Key Points
Replication exhausted human MSCs display attenuated immunosuppressive properties partly because of defective kynurenine production.
IFNγ prelicensing can rescue replicative senescence-associated immune defects of human bone marrow–derived MSCs.
Abstract
Industrial-scale expansion of mesenchymal stromal cells (MSCs) is often used in clinical trials, and the effect of replicative senescence on MSC functionality is of mechanistic interest. Senescent MSCs exhibit cell-cycle arrest, cellular hypertrophy, and express the senescent marker β-galactosidase. Although both fit and senescent MSCs display intact lung-homing properties in vivo, senescent MSCs acquire a significant defect in inhibiting T-cell proliferation and cytokine secretion in vitro. IFNγ does not upregulate HLA-DR on senescent MSCs, whereas its silencing did not reverse fit MSCs’ immunosuppressive properties. Secretome analysis of MSC and activated peripheral blood mononuclear cell coculture demonstrate that senescent MSCs are significantly defective in up (vascular endothelial growth factor [VEGF], granulocyte colony-stimulating factor [GCSF], CXCL10, CCL2) or down (IL-1ra, IFNγ, IL-2r, CCL4, tumor necrosis factor-α, IL-5) regulating cytokines/chemokines. Unlike indoleamine 2,3 dioxygenase (IDO), silencing of CXCL9, CXCL10, CXCL11, GCSF, CCL2, and exogenous addition of VEGF, fibroblast growth factor-basic do not modulate MSCs’ immunosuppressive properties. Kynurenine levels were downregulated in senescent MSC cocultures compared with fit MSC counterparts, and exogenous addition of kynurenine inhibits T-cell proliferation in the presence of senescent MSCs. IFNγ prelicensing activated several immunomodulatory genes including IDO in fit and senescent MSCs at comparable levels and significantly enhanced senescent MSCs’ immunosuppressive effect on T-cell proliferation. Our results define immune functional defects acquired by senescent MSCs, which are reversible by IFNγ prelicensing.
Introduction
Bone marrow–derived mesenchymal stromal cells (MSCs) are under clinical investigation to test their use in adoptive immunosuppressive cell therapy for auto- and allo-immune disorders.1 Although early-phase clinical trials demonstrated that MSC infusion is safe, variations in efficacy is an ongoing issue.2,3 Defining the functionality of in vitro expanded MSCs will inform not only the surrogate measure of in vivo potency but also the cause and source of clinical inconsistency arising from infused cell preparations. In bone marrow, native endogenous MSCs are present at a low frequency (0.01%-0.001% of nucleated cells) and their robust in vitro proliferative potential under standard cell culture conditions make them as an attractive candidate for cell therapy studies.4 However, the extent of in vitro culture expansion of MSCs can vary dramatically between low passage manufacture of MSCs, as is typical of many academic-sponsored trials relative to large-scale expansion of MSC-like cells typical of industry-sponsored studies. Considering that an average cell dose for most clinical studies is ∼1 to 2 million MSCs per kilogram of body weight, most academic cell processing centers will typically manufacture 5 to 10 doses from an allogeneic donor, whereas industrial-scale expansion of MSC-like cells can be as high as 10 000 to 1 000 000 doses per donor. Considering that advanced-phase clinical trials using industrial-scale expanded MSCs have not met primary efficacy end points, it is worthwhile, considering the possibility that prolonged culture expansion and companion replicative senescence may affect the potency of MSC-like cells when compared with low-passage products. In support of this hypothesis, it was observed retrospectively that late passage MSCs were clinically shown to be less effective in ameliorating graft-versus-host disease than early passage cells.3,5
Prolonged culture expansion leads to the replicative exhaustion/senescence of MSCs like any other primary somatic cells in culture as defined by the “Hayflick limit.”6 Cellular senescence is an antitumorigenesis process that replicating cells use in conjunction with cell death programs to prevent malignant transformation. In vitro expanded human MSCs neither exhibit chromosomal aberrations nor undergo tumorigenic transformation while they acquire replicative senescence as part of extensive culture expansion procedures.7-11 Replicative senescence on MSCs has been shown to be associated with reduced telomere length, increased P16INK4A expression, alterations in differentiation potential, global gene expression profile, microRNA expression, DNA methylation pattern, and reactive oxygen species accumulation.12-18 Thus, replicative senescence induces alterations in MSC functionality, but the effect of these alterations on MSCs’ interaction with immune responders and responsiveness to inflammatory cues is largely unknown. An important unanswered question is: Are MSCs’ interactions with immune responders different based on their replicative history and relative immunologic fitness? In the present study, we have characterized early- and late-passage MSCs for their comparative and distinct immunomodulatory properties and suppressive mechanisms through phenotype, transcriptome, and immune functional analysis. In addition, to improve MSCs’ function, we investigated whether cytokine prelicensing rescues senescence-induced defects in MSC immunosuppressive functions.
