into double congenic (CD45.1+/CD45.2+) recipient mice (Figure 2A). Multilineage donor contribution in the bone marrow of all secondary recipient mice was observed only in presence of V−P+ stromal layers. The V+P− stromal layers supported the proliferation of hematopoietic cells but failed to maintain either short-term (supplemental Figure 5D) or long-term repopulating cells (Figure 2B). Light microscopy observation of the cocultures at 2 weeks revealed the presence of large cells (∼30 μm in diameter) in the V+P− condition, suggesting that this stromal cell population has the unique ability to support megakaryopoiesis (Figure 2C).
V+P− stromal cells do not support maintenance of long-term repopulating HSCs. (A) Outline of the long-term HSC repopulating assay. Bone marrow–derived CD45.1+ SLAM cells cultured in low-cytokine medium onto stromal layers were transplanted into F1 double congenic CD45.1+/CD45.2+ irradiated mice. Bone marrow cells from primary recipient mice were transplanted into F1 secondary recipient mice. (B) Percentage of donor cells in the bone marrow of secondary recipient mice 18 weeks posttransplant are presented (# indicates groups for which all recipient animals displayed multilineage engraftment). Values represent mean ± standard deviation (SD) from 2 independent experiments. (C) Representative images of the cocultures after 14 days. Arrows point to putative MKs. Scale bars represent 80 μm. CK, cytokine.
V+P− stromal cells do not support maintenance of long-term repopulating HSCs. (A) Outline of the long-term HSC repopulating assay. Bone marrow–derived CD45.1+ SLAM cells cultured in low-cytokine medium onto stromal layers were transplanted into F1 double congenic CD45.1+/CD45.2+ irradiated mice. Bone marrow cells from primary recipient mice were transplanted into F1 secondary recipient mice. (B) Percentage of donor cells in the bone marrow of secondary recipient mice 18 weeks posttransplant are presented (# indicates groups for which all recipient animals displayed multilineage engraftment). Values represent mean ± standard deviation (SD) from 2 independent experiments. (C) Representative images of the cocultures after 14 days. Arrows point to putative MKs. Scale bars represent 80 μm. CK, cytokine.
Fetal liver V+P− stroma supports the production of MKs from murine HSCs
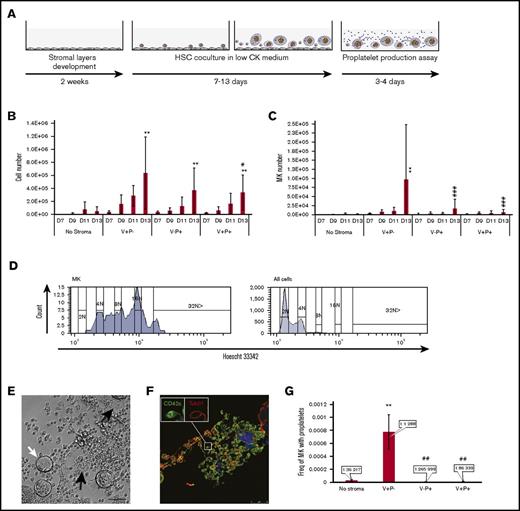
The capacity to support MK production was further evaluated by analyzing CD41 and CD42c expression and ploidy levels. While the total number of cells produced from 50 HSCs showed a similar increase in the 3 stromal cocultures when compared with no stroma (Figure 3B), clear differences were observed for MK production. A CD41+CD42c+ population was identified at days 7 and 9 of coculture for the 3 stromal layers, but a striking increase was observed in the V+P− coculture at day 13. An average of 105 MKs per 50 HSCs was produced, which was 100 times greater than in the absence of stroma (Figure 3C). By comparison, V−P+ and V+P+ cocultures showed a 17- and 6-fold increase, respectively. No hematopoietic cells were produced from any of the established stromal layers when grown for 13 days in coculture medium (data not shown). The CD41+CD42c+ cells from the 3 coculture conditions were polyploid. A representative histogram of the ploidy analysis is presented in Figure 3D. Myeloid and lymphoid lineages identified using CD11b and Gr-1 markers and CD3 and B220, respectively, were equivalent in all stromal conditions (data not shown).
