Key Points
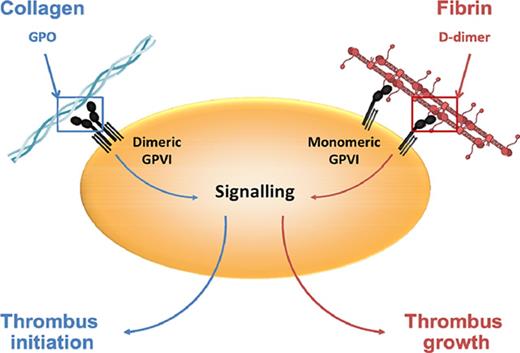
GPVI is the major signaling receptor for fibrin in human platelets; the GPVI binding site is located in the fibrin D-dimer region.
D-dimer blocks platelet aggregation by fibrin and collagen but not by a collagen-related peptide, suggesting a distinct binding epitope.
Abstract
Fibrin has recently been shown to activate platelets through the immunoglobulin receptor glycoprotein VI (GPVI). In the present study, we show that spreading of human platelets on fibrin is abolished in patients deficient in GPVI, confirming that fibrin activates human platelets through the immunoglobulin receptor. Using a series of proteolytic fragments, we show that D-dimer, but not the E fragment of fibrin, binds to GPVI and that immobilized D-dimer induces platelet spreading through activation of Src and Syk tyrosine kinases. In contrast, when platelets are activated in suspension, soluble D-dimer inhibits platelet aggregation induced by fibrin and collagen, but not by a collagen-related peptide composed of a repeat GPO sequence or by thrombin. Using surface plasmon resonance, we demonstrate that fibrin binds selectively to monomeric GPVI with a KD of 302 nM, in contrast to collagen, which binds primarily to dimeric GPVI. These results establish GPVI as the major signaling receptor for fibrin in human platelets and provide evidence that fibrin binds to a distinct configuration of GPVI. This indicates that it may be possible to develop agents that selectively block the interaction of fibrin but not collagen with the immunoglobulin receptor. Such agents are required to establish whether selective targeting of either interaction has the potential to lead to development of an antithrombotic agent with a reduced effect on bleeding relative to current antiplatelet drugs.
Introduction
Until recently, glycoprotein VI (GPVI) was thought to function only as the principal signaling receptor in platelets for collagen, with a critical role in the initiation of thrombus formation at sites of damage to the vessel wall. However, the recent discovery that fibrin, the final product of the coagulation cascade, activates GPVI suggests that the immunoglobulin receptor plays a critical role in thrombus propagation.1,2 This provides an explanation for the paradoxical observation that mice deficient in GPVI fail to form an occlusive thrombus in a FeCl3 injury model, even though the onset of thrombosis, which is where collagen is exposed, is not altered.3 Given the relatively mild bleeding diathesis of mice and patients deficient in GPVI,1,4,5 these findings raise the possibility that activation of GPVI by fibrin represents a target for development of a new class of antithrombotic drug that may have a reduced effect on bleeding relative to current antiplatelet drugs.
Fibrinogen plays a critical role in thrombus formation through the formation of a fibrin clot and by contributing to platelet aggregation via interaction with integrin αIIbβ3. Fibrinogen has a molecular weight of 340 kDa and is composed of 2 repeats of 3 polypeptides consisting of Aα, Bβ, and γ chains. Fibrin is an insoluble polymer that is generated from fibrinogen by the proteolytic action of thrombin. Thrombin cleaves fibrinogen at 2 distinct sites in the central E domain of fibrinogen, situated at the N-termini of the α chain and the β chain, releasing fibrinopeptides A and B, respectively. Proteolytic cleavage induces conformational changes that lead to formation of fibrin monomers and exposure of new N-termini,6 which interact with a constitutive complementary binding pocket in the D-domain in the γ and β chains, respectively, leading to protofibril formation through D-E-D interactions.7 Fibrinopeptide B cleavage is associated with release of the α C-terminal domains, which leads to lateral aggregation of protofibrils, increasing fibrin fiber thickness.8 Thrombin also activates FXIII, which catalyzes the formation of γ-γ and α-α isopeptide bonds, thereby stabilizing the clot and improving resistance to fibrinolysis.9 Lysis of crosslinked fibrin by plasmin produces D-dimer containing γ-γ crosslinks that hold 2 D-regions together.
In this study, we have mapped the binding sites between fibrin and GPVI by investigating the action of plasmin-derived proteolytic fragments of fibrin on platelet activation. Using this approach, we have localized the binding site of GPVI to the D-dimer region in fibrin. We show that immobilized D-dimer induces platelet spreading, whereas soluble D-dimer inhibits collagen- and fibrin-induced platelet activation. Further, we provide evidence that the 2 ligands bind to distinct configurations of GPVI and possibly to distinct epitopes, suggesting that it may be possible to generate inhibitors that selectively inhibit binding of collagen or fibrin.
