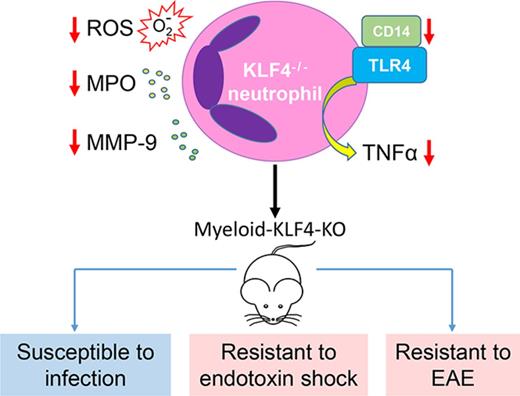
Key Points
KLF4 deficiency impairs neutrophil function in vitro and in vivo.
This is the first demonstration that KLF4 plays a crucial role in neutrophils.
Abstract
Neutrophils are the most abundant white blood cells in circulation and are key components of the innate immune response. Clinical and experimental studies support an important role for the neutrophils in a broad spectrum of acute and chronic inflammatory conditions. However, our understanding of nodal points that control neutrophil activation remains incompletely understood. Over the past decade, studies have linked members of the Kruppel-like family of transcription factors (KLFs) to myeloid cell differentiation and function. Here we show that KLF4 is a critical transcriptional regulator of neutrophil biology. KLF4-deficient neutrophils exhibited impaired responses to inflammatory stimulation ex vivo, including reduced production of cytokines and reactive oxygen species, impaired degranulation, and impaired bacterial killing and clearance. Consequently, mice bearing myeloid-specific conditional KLF4 deficiency (K4-cKO) exhibited enhanced susceptibility to bacterial infection but resistance to lipopolysaccharide-induced septic shock and experimental autoimmune encephalomyelitis. Finally, mechanistic studies revealed that the defects in KLF4-deficient neutrophils likely resulted from the defective Toll-like receptor 4–NF-κB signaling. Collectively, these findings identify KLF4 as a novel transcriptional regulator of neutrophil activation.
Introduction
Neutrophils are key components of the innate immunity and important regulators of the adaptive immunity.1-5 Patients with congenital neutrophil deficiencies or neutropenia secondary to chemotherapy and other diseases suffer from life-threatening infections, underscoring the importance of neutrophils in health.6-8 However, the transcriptional mechanisms that regulate neutrophil biology remain incompletely understood.
As the front line of host defense against pathogen attack, neutrophils are equipped with an arsenal of antimicrobial agents that also can be destructive to host tissue. Therefore, their deployment must be executed with exquisite precision and timing, at locations where they are both contained and effective.1 A classic infection disease is sepsis in which neutrophils play diverse roles.5 At early stage or nonsevere sepsis, neutrophils are attracted to and activated at the infection sites where they kill the microbes and control infection. Thus, efficient neutrophil function would benefit the host with better pathogen clearance leading to recovery. However, at the late stage of severe sepsis, uncontrolled infection leads to systemic inflammation and activation of neutrophils in the blood vessels, resulting in systemic inflammatory response syndrome, multiple organ dysfunction syndrome, and high mortality. In this latter setting, reduced neutrophil activation could be beneficial.
Neutrophils also mediate chronic inflammation in which complicated interplays between neutrophils and other immune cells are important to disease pathogenesis. Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS), characterized as demyelination, inflammation, and neurodegeneration of the spinal cord and brain.9 Experimental autoimmune encephalomyelitis (EAE) is a mouse model of MS. Recent studies in both MS patients and EAE mice suggest a critical role for neutrophils in disease pathogenesis.10,11 Neutrophils in the blood of MS patients exhibit a primed phenotype, and both neutrophil number and biomarkers of neutrophil activity increase during relapses.11 In EAE, neutrophils comprise a significant percentage of CNS-infiltrating leukocytes prior to disease onset and relapse, and disease was ameliorated when neutrophils were depleted prior to, but not after, disease onset or relapse, suggesting important neutrophil function during the initial formation of MS lesions.12 It has been reported that neutrophils may play a role in mediating blood-brain barrier breakdown.13 In addition, neutrophils isolated from the CNS at onset of EAE produce tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), IL-12, IL-1β, and interferon γ, cytokines important to the maturation of antigen-presenting cells and activation of adaptive immune system.12 Therefore, neutrophils may influence MS and EAE at multiple levels.