HSC culture with V+P− stromal cells supports the expansion of CD41+CD42+polyploid MKs that efficiently produce proplatelets. (A) Fetal liver stromal cells were sorted confluent adherent layers developed over 2 weeks. HSCs (50 SLAM cells) were cocultured with sorted stromal cells for 7 to 13 days and assayed for MK production. At days 9 to 13, a fraction of the cells were subcultured in proplatelet medium. (B) Total number of cells produced from 50 SLAM cells in the absence of stroma or with the different stroma. Values represent mean ± SD from 5 independent experiments. *P < .01 vs the nonstroma group for the same day; # P < .05 vs stroma V+P− for the same day. Other comparisons were not statistically significant. (C) Total number of MKs, identified as cells expressing CD41 and CD42c, produced from 50 SLAM cells. Values represent mean ± SD from 5 independent experiments *P < .01 vs the nonstroma group for the same day; #P < .001 vs stroma V+P− for the same day. Other comparisons were not statistically significant. (D) Representative histogram of the DNA content analysis of MKs (CD41+CD42c+) top) and all cells (bottom) produced during a 13-day coculture on V+P− cells. (E) Representative phase contrast image of cells first cultured on V+P− cells and replated for 3 to 4 days in proplatelet medium. White arrows indicate MKs, and black arrows indicate proplatelet extension. Scale bar represents 100 μm. (F) Immunostaining confirmed the presence of tubulin β1 in the proplatelet extension. Scale bar represents 25 μm. (G) Cells from day 11 coculture were harvested and plated in proplatelet medium using a limiting dilution scheme. The frequency of cells produced from day 11 cocultures capable of producing proplatelet-bearing MKs is presented. Values represent mean ± SD from 4 independent experiments *P < .01 vs the nonstroma group for the same day; #P < .01 vs stroma V+P− for the same day. Other comparisons were not statistically significant. CK, cytokine; D, day.
HSC culture with V+P− stromal cells supports the expansion of CD41+CD42+polyploid MKs that efficiently produce proplatelets. (A) Fetal liver stromal cells were sorted confluent adherent layers developed over 2 weeks. HSCs (50 SLAM cells) were cocultured with sorted stromal cells for 7 to 13 days and assayed for MK production. At days 9 to 13, a fraction of the cells were subcultured in proplatelet medium. (B) Total number of cells produced from 50 SLAM cells in the absence of stroma or with the different stroma. Values represent mean ± SD from 5 independent experiments. *P < .01 vs the nonstroma group for the same day; # P < .05 vs stroma V+P− for the same day. Other comparisons were not statistically significant. (C) Total number of MKs, identified as cells expressing CD41 and CD42c, produced from 50 SLAM cells. Values represent mean ± SD from 5 independent experiments *P < .01 vs the nonstroma group for the same day; #P < .001 vs stroma V+P− for the same day. Other comparisons were not statistically significant. (D) Representative histogram of the DNA content analysis of MKs (CD41+CD42c+) top) and all cells (bottom) produced during a 13-day coculture on V+P− cells. (E) Representative phase contrast image of cells first cultured on V+P− cells and replated for 3 to 4 days in proplatelet medium. White arrows indicate MKs, and black arrows indicate proplatelet extension. Scale bar represents 100 μm. (F) Immunostaining confirmed the presence of tubulin β1 in the proplatelet extension. Scale bar represents 25 μm. (G) Cells from day 11 coculture were harvested and plated in proplatelet medium using a limiting dilution scheme. The frequency of cells produced from day 11 cocultures capable of producing proplatelet-bearing MKs is presented. Values represent mean ± SD from 4 independent experiments *P < .01 vs the nonstroma group for the same day; #P < .01 vs stroma V+P− for the same day. Other comparisons were not statistically significant. CK, cytokine; D, day.