Materials and methods
Bone marrow MSC isolation and passage
MSCs were obtained from the bone marrow of consenting healthy individuals compliant with Emory University Institutional Review Board guidelines. Harvested bone marrow was separated by Ficoll density gradient and plated on Minimum Essential Medium Eagle α modification (α-MEM) containing 15% to 20% fetal calf serum and 100 U/mL penicillin/streptomycin (200 000 cell/cm2). Three days post–flask seeding, nonadherent cells were removed and adhered MSCs were allowed to expand for an additional 7 days (passage 0). Subsequently, MSCs were passaged weekly and replated at a seeding density of 1000 cells/cm2. During early passage, cells were split in 80% confluence or at every seventh day. Doubling time was calculated using a formula as described previously.19 Fit MSCs are derived from passage 2 to 4 (doubling time 1.6 ± 0.2 days) and senescent MSCs are derived from passages 10 to 15 (doubling time 10.9 ± 3.1 days).
Assays to determine replicative senescence
Fit or senescent MSCs were seeded on to 96-well plates at a density of 5000 cells/cm2. Cells were cultured with either medium containing human platelet lysate or fetal calf serum. MTT assays were performed as described previously.20 Senescence-associated β-galactosidase activity was determined using X-gal staining as described previously.21 Cell-cycle analysis was performed in flow cytometer using standard propidium iodide staining in ice-cold methanol-fixed fit and senescent MSCs.
Phenotyping of MSCs by flow cytometry
Fit or Senescent MSCs (± IFN-γ activated) were subjected to flow cytometry analysis for the expression of HLA-ABC APC, HLADR PerCP, CD80 PE, CD86 PE, B7H1 PE, B7DC PE, CD105 PE, CD44 PE, CD73 PE, CD90 APC CD45 PE, CD119 PE, and appropriate isotype controls (BD Biosciences, San Jose, CA). Mean fluorescent intensity and histogram analysis for the marker expression was performed with Flow Jo software.
MSC and T-cell coculture
Coculture of MSCs and T cells has been described previously.22 Briefly, ± IFNγ-activated, fit (low passage), or senescent (high passage) MSCs were seeded onto 96-well plates and cocultured with carboxyfluorescein succinimidyl ester (CFSE)labeled or –nonlabeled random donor human PBMCs with indicated ratio. Dynabeads Human T-Activator CD3/CD28 (Life Technologies AS, Norway) was used to stimulate the T cells. For intracellular cytokine staining, Brefeldin A was added at the concentration of 10 μg/mL (Sigma-Aldrich, St. Louis, MO) for 12 to 14 hours, and intracellular flow cytometry staining was performed with BD Cytofix and Cytoperm procedure with the antibodies CD3APCCy7 and IFNγAPC. Ki67 proliferation assay was performed after 4 days according to manufacturer instructions (BD Biosciences).
Tracking of human MSCs in mice
Tracking of human MSCs in C57BL/B6 mice was performed as described previously.23 Fit or senescent MSCs (0.5 × 106 cells/mouse) were infused in to C57BL/B6 mice (8-10 weeks old; The Jackson Laboratories) through tail vein. Twenty-four hours post infusion, the mice were killed and the lungs were collected and total genomic DNA was extracted using a QIAamp DNA Mini Kit (QIAGEN). 100 ng of DNA were used for real-time polymerase chain reaction (PCR) in Applied Biosystems 7500 fast real-time PCR using RT2 SYBR Green ROX qPCR master mix and mouse RT2 qPCR (QIAGEN) and human gDNA primers (SA Biosciences).