Materials and methods
Reagents
Human, mice, and bovine fibrinogens were from Enzyme Research Laboratories (Swansea, United Kingdom). Goat anti-human immunoglobulin G (IgG) HRP conjugated was from ThermoFisher Scientific (Glasgow, United Kingdom). Rabbit anti-6-His HRP conjugated (Bethyl A190-114P) and PPACK were from Cambridge Bioscience (Cambridge, United Kingdom). Alexa-488 phalloidin was from Molecular Probes (Life Technologies, Paisley, United Kingdom). Collagen and collagen diluent were from Nicomed. Collagen-related peptide (CRP; 10 glycine-proline-hydroxproline [GPO] repeats) was crosslinked as described.10 PAR-1 peptide (TFLLR) was produced by Alta Bioscience (Birmingham, United Kingdom). The fibrin fragment E and D-dimer were from Quadratech (Epsom, United Kingdom). FXIIIa was from Zedira (Darmstadt, Germany). Dasatanib was from LC Laboratories (Woburn, MA). PRT-060318 was from Caltag Medsystems (Buckingham, United Kingdom). Recombinant fibrinogen with γA/γA or γ’/γ’ domains was generated as described.11 γA/γ’ fibrinogen was purified from human placenta as described.12 Revacept was provided by advanceCOR (Munich, Germany). All other reagents were purchased from Sigma-Aldrich (Poole, United Kingdom) or described sources.1
Generation of fibrin
Fibrin was freshly prepared in solution by incubating fibrinogen (1 mg/mL; 4.17 µM) with 1 unit per mL of thrombin and 0.7 μg/mL of FXIIIa in phosphate-buffered saline (PBS) with 10 mM Ca2+ for 1 hour at room temperature. Thrombin was blocked by addition of PPACK (20 μM) for 15 minutes at room temperature. The fibrin fiber was sonicated on ice to aid dispersal and concentrated by centrifugation and resuspension in Tyrode’s N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer. Fibrin was generated on coverslips and precoated with fibrinogen, using thrombin and neutralization by PPACK as described previously.1
GPVI constructions
Soluble GPVI collagen-binding domain (CBD; comprising residues S2-T183) was prepared as follows. GPVI-Fcα13 was used as a template to amplify the GPVI CBD by polymerase chain reaction. A forward primer 5′-GGAGACCCAAGCTTGCGGCCGCCACCATGAAGTTATGGCTGAAT-3 containing an HindIII sequence and the rat IgG2b secretion signal were used with 5′-GCTGCTGAATTCCTAGTGGTGGTGGTGGTGGTGGCTACCCTGGAAATACAGGTTCTCGGA-3′ as the reverse primer, adding a tobacco etch virus protease–cleavable hexahistidine tag at the C-terminus. This polymerase chain reaction product was then inserted into the eukaryotic expression vector pEE12.4 (Lonza Biologics) by HindIII/EcoRI restriction endonuclease digestion followed by ligation with T4 DNA ligase.
GPVI-Fcγ was prepared by subcloning the GPVI CBD into the SigpIg+ mammalian expression vector (R&D) that encodes an N-terminal CD33 signal sequence and a C-terminal human IgG1-Fc sequence relative to the insert.14,15 Proteins were expressed by transient transfection of HEK293T cells. Cell-culture medium containing GPVI CBD or GPVI-Fcγ was purified by Ni-NTA or protein-A affinity chromatography, respectively. Eluted proteins were further purified by gel filtration using Superdex S75 or S200 columns for GPVI CBD or GPVI-Fcγ, respectively.
Revacept was cloned and expressed in Chinese hamster ovary cells by fermentation in large scale under good manufacturing practice conditions as described.16
Human platelets
Blood was taken using 3.8% sodium citrate (volume/volume ratio of 1:9) as the anticoagulant. Informed consent was obtained according to the guidelines of the local ethics committee. All steps of this study complied with the ethical principles according to the Declaration of Helsinki. Washed platelets were obtained by centrifugation using prostacyclin (2.8 μM) and resuspended in modified Tyrode’s HEPES buffer (134 mM of NaCl, 0.34 mM of Na2HPO4, 2.9 mM of KCl, 12 mM of NaHCO3, 20 mM of HEPES, 5 mM of glucose, 1 mM of MgCl2; pH7.3) as described.1 Platelets were used at 20 × 109/L for static adhesion or 500 × 109/L for other studies.
Mice platelets
Gp6−/− mice were provided by Jerry Ware (University of Arkansas for Medical Sciences, Little Rock, AR).17 Wild-type mice were generated from breeding of heterozygotes or purchased from Harlan Laboratories (Hillcrest, United Kingdom). All procedures were undertaken with UK Home Office approval. Blood was drawn from narcosed and CO2-asphyxiated mice into 10% acid citrate dextrose and platelet-rich plasma generated as described.1 Washed platelets were obtained by centrifugation using prostacyclin (2.8 μM) and resuspended in modified Tyrode’s HEPES buffer. Platelets were used at 20 × 109/L for static adhesion or 500 × 109/L for other studies.
Protein phosphorylation
Washed platelets were stimulated with different agonists. Activation was terminated with ice-cold lysis buffer (300 mM of NaCl, 20 mM of Tris, 2 mM of EGTA, 2 mM of EDTA, and 2% IGEPALCA-630 [pH 7.4], plus 2.5 mM of Na3VO4, 100 mg/mL of AEBSF, 5 mg/mL of leupeptin, 5 mg/mL of aprotinin, and 0.5 mg/mL of pepstatin). Whole-cell lysates were prepared by boiling a sample of lysate with sodium dodecyl sulfate (SDS) sample buffer. Syk was immunoprecipitated with α-Syk antibody and protein A-Sepharose beads for 2 hours. The beads were then washed, and proteins were eluted by boiling in SDS sample buffer. Samples were separated by SDS-polyacrylamide gel electrophoresis, electrotransferred, and western blotted before exposure to film.