Over the past decade, studies have linked members of the Kruppel-like family of transcription factors (KLFs) to immune cell function.14 In particular, our group and others identified KLF4 as an essential transcriptional regulator of myeloid cells, including monocytes, macrophages, and dendritic cells (DCs).15-18 However, its role in neutrophils remains completely unknown. Here we show multiple lines of evidence supporting that KLF4 is requisite for neutrophil biology.
Methods
Animals
Myeloid-specific Klf-4-knockout (K4-cKO) mice were generated by breeding Klf4fl/fl mice with Lyz2cre/cre mice as described previously.17 Lyz2cre/cre (Cre) mice were used as control mice. All studies were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University.
Bone marrow neutrophil isolation and stimulation
Bone marrow cells were flushed and dispersed to single cell suspension with phosphate-buffered saline (PBS; Ca2+/Mg2+ free). After red blood cell lysis in 20 mL 0.2% NaCl for 20 seconds and neutralized in 20 mL 1.8% NaCl, cells were pelleted down and subjected to gradient centrifugation in 6 mL 1119/1077 Histopaque (Sigma). Neutrophils were collected from the interface and used for in vitro studies. Neutrophils were stimulated with either lipopolysaccharides (LPS) (50 ng/mL) or Escherichia coli (10 multiplicity of infection [MOI]) for 30 minutes. Matrix metallopeptidase 9 (MMP-9) and myeloperoxidase (MPO) in serum and condition medium were measured by mouse MPO enzyme-linked immunosorbent assay (ELISA) Kit (Thermo Fisher, EMMPO) and mouse pro-MMP-9 ELISA Kit (Thermo Fisher, EMMMP9).
Measurement of ROS production
Bone marrow neutrophils from gradient isolation were stimulated with 100 μM N-Formyl-Met-Leu-Phe (f-MLF) (Sigma) in 12-well plate for reactive oxygen species (ROS) production using Cytochrome C Reductase (reduced NAD phosphate [NADPH]) Assay Kit (Sigma).19 Briefly, 5 × 105 neutrophils were added to each well with f-MLF or without f-MLF (as background), and NADPH was added to each well. Positive control was rabbit liver lysate, and a reagent-only well was used as blank control. The kinetic reading of optical density at 550 nm (OD550) was performed immediately after adding NADPH, and OD550 (fMLF-treated well − vehicle-treated well) was shown.
Caspase 3 activation assays
Caspase 3 activation was determined by detecting its cleaved fragments by western blotting.20 Pro-caspase 3 (35 kD) is cleaved into 19/17-kD fragments upon activation. Both pro-caspase 3 and activated caspase 3 can be detected by an anti-caspase 3 antibody (Cell Signaling). Caspase 3 protease activity was determined using the fluorogenic substrate acetyl Asp-Glu-Val-Asp 7-amido-4-methylcoumarin (Ac-DEVD-AMC, BD Biosciences). Enzyme-catalyzed release of fluorescent 7-amino-4-methylcoumarin was measured with the excitation and emission wavelengths of 360 nm and 460 nm, respectively. Fluorescence readings were normalized to protein concentration to correct loading difference.
RNA isolation and qPCR
Total RNA from cells was isolated using TRIzol reagent (Invitrogen). First-strand complementary DNA was synthesized and subjected to quantitative polymerase chain reaction (qPCR) with either SYBR green or Roche universal probe reagents (Universal ProbeLibrary, Roche Applied Science) on a StepOnePlus Real-Time PCR System (Applied Biosystems). Gene expression was normalized to GAPDH, 18SRNA, or β-actin using the ΔΔCt method. Toll-like receptor (TLR) pathway qPCR array was performed with Qiagen RT2 Profiler PCR Array (mouse TLR signaling pathway).