siRNA knockdown on human MSCs
MSCs were seeded in 96-well plates at a concentration of 5000 cells per well one day before transfection with nontargeting control siRNA or HLA-DR, CXCL9, CXCL10, CXCL11, granulocyte colony-stimulating factor (GCSF), CCL2, kynureninase (KYN), kynurenine 3-monooxygenase (KMO), IDO SMART Pool siRNA (Dharmacon, Lafayette, CO). During transfection, the cells were conditioned with serum-free 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) containing α-MEM for 30 minutes. 2 μL of 100 uM–specific/control siRNA solution (A) or 3 μL Dharmafect 1 reagent (B) was added to 250 μL α-MEM–containing 10 mM HEPES. A and B were mixed and incubated at room temperature for 30 minutes. 50 μl of the siRNA transfection cocktail was added to each well. The cells were then incubated for 5 hours and the transfection medium was replaced with MSC culture medium. After 12 hours, MSCs were cultured with Dynabead-activated PBMCs on the next day of transfection.
Secretome analysis
Supernatants were collected from fit and senescent MSCs cocultured with activated PBMCs for 4 days and stored at −80 °C. Thawed supernatants were centrifuged at 1500 rpm for 3 minutes to eliminate cell debris and analyzed by magnetic bead–based multiplex luminex assays for cytokines, chemokines, and certain growth factors (supplemental Figures 1 and 3) (human cytokine 30-plex panel, Life Technologies) according to the manufacturer’s instructions using Luminex xMAP (multi-analyte profiling) technology. Kynurenine enzyme-linked immunosorbent assay was performed as described in the manufacturer’s instructions (US Biological Sciences).
Fluidigm nanoscale 48*48 PCR array
Quantitative reverse transcription (RT)-PCR was performed using Fluidigm 48 × 48 nanofluidic arrays.20 Briefly, ± IFNγ (20 ng/mL)-stimulated cDNA samples from fit and senescent MSCs were pre-amplified with 14-cycle PCR reaction for each sample with the combination of 100 ng cDNA with pooled primers as described by TaqMan Pre-Amp Mastermix (Fluidigm BioMark) manufacturer protocols. Two-thousand three-hundred-four parallel quantitative RT-PCR reactions were performed for each primer pair on each sample on a 48 × 48 array. Amplification was detected in Eva Green detection assay on a Biomark I machine based on standard Fluidigm protocols. PCR data were normalized and analyzed with SAS/JMP Genomics software.
IDO assays
Fit or senescent MSCs (± IFNγ-activated) were lysed and total RNA was extracted using an RNeasy plus mini kit (QIAGEN). Normalized RNA was used to convert cDNA using Quantitect reverse transcription kit (QIAGEN). Sybr green (Perfecta Sybr green fast mix, Quanta Biosciences) real-time PCR was performed with IDO primer pairs as described previously.22 IDO protein were detected using primary rabbit anti-human IDO1 (1:1,000; EMD Millipore Corporation, Billerica, MA) or rabbit anti-human β-actin (1:1000; Cell Signaling Technology, Inc, Danvers, MA), and secondary horseradish peroxide–coupled goat anti-rabbit IgG h+l (1:10 000; Bethyl Laboratories, Inc, Montgomery, TX). ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ) was used to detect immunoreactive blots. IDO activity was blocked using 1-methyl-DL-tryptophan (1 mM concentration) (Sigma-Aldrich) in MSC and T-cell coculture.
Statistical analysis
Data were analyzed with the GraphPad Prism 5.0 software. For the comparison of 2 groups, paired Student t test was applied. A 2-sided P value < .05 was considered statistically significant.