Platelet aggregation
Washed platelets were incubated in the presence of dasatinib (3 μM) and PRT-060318 (10 μM), D-dimer (30 μg/mL; 1.67 × 10−7 M), or solvent controls. Platelets were stimulated by fibrin, collagen, CRP, or PAR1. Light transmission was recorded at 37°C with stirring (1200 rpm) in an aggregometer (Chrono-Log Stago, Parsippany, NJ). ATP secretion was monitored in washed platelets in parallel with platelet aggregation by adding firefly luciferase and luciferin (Chrono-Lume; Chrono-Log Stago) and comparing the luminescence generated by platelet ATP release with an ATP standard.18
Platelet spreading
Glass coverslips were coated in the presence of 10 ug/mL of collagen, fibrinogen, D-dimer, or fibrin generated as described in the previous paragraph. After washing with PBS, coverslips were blocked with 5 mg/mL of heat-inactivated bovine serum albumin in PBS for 60 minutes. Human or mice platelets were allowed to spread for 30 or 45 minutes, respectively, at 37°C before washing with PBS followed by fixation with paraformaldehyde (3.7%) for 10 minutes. For actin staining, platelets were permeabilized with 0.1% Triton X-100 for 5 minutes and stained with Alex-488-phalloidin for 45 minutes in the dark. Platelets were imaged on a Zeiss Axiovert 200M microscope. Platelet surface area was analyzed using ImageJ (National Institutes of Health, Bethesda, MD). In each independent experiment, 5 random pictures were analyzed (100 platelets in total).
Solid-phase binding assay
Two recombinant proteins were used in the binding studies: GPVI-Fc fusion (dimer) and GPVI-His tagged (monomer). Coverslips were coated with collagen, fibrinogen, fibrin, and fibrin fragments (10 μg/mL) overnight at 4°C. The plates were blocked with 3% bovine serum albumin–PBS for 1 hour, washed, and incubated with monomeric or dimeric GPVI (100 nM) for 1 hour. After washing, secondary antibodies HRP-conjugated goat anti-human IgG-Fc or HRP-conjugated anti-His Tag were added at 4 μg/mL for 1 hour to detect GPVI dimers or monomers, respectively. The binding of GPVI to the immobilized substrate was visualized using 3,3′,5,5′-tetramethylbenzidine. The reaction was stopped by addition of H2SO4 (2 M), and absorbance was measured at 450 nm with a spectrofluorometer. For the competitive binding assay, monomeric GPVI was incubated with a different concentration of D-dimer for 1 hour before addition to the immobilized substrate.
SPR
Surface plasmon resonance (SPR) ligands and analytes were prepared as detailed in supplemental Materials. SPR was performed on a Pioneer platform from PALL FortéBio (Portsmouth, United Kingdom) using a OneStep titration function based on Taylor dispersion injection theory.19,20 All kinetic binding assays were performed with both the analysis and sample rack temperatures at 37°C. GPVI analytes were diluted to 1 µM using the same batch of running buffer for the blanks and the OneStep titration function. The GPVI analytes were injected into the sensor chamber at a flow rate of 30 µL per minute using the OneStep titration function with a loop inject of 100% after 8 leadoff blanks and 3 bulk standard injects of 3% sucrose in running buffer for n = 3 (3 replicates/cycles) using group replicates. The GPVI analytes had a dissociation time of 400 seconds, and the chip surface was regenerated with 1 M of NaCl with a flow rate of 60 µL per minute for 10 seconds followed by a 400-second dissociation period. One periodic blank was performed every 3 cycles. The binding data were zeroed to the preinject resonance units to obtain a starting response unit of 0, the reference curve was subtracted, and the data were blanked to the closest buffer blank. Data were analyzed with Qdat data analysis software (PALL FortéBio). Binding data were fit using a simple KA/KD model, and aggregation/retention parameters were adjusted per binding curve according to goodness of fit and curve type.
Data analysis
Statistical analysis was realized by ANOVA with Tukey posttest. Results are shown as the mean ± standard deviation of a minimum of 3 experiments.
Results
Fibrin does not activate GPVI-deficient human platelets
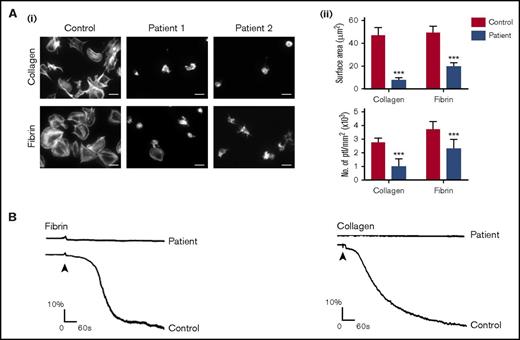
We have shown that fibrin binds to human and mouse GPVI and that spreading of mouse platelets on fibrin is abolished in the absence of GPVI.1 In the present study, we have investigated spreading in human platelets deficient in GPVI through access to 2 unrelated patients who do not express the immunoglobulin receptor (Figure 1A). The 2 patients are homozygous for an adenine insertion in exon 6 at position 242, which generates a stop codon before the transmembrane domain and therefore prevents surface expression of GPVI.4 Platelets from either patient do not aggregate to collagen and do not express detectable GPVI when measured by flow cytometry (Figure 1B; data not shown).