The expression of CD42c and polyploidy indicated that these MKs were well differentiated. This was confirmed following replating of cells from a 9- to 13-day coculture in a TPO-rich medium to favor full MK maturation.19 Under this condition, MKs derived from HSC/V+P− cocultures efficiently developed proplatelet extensions after 3 days with typical shafts and platelet swellings that were positive for β1-tubulin labeling (Figure 3E-F). The proplatelet capacity was quantified using a limiting dilution scheme that allowed for a comparison of the frequency of cells capable of producing proplatelets in cells generated in the different coculture conditions. Whereas the cells produced in the absence of stroma or in the presence of V−P+ and V+P+ stromal layers displayed a frequency of proplatelet-forming MKs of 1:36 217, 1:265 999, and 1:86 339 cells, respectively, the cells produced in the presence of V+P- stromal layers exhibited a frequency of proplatelet-forming MKs of 1:1288 cells Figure 3G.
These results indicate that stromal layers derived from V+P− cells provide a unique microenvironment favoring the production of mature MKs from HSCs.
The V+P− stromal layers promote MK differentiation via cell contact with hematopoietic progenitors
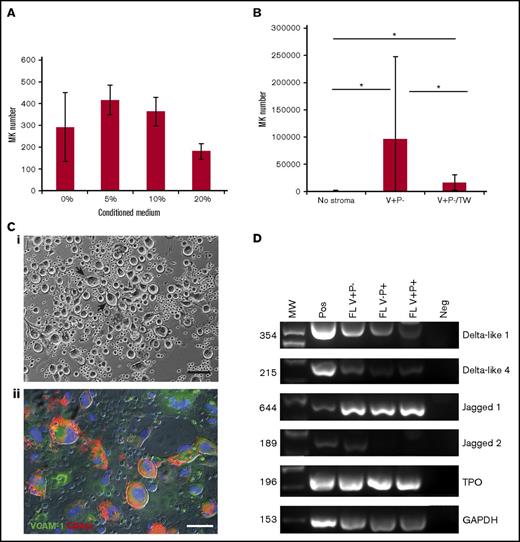
Having established that V+P− stromal cells supported MK production, we evaluated whether this was mediated by secreted factors, cell–cell contact, or both. The role of secreted factors was first assessed by culturing HSCs in low-cytokine medium supplemented with an increasing proportion (5% to 20%) of conditioned media collected from V+P− stromal cells. The number of CD41+CD42c+ cells produced after 9 days of culture was not increased in the presence of conditioned media compared with the control, suggesting minimal implication of secreted factors (Figure 4A). In agreement with this, the potential importance of cell–cell contact was suggested in Transwell assays where the number of MKs produced from 50 HSCs seeded in the upper chamber, but not in contact with V+P− cells (16 170.4 ± 5759 MKs at day 13), was only slightly increased compared with the increase observed at day 13 in the standard coculture (96 414.4 ± 43 902.5) (Figure 4B). TPO, an essential cytokine of MK lineage differentiation, was expressed in all the fetal stromal layers tested (Figure 4Di).These data suggest that direct HSC–stromal cell contact could be the primary determinant or factor supporting the production of a large number of MKs. Additional support for the contact-dependency hypothesis came from the observation of clusters of large cells adhering to the top of the stromal layer (Figure 4Ci). These clusters were observed from day 7 in all the cocultures with V+P− cells, but only on rare instances with V+P+ stromal layers and never with V−P+ cells. Immunostaining for CD42c expression confirmed that these adherent cells were indeed MKs (Figure 4Cii). Considering that Notch signaling has been implicated in MK erythroid commitment,20-22 we investigated the expression of Notch ligands in stromal cells. While Dll1, Dll4, and Jag 1 are expressed by the 3 stromal layers, Jag2 is only detected in V+P−–derived stromal layers (Figure 4Dii).
V+P− stromal cells promote MK expansion via a contact-dependent mechanism. (A) HSCs (50 SLAM cells) were plated in low-cytokine serum-free medium in the presence of increasing proportions (vol/vol) of conditioned medium from a V+P− stromal cell layer. The bar graph represents the number of MKs produced per 50 SLAM cells plated. The mean values ± SD from 2 independent experiments were not statistically different. (B) Coculture of HSC and stromal layers were established in a noncontact setup using Transwell insert (V+P−/TW) with 0.3-μm pores and analyzed for MK content after 13 days of coculture in comparison with cultures without stroma and cocultures with a V+P− layer. Values represent mean ± SD of MKs produced per 50 SLAM cells from 3 independent experiments. *P < .01. (Ci) Phase contrast image of a cluster of large cells adherent on the stromal layers observed only during the coculture of HSC with V+P−–derived stromal layers. Representative image from a day 9 coculture is presented. Scale bar represents 100 μm. (Cii) Immunofluorescence analysis of the expression of CD42c (red) by the large cells forming clusters on V+P− stromal layers identified by the expression of VCAM-1 (green). Scale bar represents 30 μm. (D) Expression of Jag1, Jag2, Dll1, Dll4, TPO, and GAPDH transcripts in fetal liver stromal cells from adherent layers. Figures on the left side of the panel indicate amplicon size.