Results
Characteristics of senescent human MSCs
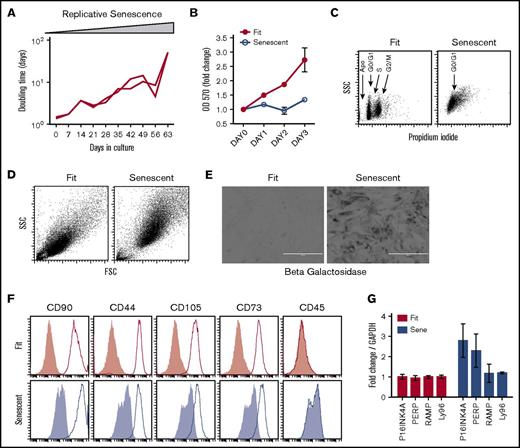
We passaged MSCs (1000 cells/cm2) derived from the bone marrow of healthy donors under standard cell culture conditions using fetal calf serum as a growth supplement. We observed that prolonged culture expansion increases the doubling time and induces replicative senescence of human MSCs (Figure 1A). Short-term growth kinetics using MTT assay demonstrated that senescent MSCs do not replicate efficiently compared with Fit MSCs (Figure 1B). Cell cycle analysis using propidium iodide staining demonstrated that senescent MSCs are in G0/G1 cycle (Figure 1C). Forward and side scatter analysis by flow cytometry demonstrated that senescent MSCs acquire a hypertrophic phenotype as evidenced by increase in cell size and granularity (Figure 1D). Detection of X-gal staining at pH 6.0 is specific for endogenous β-galactosidase activity within acidic lysosomal compartment and is considered as senescence-associated β-galactosidase activity.21,24 Our results show that senescent-associated β-galactosidase activity in replication exhausted MSCs (Figure 1E). The International Society for Cell Therapy (ISCT) has defined consensus minimal criteria for MSCs25 and found that there is no distinction in the phenotypical markers (CD90+CD44+CD105+CD73+CD45–) expressed by fit and senescent MSCs (Figure 1F). Senescent MSCs have been shown to express higher level of mRNA for genes such as P16INK4A, PERP, RAMP, and LY96,13,17 and we found that P16INK4A and PERP mRNA were upregulated (∼twofold) by senescent MSCs (Figure 1G).
Phenotypical characteristics of replicative senescent MSCs. (A) MSCs derived from the bone marrow of 2 healthy individuals were culture-passaged at the indicated time points. Doubling time was calculated based on the cell numbers at seeding and harvesting time points and duration of the culture. (B) MSCs from replicative fit and senescent phases were seeded at similar density in the 96-well plates. MTT assay was performed at the indicated time points to determine the growths of MSCs. Results are plotted as mean ± standard deviation (SD). (C) Cell-cycle analysis was performed on fit and senescent MSCs through propidium iodide staining and subjected to flow cytometry. (D) Size and granularity of fit and senescent MSCs were determined by forward and side scatter analysis by flow cytometry. (E) Fit and senescent MSCs were subjected to senescence-associated lysosomal β-galactosidase staining. Dark gray staining represents β-galactosidase staining. Scale bars represent 400 μm. (F) Replicative fit and senescent MSCs were subjected to staining for MSC markers as defined by the ISCT and acquired through flow cytometry. Open and gray histograms represent marker and isotype controls, respectively. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (G) Fold difference in the P16INK4A, PERP, LAMP, and LY96 mRNA of fit and senescent MSCs, relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were determined in quantitative sybr green real-time PCR. Cumulative is shown from 2 independent donors.
Phenotypical characteristics of replicative senescent MSCs. (A) MSCs derived from the bone marrow of 2 healthy individuals were culture-passaged at the indicated time points. Doubling time was calculated based on the cell numbers at seeding and harvesting time points and duration of the culture. (B) MSCs from replicative fit and senescent phases were seeded at similar density in the 96-well plates. MTT assay was performed at the indicated time points to determine the growths of MSCs. Results are plotted as mean ± standard deviation (SD). (C) Cell-cycle analysis was performed on fit and senescent MSCs through propidium iodide staining and subjected to flow cytometry. (D) Size and granularity of fit and senescent MSCs were determined by forward and side scatter analysis by flow cytometry. (E) Fit and senescent MSCs were subjected to senescence-associated lysosomal β-galactosidase staining. Dark gray staining represents β-galactosidase staining. Scale bars represent 400 μm. (F) Replicative fit and senescent MSCs were subjected to staining for MSC markers as defined by the ISCT and acquired through flow cytometry. Open and gray histograms represent marker and isotype controls, respectively. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (G) Fold difference in the P16INK4A, PERP, LAMP, and LY96 mRNA of fit and senescent MSCs, relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were determined in quantitative sybr green real-time PCR. Cumulative is shown from 2 independent donors.