GPVI-deficient patients are unresponsive to fibrin. Glass coverslips were coated with collagen or fibrin as described in "Materials and methods." (Ai) Human platelets (ptl; 20 × 109/L) were allowed to spread on coated coverslips, followed by staining of actin with Alexa-488 phalloidin. The pictures are representative results of a control and 2 GPVI-deficient patients. Scale bar, 5 μm. (Aii) The histograms illustrate the quantification of the surface area of platelets and the number of platelets calculated per millimeter squared. In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. ***P < .001. (B) Representative aggregation traces form a GPVI-deficient patient are shown (the control aggregation trace is representative of >20 donors).
GPVI-deficient patients are unresponsive to fibrin. Glass coverslips were coated with collagen or fibrin as described in "Materials and methods." (Ai) Human platelets (ptl; 20 × 109/L) were allowed to spread on coated coverslips, followed by staining of actin with Alexa-488 phalloidin. The pictures are representative results of a control and 2 GPVI-deficient patients. Scale bar, 5 μm. (Aii) The histograms illustrate the quantification of the surface area of platelets and the number of platelets calculated per millimeter squared. In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. ***P < .001. (B) Representative aggregation traces form a GPVI-deficient patient are shown (the control aggregation trace is representative of >20 donors).
Platelets from healthy controls generate filopodia and full lamellipodia sheets on fibrin as shown in Figure 1A and previously reported.1,2 A similar pattern of spreading is seen in platelets from donors who are heterozygous for the mutation (data not shown). In contrast, spreading on fibrin is markedly inhibited in platelets from patients who lack the glycoprotein receptor, with only a small number of platelets showing partial or in rare cases full spreading (Figure 1A). In addition, adhesion of platelets to fibrin is reduced by ∼40% in both patients (Figure 1Aii). These results demonstrate that spreading of human platelets on fibrin is critically dependent on GPVI but that platelets express additional receptors for fibrin that support adhesion, such as integrin αIIbβ3.21
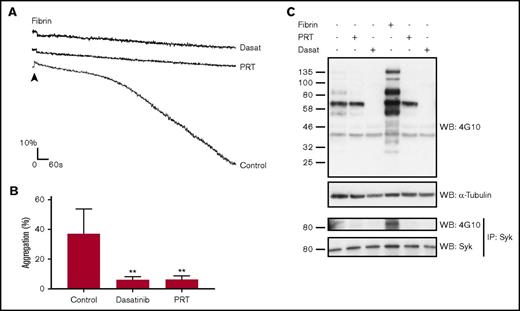
To further investigate GPVI as a signaling receptor for fibrin, we generated a suspension of fibrin and monitored aggregation of washed platelets. This approach is analogous to the generation of a suspension of collagen. Fibrin was generated from fibrinogen by addition of thrombin and, after neutralization of the protease, dispersed by sonication. Platelets underwent a slow aggregation to fibrin, which varied between donors and fibrin preparations as illustrated in Figures 1B and 2A. Aggregation was not preceded by a clear increase in optical density, which is indicative of shape change, possibly because it was masked by the relatively slow onset of response. Aggregation was abolished in the absence of GPVI or in the presence of the Src and Syk inhibitors dasatinib (3 μM)and PRT-060318 (10 μM), respectively (Figure 2A-B). Additionally, dasatinib and PRT-060318 blocked the increase in tyrosine phosphorylation in whole-cell lysates and Syk immunoprecipitates in response to fibrin (Figure 2C). These observations demonstrate that fibrin activates platelets through GPVI and Src and Syk tyrosine kinases.
Fibrin induces Syk-dependent platelet activation and aggregation. (A) Platelets (500 × 109/L), pretreated where indicated with dasatinib (dasat; 3 μM) or PRT-060318 (10 μM), were stimulated with fibrin dispersed as a suspension. (B) The histogram represents 3 independent experiments. The results are shown as mean ± standard deviation. **P < .01. (C) Stimulations were stopped with addition of 2× lysis buffer. A sample of the whole-cell lysate (WCL) was removed, and the remaining lysate was used to immunoprecipitate (IP) Syk. WCLs and IPs were separated by SDS-polyacrylamide gel electrophoresis and western blotted (WB) for pTyr and Syk. The results are shown as a representative of 3 independent experiments.
Fibrin induces Syk-dependent platelet activation and aggregation. (A) Platelets (500 × 109/L), pretreated where indicated with dasatinib (dasat; 3 μM) or PRT-060318 (10 μM), were stimulated with fibrin dispersed as a suspension. (B) The histogram represents 3 independent experiments. The results are shown as mean ± standard deviation. **P < .01. (C) Stimulations were stopped with addition of 2× lysis buffer. A sample of the whole-cell lysate (WCL) was removed, and the remaining lysate was used to immunoprecipitate (IP) Syk. WCLs and IPs were separated by SDS-polyacrylamide gel electrophoresis and western blotted (WB) for pTyr and Syk. The results are shown as a representative of 3 independent experiments.