V+P− stromal cells promote MK expansion via a contact-dependent mechanism. (A) HSCs (50 SLAM cells) were plated in low-cytokine serum-free medium in the presence of increasing proportions (vol/vol) of conditioned medium from a V+P− stromal cell layer. The bar graph represents the number of MKs produced per 50 SLAM cells plated. The mean values ± SD from 2 independent experiments were not statistically different. (B) Coculture of HSC and stromal layers were established in a noncontact setup using Transwell insert (V+P−/TW) with 0.3-μm pores and analyzed for MK content after 13 days of coculture in comparison with cultures without stroma and cocultures with a V+P− layer. Values represent mean ± SD of MKs produced per 50 SLAM cells from 3 independent experiments. *P < .01. (Ci) Phase contrast image of a cluster of large cells adherent on the stromal layers observed only during the coculture of HSC with V+P−–derived stromal layers. Representative image from a day 9 coculture is presented. Scale bar represents 100 μm. (Cii) Immunofluorescence analysis of the expression of CD42c (red) by the large cells forming clusters on V+P− stromal layers identified by the expression of VCAM-1 (green). Scale bar represents 30 μm. (D) Expression of Jag1, Jag2, Dll1, Dll4, TPO, and GAPDH transcripts in fetal liver stromal cells from adherent layers. Figures on the left side of the panel indicate amplicon size.
Considering the importance of the cellular contact between hematopoietic progenitors and stromal cells for the stimulation of megakaryopoiesis, we performed immunolocalization of the different stromal cells, hematopoietic progenitors, and MKs (supplemental Figure 6A-C). MKs were identified as cells expressing CD42c23 and CD150. We observed that 98% of MKs were in contact with VCAM-1–positive cells (supplemental Figure 6E).
V+P− stromal cells support the production of MK-committed progenitors
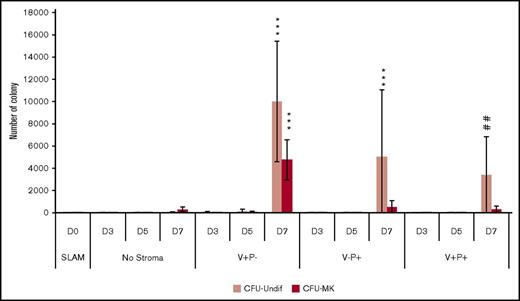
The increase in the number of MKs produced from HSCs cocultured with V+P− stromal layers could result from an increase in the commitment of HSCs toward the MK lineage or the expansion of MK progenitors. We therefore performed clonogenic assays to quantify the CFU-MKs produced in the coculture of HSCs with the different stromal layers from day 3 to day 7. We observed that V+P−–derived stromal layers promoted the production of a larger number of CFU-MKs at day 7 (4759.4 ± 1042.4) compared with the culture in the absence of stroma (322.6 ± 128.2) or in the presence of stromal layers derived from V−P+ (494.9 ± 334.4) or V+P+ (301.7 ± 175.9) cells (Figure 5). In addition, we also observed an increase in the number of undifferentiated colonies produced in presence of V+P− stromal layers. This would suggest that the action of the stromal cells was not restricted to the MK lineage. Thus, we also quantified early progenitors (colony-forming unit [CFU] granulocyte, erythrocyte, monocyte/macrophage) from day 3 to day 7, as well as CFU erythroid at days 5 and 7 of coculture (supplemental Figure 7). We observed an increased number of CFU granulocyte, erythrocyte, monocyte/macrophages generated in the V+P− coculture from day 5 and all CFUs at day 7. These data suggest that V+P− cells stimulate the production of MKs through an expansion of the hematopoietic progenitor compartment.