Replicative senescent MSCs exhibit attenuated T-cell immunosuppressive properties in vitro and maintain intact lung-homing properties in vivo
We analyzed the veto effect of fit and senescent MSCs on activated T-cell proliferation in vitro. Our results demonstrate that fit MSC populations inhibit CD3+Ki67+ T-cell proliferation more efficiently than senescent counterparts (%CD3+Ki67+:No MSC [70 ± 11], fit MSC [29 ± 11], senescent MSC [60 ± 17]) (Figure 2A-B). Next we investigated the effect of fit and senescent MSCs on cytokine production by proliferating (CFSE-diluted) T cells. We found that senescent MSCs fail to inhibit IFNγ production by CFSE-diluted T-cell populations, whereas fit MSCs exhibit inhibition (Figure 2C-D). To determine whether senescent MSCs added to fit MSC in a heterogeneous cell culture system lead to a dominant-negative effect on T-cell suppression, we mixed fit and senescent MSCs in a 1:1 ratio and investigated the immunosuppressive effect of this mixed population. We observed that mixture of fit and senescent MSCs (akin to what could be found as part of clinical cell preparations) inhibits T cells as well as fit MSCs alone, suggesting that senescent MSCs do not lead to a dominant-negative effect on fit cells (Figure 2E-F) in inhibiting T-cell proliferation, suggesting a loss-of-function defect in senescent MSCs. Our published results demonstrate that cell preparation and handling methods such as freeze-thawing compromise human MSCs’ lung-binding properties in vivo.23,26 Here we have investigated the effect of replicative senescence on human MSCs’ in vivo lung tropic properties following tail vein injection in C57BL/B6 mice. Our cumulative results of 2 independent experiments using quantitative human genomic DNA PCR demonstrated that both fit and senescent MSCs exhibit comparable short-term lung-homing properties (Figure 2G).
Replicative senescent MSCs display attenuated immunosuppressive and intact lung-homing properties. Replicative fit and senescent MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Representative fluorescence-activated cell sorting (FACS) plot and (B) cumulative effect of fit and senescent MSCs’ (n = 5 donor pairs) effect on T-cell proliferation (% CD3+Ki67+) is shown. CFSE-labeled PBMCs were cocultured with replicative fit and senescent MSCs and were stimulated with αCD3αCD28 Dynabeads. On the fourth day, intracellular cytokine staining was performed to determine the percentage of cytokine-secreting proliferated T cells CD3+CFSEdim IFNγ T cells. (C) Representative FACS plot and (D) dose-dependent effect are shown. Individual or mixed fit and senescent MSCs at 1:1 ratio were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs and 4 days post culture, T-cell proliferation was measured as indicated above. (E) Representative FACS plot and (F) cumulative effect are shown. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (G) Fit or senescent MSCs (0.5 × 106/animal) were injected IV into C57BL/B6 mice via the tail vein. At 24 hours post-infusion, the animals were killed and the lungs were excised to extract total gDNA for real-time PCR amplification of human gDNA and mouse gDNA. Human gDNA threshold cycle (CT) values were normalized with mouse gDNA values. Cumulative inverse CT values with mean ± SD were shown from 2 independent experiments (n = 6 animals per group), performed with 2 unique fit and senescent MSC donors pairs. P < .05 was considered statistically significant based on 2-tailed Student t tests.