Phagocytosis, bacteria killing, and colony formation assay
To determine phagocytosis function, fluorescein isothiocyanate (FITC)–labeled E coli (K-12 strain) BioParticles (Molecular Probes: E2861) were incubated with neutrophils for 30 minutes at MOI 10:1.17 Cells were then washed with PBS for 3 times and fixed with formalin for 5 minutes at room temperature before subjected to flow cytometry (fluorescence-activated cell sorting). Phagocytosis ability was determined by intracellular FITC fluorescence. Neutrophils without incubation with BioParticles was used as negative control. To determine bacterial killing, live E coli (ATCC-25922 strain) were incubated with neutrophils (MOI = 2) at 37°C for 3 hours. After incubation, cell culture was taken for colony formation assay with serial dilution.17 Medium and samples from in vivo sepsis models were diluted by a ratio of 10 and cultured on Luria broth–agar plates. Colony numbers were counted after 16-hour incubation at 37°C and calculated to get colony formation unit in original samples.
Sepsis models
Cre and K4-cKO mice were intraperitoneally injected with E coli (ATCC-25922 strain) for an early sepsis model and LPS for a septic shock model.21,22 To introduce bacterial infection, we injected 20 million E coli (ATCC-25922 strain) into the peritoneal cavity. Animals were monitored for survival curve. A second set of animals were euthanized at 14-hour postinjection to collect blood and peritoneal fluid for colony formation assay. Serum samples were prepared at 4-hour postinjection for cytokine levels by ELISA. To introduce septic shock, animals were intraperitoneally injected with LPS (10 mg/kg) and monitored for 5 days. All animals used for sepsis models were between 8 and 12 weeks old.
Induction and evaluation of EAE
EAE model was established using a Hooke EAE Kit in C57BL/6 mice (Hooke Laboratories). Briefly, age-matched female mice older than 10 weeks were performed with subcutaneous injection at 2 different sites with myelin oligodendrocyte glycoprotein peptide fragment (33-55) (MOG33-55) peptide emulsified in Freund’s complete adjuvant, along with 2 doses of pertussis toxin intraperitoneal injection.19 EAE mice were weighed and scored for neurological deficits daily as follows: 0, no clinical signs; 0.5, partially limp tail; 1, paralyzed tail; 1.5, hind limb paresis, uncoordinated movement; 2, 1 hind limb paralyzed, does not respond to pinch; 2.5, both hind limbs paralyzed, neither responds to pinch; 3, hind limbs paralyzed, weakness in forelimbs; 3.5, hind limbs paralyzed, 1 forelimb paralyzed; 4, hind limbs paralyzed, both forelimbs paralyzed; 4.5, moribund; 5, death. Any mouse that scored a 4.5 was immediately euthanized and was scored 5 afterward. If a mouse scored 4, it was given saline injection as supportive care and scored again 24 hours later. If it was still at a 4, the mouse was euthanized and scored 5 afterward. Paper bedding and gel food on the floor were given according to the requirement of the Animal Resource Center of Case Western Reserve University.
Histology and immunofluorescence staining
Mice were perfused with PBS (containing 2 mM EDTA) and 4% paraformaldehyde. Spinal cords were removed and postfixed overnight in 4% paraformaldehyde. After being dehydrated in 30% sucrose, samples were embedded in tissue freezing medium (Tissue Tek) and stored at −80°C until sectioning. Spinal cord sections were stained with 4′,6-diamidino-2-phenylindole (Vector) and Fluoromyelin green fluorescent myelin stain (ThermoFisher) following the manufacturer’s instruction. Hematoxylin and eosin staining was performed following standard protocol as previously described.17
Flow cytometry
For flow cytometry assay, whole spinal cord was isolated and smashed in PBS (Ca2+/Mg2+ free) on EAE day 14 and stained with anti-CD45-Percp (clone 30-F11), anti-CD11b-Alexa Fluor488 (clone M1/70), anti-CD11c-PE (clone N418), anti-Ly6G (clone 1A8)-Alexa Fluor594, or anti-CD3-Alexa Fluor488 (clone 17A2), anti-CD4-FITC (clone GK1.5), anti-CD19-PE (BD Biosciences). For peritoneal immune cells analysis, peritoneal cells were washed with 5 mL PBS 6 hours/12 hours after injection of 4% Thiglycollate. Total cells were stained with anti-Gr-1-FITC (Clone RB6-8C5) and anti-CD115-PE (Clone T38-320) for further analysis. The stained cells were analyzed on a BD LSRII flow cytometer. Data were analyzed using FlowJo software (v10.1).