V+P− stromal cells promote the expansion of hematopoietic progenitors. HSCs (50 SLAM cells) were plated in low-cytokine serum-free medium in the presence or absence of stromal layers. After 3, 5, and 7 days, the cells produced were subjected to a clonogenic assay for CFU-MKs. The graph bar represents the number of CFU-MKs (dark red) and undifferentiated CFU colonies (light red) produced per 50 SLAM cells. Values represent mean ± SD from 3 independent experiments. ***P < .001 vs the nonstroma group for the same day; ##P < .01 comparison for the number of CFU-MKs at day 7 in the stroma V+P− vs other stroma groups. Other comparisons were not statistically significant. D, day.
V+P− stromal cells promote the expansion of hematopoietic progenitors. HSCs (50 SLAM cells) were plated in low-cytokine serum-free medium in the presence or absence of stromal layers. After 3, 5, and 7 days, the cells produced were subjected to a clonogenic assay for CFU-MKs. The graph bar represents the number of CFU-MKs (dark red) and undifferentiated CFU colonies (light red) produced per 50 SLAM cells. Values represent mean ± SD from 3 independent experiments. ***P < .001 vs the nonstroma group for the same day; ##P < .01 comparison for the number of CFU-MKs at day 7 in the stroma V+P− vs other stroma groups. Other comparisons were not statistically significant. D, day.
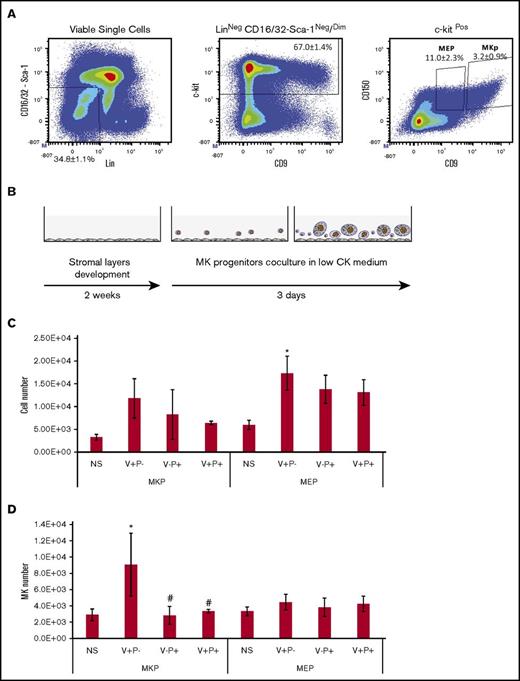
V+P− stromal cells provide lesser support to the more committed cells
To investigate the role of the stromal layers in the later steps of MK differentiation/maturation, we performed cocultures using sorted MK progenitors. Two populations representing different levels of commitment to the MK lineage were evaluated.24,25 The Lin−CD16/32−Sca-1−c-kit+ CD9brightCD150+ cell population represents a population of unipotent MK precursors, and the Lin−CD16/32−Sca-1−c-kit+ CD9dimCD150+ cell population contains MK erythroid bipotent progenitors (MEPs) (Figure 6A). Cocultures were established in serum-free medium in the presence of low cytokine levels, as for HSC-initiated cocultures (Figure 6B). All stromal layers supported an approximately eightfold proliferation of the plated cells (MEP and MK precursors), while in the absence of stroma, proliferation was less than twofold (Figure 6C). The number of MKs produced from MEPs was not increased in the presence of stromal layers, while in MK precursors, we observed a slight increase in the number of mature MKs produced only in the presence of V+P− stromal layers (Figure 6D). Therefore, when cocultures were initiated with MK-committed cells, all stromal layers provided similar support for MK differentiation and maturation.