Replicative senescent MSCs display attenuated immunosuppressive and intact lung-homing properties. Replicative fit and senescent MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Representative fluorescence-activated cell sorting (FACS) plot and (B) cumulative effect of fit and senescent MSCs’ (n = 5 donor pairs) effect on T-cell proliferation (% CD3+Ki67+) is shown. CFSE-labeled PBMCs were cocultured with replicative fit and senescent MSCs and were stimulated with αCD3αCD28 Dynabeads. On the fourth day, intracellular cytokine staining was performed to determine the percentage of cytokine-secreting proliferated T cells CD3+CFSEdim IFNγ T cells. (C) Representative FACS plot and (D) dose-dependent effect are shown. Individual or mixed fit and senescent MSCs at 1:1 ratio were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs and 4 days post culture, T-cell proliferation was measured as indicated above. (E) Representative FACS plot and (F) cumulative effect are shown. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (G) Fit or senescent MSCs (0.5 × 106/animal) were injected IV into C57BL/B6 mice via the tail vein. At 24 hours post-infusion, the animals were killed and the lungs were excised to extract total gDNA for real-time PCR amplification of human gDNA and mouse gDNA. Human gDNA threshold cycle (CT) values were normalized with mouse gDNA values. Cumulative inverse CT values with mean ± SD were shown from 2 independent experiments (n = 6 animals per group), performed with 2 unique fit and senescent MSC donors pairs. P < .05 was considered statistically significant based on 2-tailed Student t tests.
Senescence-associated defect in HLA-DR upregulation is dispensable for MSCs’ immunosuppressive properties
The immunosuppressive potential of MSCs upon transfusion in vivo is dependent on its response to IFNγ.27 We have compared fit and senescent MSCs for IFNγ-mediated activation of upstream signaling cascade and downstream key effector molecule expression. Both fit and senescent MSCs express the IFNγ receptor 1 (CD119) on their surface (Figure 3A). IFNγ activates the JAK-STAT signaling pathway in MSCs,28 and Phosflow analysis demonstrated that short-term IFNγ activation phosphorylates STAT1 in both fit and senescent MSCs at similar levels (Figure 3B). IFNγ upregulates HLA-ABC, B7H1 (PDL1), and B7DC (PDL2) molecules on the surface of both fit and senescent MSCs (Figure 3C). Neither costimulatory molecules B7-1 (CD80) nor B7-2 (CD86) were upregulated by IFNγ on fit and senescent MSCs (Figure 3C). However, senescent MSCs do not upregulate HLA-DR upon stimulation with IFNγ in contrast to what occurs in fit MSCs (Figure 3C-D). To determine whether the blunted upregulation of HLA-DR on the surface of senescent MSCs is caused by cytosolic retention or at the transcriptional level, we have performed real-time PCR analysis for the transcripts of HLA-DR. Our results demonstrate that HLA-DR mRNA was not efficiently unregulated by IFNγ (Figure 3E). To further investigate the functional role of HLA-DR in maintaining the veto properties of MSCs, we used a siRNA knockdown approach (Figure 3F-G). Our results demonstrate that, compared with control siRNA, HLA-DR siRNA–transfected MSCs are unaffected in regard to their inhibitory effects on CD3+CD4+ and CD3+CD8+ T-cell proliferation (Figure 3H-J).
Senescence-associated defective HLA-DR upregulation does not modulate immunosuppressive properties of MSCs. (A) Fit and senescent MSCs were subjected to staining of the receptor for IFNγ (CD119) and acquired through flow cytometry. (B) Fit and senescent MSCs stimulated with indicated concentrations of IFNγ for 15 minutes. P-STAT1 (Y701) Phosflow was performed subsequently and acquired through flow cytometry. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (C) Fit and senescent MSCs were stimulated with IFNγ for 48 hours and subsequently stained with the antibodies to the surface markers for HLA-ABC, HLA-DR, B7-1, B7-2, B7H1, and B7DC for flow cytometry. (D) Cumulative HLA-DR MFI fold change derived from 2 independent fit and senescent MSC donor pairs were shown. (E) Fit and senescent MSCs were stimulated with IFNγ for 48 hours. Expression level of HLA-DR mRNA relative to GAPDH was evaluated by the quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of HLA-DR over the unstimulated control. Control or HLA-DR siRNA-transfected MSCs were stimulated with IFNγ for 48 hours. Cells were trypsinized, stained for HLA-DR or appropriate isotype control antibodies, and analyzed by flow cytometry. (F) Representative histogram. (G) Cumulative mean fluorescent intensity of HLA-DR and isotype control antibody stains were shown on 2 independent fit and senescent MSC donor pairs. HLA-DR or control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (H) Representative FACS plot and effect of MSCs on (I) CD3+CD4+ and (J) CD3+CD8+ T-cell proliferation are shown. Similar results were obtained in a repeat experiment with another MSC donor.