Fibrin binds to monomeric GPVI
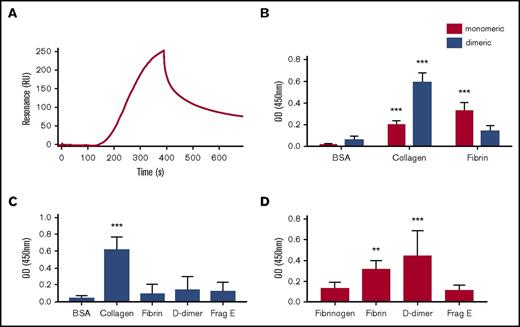
GPVI is expressed on platelets primarily as a monomer and forms dimers and higher oligomers upon activation by collagen and other agonists.22,23 Collagen binds with high affinity to dimeric but not to monomeric GPVI, with conversion of monomers to dimers being a key step in collagen-mediated platelet activation.19,24 In the present study, we measured binding of monomeric and dimeric GPVI to immobilized fibrin using SPR. Monomeric GPVI binds to fibrin with a KD of 302 ± 5 nM (Figure 3A), but there was no detectable binding of GPVI at a concentration of 1 μM (data not shown). Selectivity was confirmed in a solid-phase binding assay, whereas collagen bound preferentially to dimeric GPVI (Figure 3B) as reported.24 In line with this, Revacept, a dimeric form of GPVI in a phase II clinical trial, did not bind to fibrin (Figure 3C). These results show that fibrin and collagen bind selectively to monomeric and dimeric GPVI, respectively.
Monomeric GPVI but not dimeric GPVI binds to fibrin. SPR was performed using OneStep titration as described in "Materials and methods." (A) Representative SPR binding curve using 1 μM of GPVI. The calculated KD was 302 ± 5 nM. Solid-based binding assay was performed in nunc maxisorb 96-well plates coated overnight with 10 μM of bovine serum albumin (BSA), collagen, fibrinogen, or fibrin. (B) Monomeric (red bars) or dimeric (blue bars) GPVI (100 nM) was incubated as described. (C) Revacept, a dimeric GPVI, was allowed to bind to 10 μM of BSA, collagen, human fibrinogen, fibrin, D-dimer, or fragment E (Frag E). (D) Monomeric GPVI was allowed to bind to 10 μM of human fibrinogen, fibrin, D-dimer, or fragment E. Bound GPVI was detected using HRP coupled to an anti-6×His monoclonal antibody for monomeric GPVI or an anti-human immunoglobulin for dimeric GPVI and Revacept. The histograms (mean ± standard deviation) show the results from 5 independent experiments. **P < .01, ***P < .001 compared with a control (BSA or fibrinogen). OD, optical density.
Monomeric GPVI but not dimeric GPVI binds to fibrin. SPR was performed using OneStep titration as described in "Materials and methods." (A) Representative SPR binding curve using 1 μM of GPVI. The calculated KD was 302 ± 5 nM. Solid-based binding assay was performed in nunc maxisorb 96-well plates coated overnight with 10 μM of bovine serum albumin (BSA), collagen, fibrinogen, or fibrin. (B) Monomeric (red bars) or dimeric (blue bars) GPVI (100 nM) was incubated as described. (C) Revacept, a dimeric GPVI, was allowed to bind to 10 μM of BSA, collagen, human fibrinogen, fibrin, D-dimer, or fragment E (Frag E). (D) Monomeric GPVI was allowed to bind to 10 μM of human fibrinogen, fibrin, D-dimer, or fragment E. Bound GPVI was detected using HRP coupled to an anti-6×His monoclonal antibody for monomeric GPVI or an anti-human immunoglobulin for dimeric GPVI and Revacept. The histograms (mean ± standard deviation) show the results from 5 independent experiments. **P < .01, ***P < .001 compared with a control (BSA or fibrinogen). OD, optical density.
Mapping of the GPVI binding site in fibrin
Fibrin(ogen) shows a high level of sequence identity among species. Consistent with this, we observed a similar level of binding of GPVI to fibrin derived from human, murine, and bovine fibrinogen (supplemental Figure 1A), indicating that the binding site for GPVI is conserved. Fibrinogen exists in 2 splicing variants in the γ-chain region, which give rise to 3 possible combinations: γA/γA, γA/γ’, and γ’/γ’. The γ’ chain has a 20–amino acid insertion in its C-terminus, which replaces the last 4 amino acids in the γA chain. No observable difference in the binding of monomeric GPVI to the 3 γA/γ’ combinations was detected (supplemental Figure 1B), showing that splicing does not affect binding of fibrin to GPVI.
To further map the site of interaction in fibrin, we investigated binding of proteolytic fragments of fibrin. Using a solid-phase binding assay, we observed a similar level of binding of monomeric GPVI to immobilized fibrin and to D-dimer, but not to E fragment, indicating that the binding epitope in fibrin resides in the D region or the D-D interface (Figure 3D). Neither dimeric GPVI nor Revacept was able to bind to the D-dimer or E fragment of fibrin, consistent with the absence of binding to fibrin (Figure 3C; data not shown).