All stromal layers support the proliferation and differentiation of MK-committed progenitors. (A) Gating strategy for the isolation of 2 populations of MK-committed progenitors. Bone marrow Lin-depleted cells were labeled for sorting of a population enriched in MK erythroid bipotent progenitor (MEP) identified as Lin−CD16/32−Sca-1−ckit+CD150+CD9dim and a population of unipotent MK precursor (MKP) cells identified as Lin−CD16/32−Sca-1−ckit+CD150+CD9bright. Dot plots are representative figures with the mean ± SEM from 5 independent experiments. (B) MEP or MK precursors were plated in low-cytokine medium onto stromal layers and cultured for 3 days. (C) The bar graph represents the total number of cells produced. Values represent mean ± SD from 10 independent experiments. *P < .01 vs the nonstroma group. Other comparisons were not statistically significant. (D) The bar graph represents the total number of mature MKs. Values represent mean ± SD produced from 10 independent experiments. *P < .05 vs the nonstroma group; #P < .05 vs stroma V+P−. Other comparisons were not statistically significant. CK, cytokine; NS, no stroma.
All stromal layers support the proliferation and differentiation of MK-committed progenitors. (A) Gating strategy for the isolation of 2 populations of MK-committed progenitors. Bone marrow Lin-depleted cells were labeled for sorting of a population enriched in MK erythroid bipotent progenitor (MEP) identified as Lin−CD16/32−Sca-1−ckit+CD150+CD9dim and a population of unipotent MK precursor (MKP) cells identified as Lin−CD16/32−Sca-1−ckit+CD150+CD9bright. Dot plots are representative figures with the mean ± SEM from 5 independent experiments. (B) MEP or MK precursors were plated in low-cytokine medium onto stromal layers and cultured for 3 days. (C) The bar graph represents the total number of cells produced. Values represent mean ± SD from 10 independent experiments. *P < .01 vs the nonstroma group. Other comparisons were not statistically significant. (D) The bar graph represents the total number of mature MKs. Values represent mean ± SD produced from 10 independent experiments. *P < .05 vs the nonstroma group; #P < .05 vs stroma V+P−. Other comparisons were not statistically significant. CK, cytokine; NS, no stroma.
Murine fetal liver stromal layers support the production of human MKs and MK progenitors
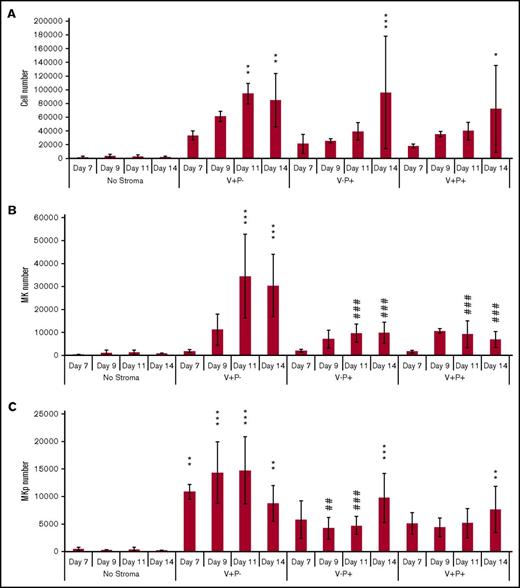
We evaluated whether the stromal cell populations isolated from mouse fetal liver exerted similar effects on human hematopoietic cells. Human HSCs/multipotent progenitors isolated from peripheral blood as CD34+CD38−/lowCD45RA− cells (supplemental Figure 8) were cultured for up to 14 days in the presence of stromal layers using a low-cytokine–containing medium, as in the mouse studies. Similar to the results obtained with mouse HSC, we observed a strong increase in the total number of cells in the presence of the 3 stromal cell populations compared with no stroma, which appeared to be faster in V+P− cocultures (Figure 7A). Phenotypic analysis of the cells produced also revealed a strong expansion of MKs (Figure 7B) identified as CD41+CD42c+ cells (supplemental Figure 9) in the V+P− coculture compared with other stromal cells. Furthermore, as observed in mouse cocultures, this appeared to be due to an increase in the production of MK-committed progenitors identified as CD41+CD34dim cells (Figure 7C).