Senescence-associated defective HLA-DR upregulation does not modulate immunosuppressive properties of MSCs. (A) Fit and senescent MSCs were subjected to staining of the receptor for IFNγ (CD119) and acquired through flow cytometry. (B) Fit and senescent MSCs stimulated with indicated concentrations of IFNγ for 15 minutes. P-STAT1 (Y701) Phosflow was performed subsequently and acquired through flow cytometry. Similar results were obtained in a repeat experiment with an additional 1 or 2 fit and senescent MSC donor pairs. (C) Fit and senescent MSCs were stimulated with IFNγ for 48 hours and subsequently stained with the antibodies to the surface markers for HLA-ABC, HLA-DR, B7-1, B7-2, B7H1, and B7DC for flow cytometry. (D) Cumulative HLA-DR MFI fold change derived from 2 independent fit and senescent MSC donor pairs were shown. (E) Fit and senescent MSCs were stimulated with IFNγ for 48 hours. Expression level of HLA-DR mRNA relative to GAPDH was evaluated by the quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of HLA-DR over the unstimulated control. Control or HLA-DR siRNA-transfected MSCs were stimulated with IFNγ for 48 hours. Cells were trypsinized, stained for HLA-DR or appropriate isotype control antibodies, and analyzed by flow cytometry. (F) Representative histogram. (G) Cumulative mean fluorescent intensity of HLA-DR and isotype control antibody stains were shown on 2 independent fit and senescent MSC donor pairs. HLA-DR or control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (H) Representative FACS plot and effect of MSCs on (I) CD3+CD4+ and (J) CD3+CD8+ T-cell proliferation are shown. Similar results were obtained in a repeat experiment with another MSC donor.
The secretome arising from interaction between senescent MSCs and immune responders is distinct from that of fit MSCs
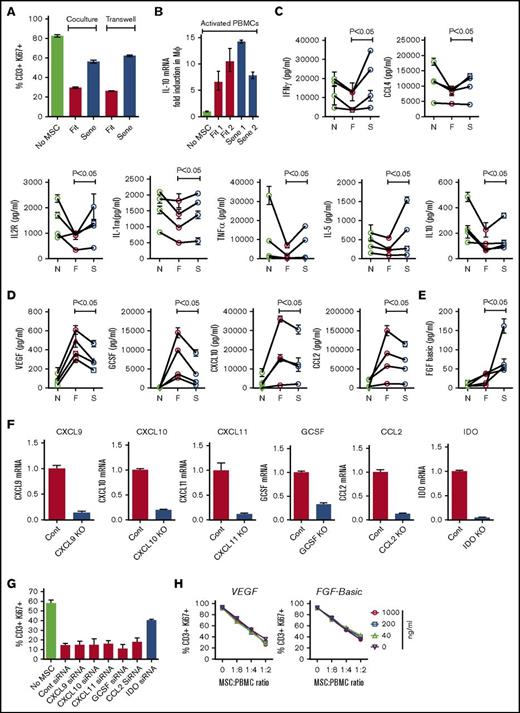
To compare contact- and noncontact-dependent senescent MSCs’ interaction with immune responders, we have cocultured fit or senescent MSCs with activated PBMCs in a 2-chamber transwell assay system. Our results demonstrate that senescent MSCs fail to inhibit T-cell proliferation in the transwell system, suggesting that senescent MSCs are defective in secreting immunosuppressive factors (Figure 4A). To define the role of senescent MSCs in deriving suppressive macrophages, we have investigated IL-10 mRNA expression in macrophages cocultured with fit or senescent MSC cultures with PBMCs. Our results demonstrate that both fit and senescent MSCs upregulate IL-10 mRNA in macrophages (Figure 4B). Although prior studies have investigated senescent MSCs’ secretome in resting status,29 their interaction with PBMCs is largely unknown, and we have further analyzed the supernatant of fit and senescent MSCs cocultured with activated PBMCs using multiplex Luminex assay with a 30-plex-cytokine profile. Our results identified 3 different patterns of cytokine profile following fit or senescent MSC cocultured with PBMCs. First, fit MSCs are more potent than senescent MSCs at quenching cytokine production by PBMCs. IL-1ra, IFNγ, IL-2R, CCL4, tumor necrosis factor-α (TNF-α), IL-5, and IL-10 were all substantially downregulated in activated PBMC cocultures with fit MSCs, whereas senescent counter led to a statistically significant attenuated effect (Figure 4C). Second, MSC-sourced vascular endothelial growth factor (VEGF), GCSF, CXCL10, and CCL2 were significantly upregulated in activated PBMC cultures with fit MSCs, whereas senescent MSCs displayed a significant defect in upregulation of these factors (Figure 4D) Finally, MSC-sourced fibroblast growth factor basic secretion (FGF-basic) is modestly increased in activated