Immobilized D-dimer induces spreading of platelets via GPVI
The observation that D-dimer binds to GPVI prompted investigation of whether it induces spreading of mouse and human platelets when immobilized on a surface. As shown in Figure 4A, D-dimer stimulates a similar pattern of spreading of mouse platelets to that induced by fibrin. In contrast, there was no spreading on fibrinogen as previously reported.1 Spreading on D-dimer and fibrin was abolished in GPVI-deficient mouse platelets, and adhesion was reduced by ∼50% in both cases (Figure 4B). Adhesion and spreading on fibrin and D-dimer were markedly inhibited or abolished in the presence of the αIIbβ3 antagonist eptifibatide (supplemental Figure 2).
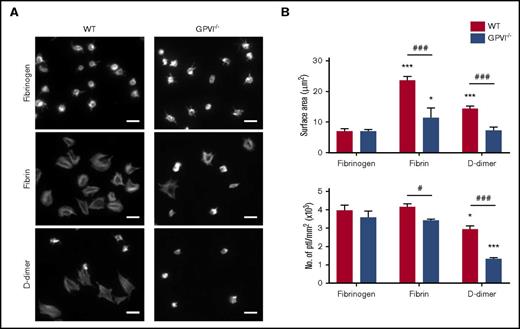
GPVI-dependent platelet spreading on D-dimer. (A) Glass coverslips were coated overnight with fibrinogen, fibrin, or D-dimer. Mouse platelets (ptl; 20 × 109/L) were allowed to spread on coated coverslips followed by staining for actin with Alexa-488 phalloidin. Scale bar, 5 μm. The figure is representative of 3 similar experiments. (B) Quantification of platelet surface area and platelet count per millimeter squared (n = 3). In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. *P < .05, ***P < .001 compared with fibrinogen; #P < .05, ###P < .001 compared with wild type (WT).
GPVI-dependent platelet spreading on D-dimer. (A) Glass coverslips were coated overnight with fibrinogen, fibrin, or D-dimer. Mouse platelets (ptl; 20 × 109/L) were allowed to spread on coated coverslips followed by staining for actin with Alexa-488 phalloidin. Scale bar, 5 μm. The figure is representative of 3 similar experiments. (B) Quantification of platelet surface area and platelet count per millimeter squared (n = 3). In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. *P < .05, ***P < .001 compared with fibrinogen; #P < .05, ###P < .001 compared with wild type (WT).
Spreading of human platelets on D-dimer is blocked in the presence of the inhibitors of Src and Syk inhibitors, dasatinib and PRT-060318, respectively (Figure 5). Both inhibitors also reduce adhesion by the same extent as that seen with fibrin (Figure 5). Spreading and adhesion are also blocked or markedly reduced in the presence of the αIIbβ3 antagonist eptifibatide but are not altered in the presence of monoclonal antibody (mAb) IV.3, which blocks FcγRIIA (supplemental Figure 3). Spreading of human platelets on fibrin was partially reduced in the presence of apyrase and indomethacin (data not shown). Immobilized fibrin and D-dimer stimulated tyrosine phosphorylation in whole-cell lysates, with the response to fibrin being similar to that of collagen (supplemental Figure 4).
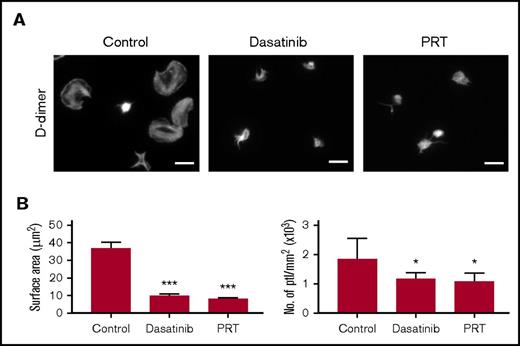
D-dimer–induced spreading is dependent on GPVI signaling. (A) Glass coverslips were coated overnight with D-dimer as described in "Materials and methods." Human platelets (ptl; 20 × 109/L) were preincubated with dasatinib (3 μM), PRT-060318 (10 μM), or vehicle before being allowed to spread on coated coverslips. Actin staining was performed on fixed platelets with Alexa-488 phalloidin. Scale bar, 5 μm. The figure is representative of 3 similar experiments. (B) Quantification of platelet surface area and platelet count per millimeter squared (n = 3). In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. *P < .05, ***P < .001 compared with control.
D-dimer–induced spreading is dependent on GPVI signaling. (A) Glass coverslips were coated overnight with D-dimer as described in "Materials and methods." Human platelets (ptl; 20 × 109/L) were preincubated with dasatinib (3 μM), PRT-060318 (10 μM), or vehicle before being allowed to spread on coated coverslips. Actin staining was performed on fixed platelets with Alexa-488 phalloidin. Scale bar, 5 μm. The figure is representative of 3 similar experiments. (B) Quantification of platelet surface area and platelet count per millimeter squared (n = 3). In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation. *P < .05, ***P < .001 compared with control.