Statistics
To analyze the difference between 2 groups, 2-tailed Student t test was used. With >2 groups, 1-way analysis of variance (ANOVA) was applied followed by Bonferroni post hoc test. Significance between survival curves was determined by log-rank test. Clinical score and body weight curves were tested by 2-way ANOVA. P value <.05 was considered significant. Data were shown as mean ± standard error of the mean.
Results
KLF4 deficiency impairs neutrophil function ex vivo
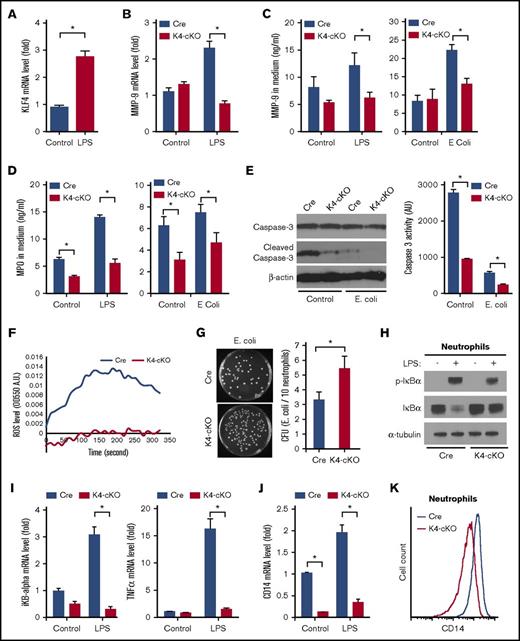
As a first step to determine if KLF4 may regulate neutrophil biology, we assessed expression of this factor following inflammatory stimulation (eg, LPS) and found that LPS induced KLF4 expression (Figure 1A). To study KLF4’s role in neutrophil biology, we used a myeloid-specific KLF4-deficient mouse line.17 The deletion of neutrophil KLF4 was confirmed by quantitative real-time PCR (qPCR), and KLF4 deficiency did not affect the circulating neutrophil numbers or their migration ability (supplemental Figure 1). We next focused on the neutrophil granules, the characteristic organelle of neutrophils. Formed during neutrophil maturation, granules contain a multitude of antimicrobial and cytotoxic substances pivotal to neutrophil function, such as MMP-9 and MPO, which are released upon activation through a process termed degranulation.4 As expected, LPS induced MMP-9 messenger RNA (mRNA) in Cre neutrophils. However, the induction was blunted in KLF4-deficient neutrophils (Figure 1B). Further, KLF4-deficient neutrophils exhibited reduced secretion of MMP-9 and MPO upon stimulation (Figure 1C-D). As the granule protein (ie, MMP-9) level was already reduced in KLF4-deficient neutrophils (Figure 1B), the reduced secretion is likely because of defects in the granule content rather than degranulation process. A unique property of neutrophils is constitutive apoptosis that is critical to neutrophil-mediated inflammation progression and resolution23,24 ; therefore, we sought to determine if KLF4-deficiency affects neutrophil apoptosis. As shown in Figure 1E, when cultured in vitro neutrophils spontaneously activated caspase-3, a hallmark of apoptosis. Incubating cells with E coli significantly blocked such activation because of the prosurvival role of LPS. However, the activation of caspase-3 was significantly blunted in KLF4-deficient neutrophils, indicating disturbed apoptosis. Another aspect of neutrophil activation is ROS generation from the NADPH oxidase complex. As shown in Figure 1F, KLF4-deficient neutrophils generated significantly less ROS upon fMLF stimulation. Finally, we found that, despite normal phagocytic ability (supplemental Figure 2), KLF4-deficient neutrophils failed to effectively kill the bacteria (Figure 1G). Collectively, these data demonstrate that KLF4 is requisite for inflammatory responses in neutrophils.