Mouse fetal liver V+P− stromal cells support the expansion of CD41+CD42+MKs from human HSCs. Human HSCs (500 CD34+CD38−CD45RA−) were cocultured with sorted stromal cells for 7, 9, 11, and 14 days and assayed for MK production. MKs were identified as CD41+CD42c+ cells, and MK progenitors were identified as CD34+CD41dim cells. (A) Total number of cells produced from 500 HSCs in the absence of stroma or with the different stroma. Values represent mean ± SD from 4 independent experiments. (B) Total number of MKs, identified as cells expressing CD41 and CD42c, produced from 500 HSCs. Values represent mean ± SD from 4 independent experiments. (C) Total number of MK progenitors, identified as CD34+CD41dim cells, produced from 500 HSCs. *P < .05, **P < .01, and ***P < .001 vs no stroma; ##P < .01 and ##P < .001 vs V+P−. Other comparisons were not statistically significant. MKp, MK progenitor.
Mouse fetal liver V+P− stromal cells support the expansion of CD41+CD42+MKs from human HSCs. Human HSCs (500 CD34+CD38−CD45RA−) were cocultured with sorted stromal cells for 7, 9, 11, and 14 days and assayed for MK production. MKs were identified as CD41+CD42c+ cells, and MK progenitors were identified as CD34+CD41dim cells. (A) Total number of cells produced from 500 HSCs in the absence of stroma or with the different stroma. Values represent mean ± SD from 4 independent experiments. (B) Total number of MKs, identified as cells expressing CD41 and CD42c, produced from 500 HSCs. Values represent mean ± SD from 4 independent experiments. (C) Total number of MK progenitors, identified as CD34+CD41dim cells, produced from 500 HSCs. *P < .05, **P < .01, and ***P < .001 vs no stroma; ##P < .01 and ##P < .001 vs V+P−. Other comparisons were not statistically significant. MKp, MK progenitor.
Discussion
The present report provides new data on the cellular microenvironment in the developing mouse fetal liver during the critical period of MK differentiation. The major outcome is the delineation of a sorting strategy to obtain a new stromal cell population harboring a hepatocyte progenitor phenotype contributing a favorable microenvironment for MK progenitor expansion.
Recent studies aiming to identify the cellular components of the niche in the bone marrow have revealed its phenotypic and functional heterogeneity.26,27 In comparison, less is known about the niche in the developing liver. It has been suggested that the fetal liver hematopoietic microenvironment consists of cells in epithelial-to-mesenchymal transition.28 Our sorting strategy allowed discrimination between epithelial and mesenchymal components of the fetal liver stroma, where the V+P− population presents a phenotype compatible with an epithelial/ hepatic identity.14,15 The presence of such a population in the fetal liver at E13.5 is consistent with the literature reporting the time of emergence of the first hepatic progenitors.18,29-32 This hypothesis is further supported by the large increase in V+P− cells at E16.5, a stage at which the hepatic program is well underway.16,17 By comparison V+P− cells are absent or rarely observed in the rest of the embryo at the same developmental stage or in the liver from newborn and adult mice. It would be relevant to test whether a population of this phenotype can increase in a regenerating liver or an ectopic site of megakaryopoiesis.
Although V+P− stromal layers did not support HSC maintenance, they favored the expansion of MK progenitors. In the literature, there is an apparent contradiction pertaining to the role of stromal cells in the regulation of megakaryopoiesis,3-9 which most likely reflects the complexity of this process. Transition from an HSC to a mature MK involves a number of steps that likely require different elements of the microenvironment, and it is possible that discrepancies in previous studies could reside in the type of stromal cells that have been used. Our sorting strategy allowed us to discriminate stromal cells and delineate a population with a clear positive effect on megakaryopoiesis. Clonogenic assays provided evidence for a role of V+P− cells in the early stages rather than on more committed MK progenitors. On the other hand, 2 other populations (V+P+ and V−P+) did not stimulate the production of CFUs. This observation suggests that the mesenchymal stromal layers may repress or delay the production of the highly proliferative progenitors identified by the CFU assay.