Soluble D-dimer inhibits spreading and aggregation of human platelets induced by fibrin and collagen
Although, when immobilized, D-dimer stimulates spreading of platelets, it does not induce aggregation or secretion in platelet suspensions at concentrations up to 100 μg/mL or binding of annexin-V (procoagulant activity) or mAb PAC-1 (αIIbβ3 activation; data not shown). In contrast, D-dimer (100 μg/mL) inhibits adhesion and spreading of human platelets on collagen- and fibrin-coated surfaces, with an EC50 value in the order of 250 nM (50 μg/mL) for both ligands (Figure 6A-B). D-dimer (10-100 μg/mL) also inhibits aggregation and secretion in washed platelets induced by collagen (0.1-0.3 μg/mL), with full recovery at higher concentrations (Figure 7A; supplemental Figure 5), but not to a PAR1 receptor–activating peptide or to CRP consisting of GPO repeats (Figure 7B). As expected, D-dimer blocks aggregation induced by fibrin (Figure 7Bii). A similar inhibitory effect of D-dimer against collagen but not CRP was seen in platelet-rich plasma (supplemental Figure 6). These results demonstrate that the epitope for D-dimer overlaps with that for fibrin (as expected) and possibly with that for collagen but not that for CRP.
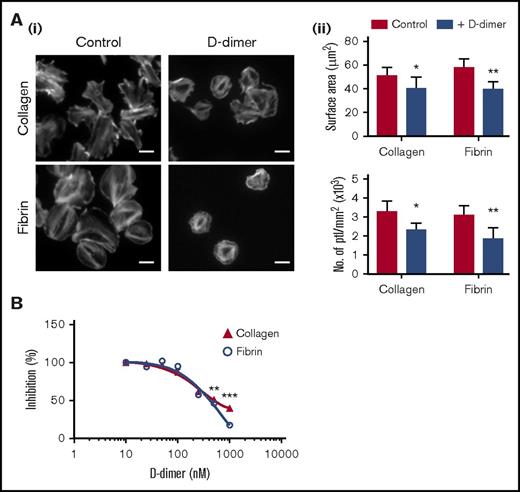
D-dimer in suspension reduces platelet spreading on collagen and fibrin. (Ai) Glass coverslips were coated with collagen or fibrin as described in "Materials and methods." Human platelets (ptl; 20 × 109/L) were preincubated with D-dimer (100 μg/mL) before being allowed to spread on coated coverslips. Actin staining was performed on fixed platelets with Alexa-488 phalloidin. Scale bar, 5 μm. (Aii) Quantification of the surface area of platelets and the number of platelets per millimeter squared of 3 independent experiments. In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation (SD). *P < .05, **P < .01. (B) The graph represents the competition binding assay between monomeric GPVI (100 nM) and D-dimer on collagen- or fibrin-coated surfaces. Binding in the absence of D-dimer is represented as 100%. The results are shown as mean ± SD and are representative of 3 experiments. **P < .01, ***P < .001.
D-dimer in suspension reduces platelet spreading on collagen and fibrin. (Ai) Glass coverslips were coated with collagen or fibrin as described in "Materials and methods." Human platelets (ptl; 20 × 109/L) were preincubated with D-dimer (100 μg/mL) before being allowed to spread on coated coverslips. Actin staining was performed on fixed platelets with Alexa-488 phalloidin. Scale bar, 5 μm. (Aii) Quantification of the surface area of platelets and the number of platelets per millimeter squared of 3 independent experiments. In each independent experiment, 5 random pictures were analyzed (100 platelets in total). The results are shown as mean ± standard deviation (SD). *P < .05, **P < .01. (B) The graph represents the competition binding assay between monomeric GPVI (100 nM) and D-dimer on collagen- or fibrin-coated surfaces. Binding in the absence of D-dimer is represented as 100%. The results are shown as mean ± SD and are representative of 3 experiments. **P < .01, ***P < .001.
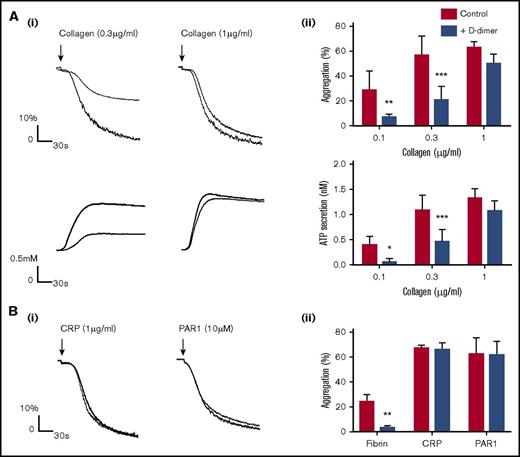
D-dimer inhibits platelet activation by collagen and fibrin in washed platelets. Platelets (500 × 109/L) in the presence of D-dimer (30 μg/mL) were stimulated with collagen (Ai-ii) or CRP, PAR1-peptide, or fibrin (Bi-ii). Aggregation and secretion were monitored by lumiaggregometry (Chrono-Log). The figures show representative aggregation traces with histograms summarizing 3 independent experiments. The results are shown as mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
D-dimer inhibits platelet activation by collagen and fibrin in washed platelets. Platelets (500 × 109/L) in the presence of D-dimer (30 μg/mL) were stimulated with collagen (Ai-ii) or CRP, PAR1-peptide, or fibrin (Bi-ii). Aggregation and secretion were monitored by lumiaggregometry (Chrono-Log). The figures show representative aggregation traces with histograms summarizing 3 independent experiments. The results are shown as mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
Discussion
The present study reports several new observations on the interaction of GPVI with fibrin. Firstly, we demonstrate that GPVI is the major signaling receptor for fibrin in human platelets, using platelets from patients who lack GPVI. This supports previous observations with the anti-GPVI blocking mAb 9012 and shows that its inhibitory effect is not due to steric hindrance.2 Secondly, the retention of adhesion to immobilized fibrin in GPVI-deficient platelets and the inhibitory effect of eptifibatide confirm integrin αIIbβ3 as a fibrin receptor, indicating that it may underlie the limited spreading in GPVI-deficient platelets.21 Thirdly, we show that fibrin binds selectively to monomeric GPVI, in contrast to collagen, which binds to dimeric GPVI. The interconversion of GPVI between monomeric and dimeric forms will therefore govern the interaction with collagen and fibrin, with nonactivated platelets, which express predominately monomeric GPVI, binding selectively to fibrin.22,23 The polymeric nature of fibrin can support binding to several GPVI receptors and in this way lead to activation. Fourthly, we show that fibrin recognizes GPVI through its D-dimer region and that D-dimer blocks activation by collagen and fibrin in suspension, suggesting a shared motif or closely located site of interaction. Alternatively, D-dimer may inhibit the response to collagen by preventing formation of GPVI dimers. Fifthly, the observation that D-dimer has no effect on platelet activation by CRP indicates that collagen and fibrin may bind to distinct epitopes in GPVI and that it may be possible to develop agents that selectively block binding of 1 or the other ligand. Such agents are required to determine the role of GPVI in platelet activation by collagen and fibrin in hemostasis and thrombosis.