KLF4-deficient neutrophils exhibited impaired function ex vivo. (A) Wild-type bone marrow neutrophils (BMNs) showed increased KLF4 expression after 30-minute LPS stimulation. (B) LPS-induced MMP-9 mRNA expression was blunted in KLF4-deficient BMNs. (C-D) The secreted MMP-9 and MPO in the condition medium from BMNs stimulated with LPS or E coli were measured by ELISA. (E) Caspase-3 activation in neutrophils assessed by western blot and fluorescent enzymatic activity assay. BMNs were incubated in culture medium without (Control) or with E coli (E. coli) for 2 hours at 37°C. (F) Extracellular ROS generation from Cre and K4-cKO BMNs in response to fMLF was determined by kinetic absorbance OD550. (G) KLF4-deficient neutrophils were defective in bacterial killing assay. The images on the left are 1× scans of 10-cm dishes. (H) LPS-induced iKBα phosphorylation and degradation. (I) LPS-induced gene expression of IκBα and TNF-α was attenuated in KLF4-deficient neutrophils. (J) CD14 mRNA expression in neutrophils. (K) CD14 protein expression on neutrophil cell surface as measured by flow cytometry. All gene expression levels were determined by qPCR. n = 3 to 5 in each group. *P < .05.
KLF4-deficient neutrophils exhibited impaired function ex vivo. (A) Wild-type bone marrow neutrophils (BMNs) showed increased KLF4 expression after 30-minute LPS stimulation. (B) LPS-induced MMP-9 mRNA expression was blunted in KLF4-deficient BMNs. (C-D) The secreted MMP-9 and MPO in the condition medium from BMNs stimulated with LPS or E coli were measured by ELISA. (E) Caspase-3 activation in neutrophils assessed by western blot and fluorescent enzymatic activity assay. BMNs were incubated in culture medium without (Control) or with E coli (E. coli) for 2 hours at 37°C. (F) Extracellular ROS generation from Cre and K4-cKO BMNs in response to fMLF was determined by kinetic absorbance OD550. (G) KLF4-deficient neutrophils were defective in bacterial killing assay. The images on the left are 1× scans of 10-cm dishes. (H) LPS-induced iKBα phosphorylation and degradation. (I) LPS-induced gene expression of IκBα and TNF-α was attenuated in KLF4-deficient neutrophils. (J) CD14 mRNA expression in neutrophils. (K) CD14 protein expression on neutrophil cell surface as measured by flow cytometry. All gene expression levels were determined by qPCR. n = 3 to 5 in each group. *P < .05.
KLF4 deficiency in neutrophils impaired TLR4 signaling
Because KLF4 deficiency significantly diminished multiple hallmarks of LPS-induced neutrophil activation, we hypothesized that the defects should be upstream signal transducers rather than specific downstream effecters. LPS (and E coli) signals through the TLR4 pathway. Through a series of intracellular adaptors, TLR4 activates the IκBα kinase complex, which phosphorylates IκBα leading to its degradation and subsequent liberation of NF-κB. Compared with Cre group, LPS-induced phosphorylation and degradation of IκBα was markedly attenuated in KLF4-deficient neutrophils (Figure 1H). As a result, transcription of IκBα and TNF-α, direct targets of NF-κB,25 were blunted in KLF4-deficient neutrophils (Figure 1I). These data demonstrated that KLF4 deficiency impaired the TLR4–NF-κB signaling in neutrophils. We then examined the expression of the TLR4 receptor complex and found that the expression of CD14 mRNA and surface protein was reduced in KLF4-deficient neutrophils at baseline and after LPS activation (Figure 1J-K; supplemental Figure 3A). In contrast, TLR4 and other adaptors were not affected by KLF4 deficiency (supplemental Figure 3B-D). To obtain a broader understanding of KLF4’s role in neutrophils, we profiled the TLR pathway by a Qiagen RT2 Profiler qPCR Array. At baseline, CD14 and c-Jun were reduced whereas 5 other genes were increased in KLF4-deficient neutrophils (supplemental Figure 4A). Upon LPS stimulation, we observed 17 genes that were reduced in KLF4-deficient neutrophils (supplemental Figure 4B). These data demonstrated that KLF4 deficiency in neutrophils impaired TLR4 signaling partly through downregulation of CD14. Of note, such a CD14-mediated TLR4 pathway may only control a small set of genes in neutrophils, and potentially, KLF4 deficiency could affect more pathways that are independent of CD14 or TLR4. Further investigation is warranted to uncover the full spectrum of KLF4-regulated genes in neutrophils.