Another possible cause for discrepancies in previous coculture studies could be the use of different types of hematopoietic progenitors. Here, we observed that the V+P− stromal cells, which supported massive expansion of MK progenitors from mouse HSCs, only minimally increased the number of MK produced from more committed progenitors. Therefore, it is possible that previous reports of a negative role of mesenchymal cells in megakaryopoiesis could stem from the type or degree of commitment of the hematopoietic cells used in the cocultures. For example, human CD34+ cells comprise a large proportion of committed progenitors that could mask the effect of the stroma on a more primitive population.5,6 Using a CD34+CD38−/lowCD45RA− population corresponding to less committed cells, we observed a very similar capacity of V+P− stromal layer to support MK expansion, illustrating the importance of progenitor selection for correct interpretation.
Other factors to consider are the type, timing, and concentration of cytokines added to the media. We chose to use suboptimal concentrations of cytokines and growth factors, including TPO, the essential factor for MK commitment and differentiation. This allowed us to reveal the potential effect provided by stromal cells, an effect that could have been masked by cocktails of cytokines already optimized to favor proliferation or MK differentiation.
Considering the hepatic identity of V+P− cells, a mode of action via the secretion of TPO could have been anticipated. However, TPO transcripts were detected in the 3 types of stromal layers. Furthermore, the addition of condition media and Transwell assays indicated that the main factor stimulating MK production was cell–cell contact and not secreted factors. The observation of some colonies of adherent MK also favors contact-dependent mechanisms and additionally raises the possibility of distinct properties of this particular population of MKs. Whether the contact is mediated by cell-surface receptors, membrane-bound growth factors, or secreted components of an extracellular matrix remains to be clarified. Notch signaling has been implicated in the commitment toward the MK lineage.22 We investigated whether differential expression of Notch ligands by stromal cells could shed light on the specific effect of the V+P− stromal cell population. Jag2 was only detected in stromal layers derived from the V+P− stromal population and could therefore mediate the action of the stromal population.
VCAM-1 has been used in the sorting scheme to discriminate between different stromal cells. Previous work suggested that this marker could also have functions in the fetal liver microenvironment. A role of VCAM-1 in the HSC niche has long been suspected, and studies have demonstrated that the expression of VCAM-1 by bone marrow stromal cells mediates the adhesion and expansion of early hematopoietic progenitors.33,34 However, later studies showed that adherence of hematopoietic progenitors to fetal liver–derived stromal cells lines was not mediated by VCAM-1, contrary to cell lines derived from the bone marrow.35 This is in agreement with our observation that VCAM-1–negative cells (V−P+) provided better support for the maintenance of long-term repopulating cells than the V+P+ cells. However, the role of VCAM-1 expression in the V+P− fetal liver population remains to be investigated.
Considering the recent studies suggesting a role for MKs as a HSC niche,36-40 it is tempting to postulate that MKs generated upon the influence of hepatic progenitors could in turn contribute to the expansion of the HSC pool that occurs is the fetal liver through factors such as PF4/CXCL4 that control HSC quiescence.39 MKs could represent a feedback loop mechanism that maintains stemness and quiescence in the highly proliferative context of the fetal liver. The existence of this amplification loop in the fetal liver remains to be demonstrated. We observed that the MKs produced from mouse bone marrow progenitors exhibited all the expected features (cell-surface receptor expression, polyploidy, capacity to produce proplatelets in vitro), but it cannot be excluded that MKs produced in the fetal liver have different properties. This would also be relevant in the light of studies demonstrating different waves of MK production in the developing liver.13
In conclusion, this study provides direct demonstration that one distinct cellular component of the fetal liver supports expansion of MKs. The strong support of murine megakaryopoiesis shown in this study could also have applications in the culture of human progenitors to improve the yield of platelets produced in vitro. Our observation that that the unique effects of the V+P− stromal cell population also applies to human HSCs could open doors to such applications.
The full-text version of this article contains a data supplement.
Authorship
Contribution: N.B., C.J., N.M., C.A., P.G., and S.E. performed experiments; T.I. and D.S. performed Dlk1 phenotypic analysis; N.B. designed the study, analyzed results, and made the figures; N.B. and F.L. wrote the paper; and C.S., P.J.S., C.G., and F.L. helped design the study and contributed to writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Brouard, UMR_S949, INSERM, Etablissement Français du Sang-Alsace, 10, rue Spielmann, BP 36, F-67065 Strasbourg Cedex, France; e-mail: nathalie.brouard@efs.sante.fr.