Cocrystallization studies will be crucial in determining the degree to which the binding sites for fibrin and collagen overlap. Knowledge of the structure of GPVI, along with mutagenesis studies, predicts that CRP binds to a groove in the D1-domain of the immunoglobulin receptor.25 The spacing and orientation of the groove match the spacing and orientation of triple helices in collagen, suggesting a plausible mechanism by which dimeric GPVI recognizes collagen. There are, however, a number of unresolved questions regarding the interaction, because mutations at the distal end of D1 (far from the proposed binding groove) diminish binding of CRP or collagen.26,27 Furthermore, mutagenesis and NMR mapping studies show an analogous distal site of D1 for collagen in LAIR-1, a related immunoglobulin collagen receptor.28 The site of interaction of fibrin with GPVI is not yet known.
D-dimer levels are elevated in patients who have experienced trauma, cancer, or thromboembolism.29 The peak concentrations are in the order of 50 μg/mL,30-34 which is sufficient to induce only weak inhibition of aggregation and secretion to low concentrations of collagen. This inhibitory effect is readily overcome by moderate increases in the collagen concentration. Thus, the physiological and pathological significance of D-dimer binding to GPVI in patients is likely to be minimal.
We have previously reported that the generation of an occlusive thrombus in an FeCl3 injury model is blocked in GPVI-deficient mice, whereas the onset of thrombosis, where collagen is exposed, is not altered.3 This suggests that the binding of fibrin to GPVI plays a critical role in formation of an occlusive thrombosis. The development of an inhibitor that selectively blocks activation by fibrin but not collagen is required to confirm this. Revacept, a recombinant dimer form of GPVI that inhibits thrombosis but not hemostasis in mouse models is in a phase II clinical trial for prevention of ischemic complications in patients with symptomatic carotid artery stenosis.16 As a dimer of GPVI, Revacept blocks the interaction of GPVI with collagen but not with fibrin.
Mice and patients deficient in GPVI have a mild bleeding diathesis. The 2 GPVI-deficient patients in this study are from unrelated families in Chile and are homozygous for the same mutation. Although both index cases were discovered because of a bleeding diathesis, their bleeding is mild, and it is unclear whether the bleeding is due to a coinherited or acquired defect. The discovery of additional patients deficient in GPVI is required to provide a more complete understanding of the role of the glycoprotein receptor in hemostasis and thrombosis.
In summary, the present study confirms GPVI as the major signaling receptor for fibrin in human platelets and demonstrates that binding occurs selectively to monomeric GPVI and through the D-dimer region of fibrin. This suggests that it may be possible to develop inhibitors that selectively block platelet activation by fibrin. Such inhibitors can be used to establish the contribution of GPVI activation by fibrin to thrombosis and may represent a new class of antiplatelet agent with a minimal effect on hemostasis given the mild bleeding diathesis of GPVI-deficient patients.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the British Heart Foundation (RG/13/18/30563); S.P.W. holds a British Heart Foundation (BHF) Chair (CH03/003); A.T.H. holds a BHF Studentship (FS/15/71/31677); and AdvanceCOR provided Revacept for experimental use.
Authorship
Contribution: M.-B.O. designed and performed research, analyzed and interpreted data, made the figures, and wrote the manuscript; A.T.H., C.W., C.N.W., and S.K.W. performed experiments and analyzed and interpreted data; A.K.B., J.L.C.M., and A.B.H. contributed reagents, designed research, and edited the manuscript; A.B. contributed essential reagents; H.P. and R.A.S.A. designed research, interpreted data, and edited the manuscript; X.S. and D.M. provided essential tools, collected data, and edited the manuscript; and S.P.W. designed and supervised research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steve P. Watson, Institute of Cardiovascular Sciences, IBR Building, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: s.p.watson@bham.ac.uk.