Myeloid KLF4 deficiency renders animals susceptible to bacterial infection but resistant to septic shock
In case of acute infection, neutrophils are the major phagocytes for pathogen clearance.5 The impaired bacterial killing function observed from KLF4-deficient neutrophils ex vivo suggests the K4-cKO animals may be susceptible to infection. To test this hypothesis, we introduced bacterial infection in Cre and K4-cKO mice by intraperitoneal injection of E coli. The K4-cKO group exhibited strikingly high mortality compared with the Cre group (Figure 2A). Bacterial burden was also higher in K4-cKO mice, indicating uncontrolled infection and development of bacteremia (Figure 2B). In addition, the circulating levels of TNF-α, MPO, and MMP-9 were significantly lower in K4-cKO mice (Figure 2C; supplemental Figure 5). These data demonstrated that KLF4-deficient neutrophils failed to mount effective host defense.
K4-cKO mice are susceptible to bacterial infection but resistant to EAE. (A) K4-cKO mice exhibited higher mortality rate than the Cre group after E coli intraperitoneal injection. n = 12 in each group. (B) Bacteria burden in the blood and peritoneal fluid harvested 14 hours postinjection. (C) TNF-α levels in serum at 4 hours postinjection. K4-cKO mice showed delayed occurrence and less disease severity after EAE induction, demonstrated by clinical score (D) and weight loss (E). n = 14 in each group. Difference was determined by 2-way ANOVA. For panel D, interaction P < .0001. For panel E, interaction P < .0021. For both panels D and E: time, P < .0001; genotype, P < .0001. (F) Spinal cord from lumbar 1 to 3 were taken from Cre and K4-cKO mice at disease peak (day 28) of EAE. Fluorescent myelin staining showed less demyelination in K4-cKO group. Hematoxylin and eosin (H&E) staining confirmed the cell infiltration in the foci. Representative data from 3 independent experiments. Original magnification ×200 (taken with a Nikon microscope; 20× objective, 10× eyepiece lens). (G) Immune cell infiltration in spinal cord as measured by flow cytometry at initiation of EAE (day 14). Neutrophils (CD45+CD11b+CD11c−Ly6G+), Th cells (CD3+CD4+), B cells (CD19+CD3−), and DCs (CD45+CD11b+CD11c+Ly6G−) were shown. n = 13 in each group. P < .05 was considered significant.
K4-cKO mice are susceptible to bacterial infection but resistant to EAE. (A) K4-cKO mice exhibited higher mortality rate than the Cre group after E coli intraperitoneal injection. n = 12 in each group. (B) Bacteria burden in the blood and peritoneal fluid harvested 14 hours postinjection. (C) TNF-α levels in serum at 4 hours postinjection. K4-cKO mice showed delayed occurrence and less disease severity after EAE induction, demonstrated by clinical score (D) and weight loss (E). n = 14 in each group. Difference was determined by 2-way ANOVA. For panel D, interaction P < .0001. For panel E, interaction P < .0021. For both panels D and E: time, P < .0001; genotype, P < .0001. (F) Spinal cord from lumbar 1 to 3 were taken from Cre and K4-cKO mice at disease peak (day 28) of EAE. Fluorescent myelin staining showed less demyelination in K4-cKO group. Hematoxylin and eosin (H&E) staining confirmed the cell infiltration in the foci. Representative data from 3 independent experiments. Original magnification ×200 (taken with a Nikon microscope; 20× objective, 10× eyepiece lens). (G) Immune cell infiltration in spinal cord as measured by flow cytometry at initiation of EAE (day 14). Neutrophils (CD45+CD11b+CD11c−Ly6G+), Th cells (CD3+CD4+), B cells (CD19+CD3−), and DCs (CD45+CD11b+CD11c+Ly6G−) were shown. n = 13 in each group. P < .05 was considered significant.
Although strong neutrophil function can be protective in the context of bacterial killing, excessive systemic inflammation can lead to septic shock and death.5 We hypothesized that, those K4-cKO mice, which are susceptible to bacterial infection and exhibited a mild inflammation phenotype, might be more resistant to direct challenge with endotoxin. As shown in supplemental Figure 6, LPS-induced mortality rate is significantly reduced in the K4-cKO group compared with Cre (20% vs 70% mortality by 120 hours, P < .04 by log-rank test) coupled with lower circulating levels of MPO and TNF-α. Collectively, these studies demonstrated that myeloid KLF4 deficiency impaired neutrophil activation in response to acute infection rendering animals susceptible to bacterial infection but resistant to LPS-induced septic shock.
Myeloid KLF4 deficiency protects animals from EAE
Neutrophils are not only key to host defense during acute infection, but also important to chronic inflammation, in which complicated interplay between neutrophils and other immune cells determines disease pathogenesis and progression.1 We sought to determine the role of KLF4-deficient neutrophils in regulating chronic inflammation using the EAE model. EAE results in inflammation and demyelination in the CNS mimicking MS in humans.9 Accumulating clinical and experimental evidence implicates neutrophils in the pathogenesis of MS and EAE.11-13,26,27
Upon immunization, Cre mice developed EAE with disease onset at ∼7 to 14 days and peak at ∼21 to 28 days (Figure 2D). In contrast, K4-cKO mice showed a delayed onset and significant reduction in severity of EAE despite 100% disease incidence (Figure 2D; supplemental 7A). Body weight loss was consistent with clinical score (Figure 2E). Notably, 2 out of 14 (∼14%) Cre mice died during 4 weeks of EAE, but no mortality was observed in the K4-cKO group (supplemental Figure 7B). Consistently, there was significantly decreased demyelination and immune cell infiltration in the spinal cords of the K4-cKO group (Figure 2F). Finally, at the disease onset phase, we found a significant reduction in CNS infiltrating neutrophils, T helper (Th) cells, B cells, and DCs in the spinal cords of K4-cKO group (Figure 2G). These data were consistent with delayed onset and reduced severity of EAE in K4-cKO mice and further indicated that KLF4-deficient neutrophils were defective in mediating chronic inflammation that deteriorates EAE.
Discussion
Studies over the past decade support a key role for the KLF family in general, and KLF4 in particular, in the context of myeloid cell biology. Foundational work by Feinberg et al revealed an absolute requirement for KLF4 in the development of monocytes.15,16 Further, at the mature macrophage stage, KLF4 is required for M2 polarization and inhibitory to M1 polarization,17 the net effect being that KLF4-deficient macrophages are more proinflammatory.17 More recently, in the context of DC biology, KLF4 deficiency was found to significantly attenuate the development of the interferon regulatory factor 4–expression convectional DCs, leading to impaired Th2 immunity.18 The current findings that include cell-based and in vivo studies strongly suggest that KLF4 deficiency attenuates neutrophil proinflammatory activation. The fact that KLF4 appears to have differential effects on myeloid cell development and activation suggests that the underlying transcriptional mechanisms mediated by KLF4 are unique in each cell type because of specific cellular context. Although additional studies will be needed, the current work focused on neutrophil biology adds critical information to a growing body of literature underscoring the importance of KLF4 in myeloid biology and innate immunity. Further, these findings suggest that KLF4 may be a viable target to treat neutrophil-mediated diseases.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by an American Heart Association National Scientist Development Grant (12SDG12070077) (X.L.); an American Heart Association Established Investigator Award (M.K.J.); the National Heart, Lung, and Blood Institute, National Institutes of Health (grants HL086548, HL123098, and HL119195 [M.K.J.]; K08HL123551 [L.Z.]); and the Chinese Key Program of Applied Basic Research Foundation of Tianjin (14JCZDJC33000) (Y.S.). This work was also generously supported by Tom F. Peterson (M.K.J.).
Authorship
Contribution: Y.S. and X.L. designed the study; Y.S., X.L., H.H., and P.S. conducted the experiments; L.Z., S.L., and L.N. assisted with experiments; Y.S., X.L., and M.K.J. analyzed the data and wrote the manuscript; and all authors read and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xudong Liao, Iris S. Bert L. Wolstein Research Building, 2103 Cornell Rd, Room 4528a, Cleveland, OH 44106-7290; e-mail: xudong.liao@case.edu; and Mukesh K. Jain, Iris S. Bert L. Wolstein Research Building, 2103 Cornell Rd, Room 4537, Cleveland, OH 44106-7290; e-mail: mukesh.jain2@case.edu.