PBMC cultures with fit MSCs, whereas this effect is substantially and significantly upregulated by senescent MSCs (Figure 4E). We did not see a significant difference in other cytokines (IL-7, IL-13, IL-17, CXCL9, CCL3, IL-15, IL-6, IL-1β, RANTES, eotaxin, IL-12, IL-8, IL-4, granulocyte macrophage-CSF, IFNα, human growth factor) between cultures containing fit vs senescent MSCs (supplemental Figure 1). To examine the role of CXCL9, CXCL10, CXCL11, GCSF, CCL2, and IDO in MSCs’ immunosuppressive properties, we used an siRNA knockdown approach (Figure 4F). Our results demonstrate that, unlike IDO, knocking down of other cytokines individually does not reverse fit MSCs’ inhibitory effect on T cells (Figure 4G). In addition, the exogenous addition of VEGF and FGF-basic do not inhibit fit MSCs’ inhibitory potential on T cells (Figure 4H), suggesting that upregulation of these factors was a bystander effect of MSC/PBMC interaction, which does not directly affect T-cell proliferation.
Differential secretome of activated PBMCs cocultured with fit and senescent MSCs. Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Relative effect of fit and senescent MSCs’ effect on T-cell proliferation (% CD3+Ki67+) in coculture and transwell culture are shown. Similar results were obtained in a repeat experiment with an additional 2 fit and senescent MSC donor pairs. (B) CD14+ purified macrophages were cultured on transwell and in the bottom PBMCs were activated with 2 independent fit or senescent MSC pairs. 48 hours later, macrophages in the transwell were harvested and IL-10 mRNA was measured with GAPDH mRNA as endogenous housekeeping control. Fold induction of IL-10 mRNA was derived through δ-δ CT method. Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, supernatant was collected and human cytokine 30-plex panel Luminex assays were performed according to the manufacturer’s instructions. Fit and senescent MSC pairs from 4 independent donors were tested against different PBMC donors. Cytokine levels were shown in pg/mL. F, fit MSCs and activated PBMCs; N, no MSCs and activated PBMCs; S, senescent MSCs and activated PBMCs. (C) IL-1ra, IFNγ, IL-2r, CCL4, TNFα, IL-5, and IL-10 were decreased efficiently by fit but not senescent MSCs. (D) VEGF, GCSF, CXCL10, and CCL2 were upregulated by fit MSCs cocultured with activated PBMCs and the senescent MSCs display attenuated upregulation. (E) FGF-basic was substantially increased by senescent MSCs compared with fit MSCs. (F) Control, CXCL9, CXCL10, CXCL11, GCSF, CCL2, and IDO siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates for 3 to 4 days. Expression level of appropriate silenced mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of silenced gene over the control siRNA-transfected MSCs. (G) CXCL9, CXCL10, CXCL11, GCSF, CCL2, IDO, and control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. Proliferation of T cells (CD3+Ki67+) in the presence and absence of siRNA transfected MSCs is shown. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (H) VEGF and FGF were added exogenously to fit MSCs cocultured with activated PBMCs at indicated ratios. Four days later, CD3+ T-cell proliferation was measured by Ki67 intracellular staining. Similar results were obtained in a repeat experiment with an additional independent MSC donor.
Differential secretome of activated PBMCs cocultured with fit and senescent MSCs. Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Relative effect of fit and senescent MSCs’ effect on T-cell proliferation (% CD3+Ki67+) in coculture and transwell culture are shown. Similar results were obtained in a repeat experiment with an additional 2 fit and senescent MSC donor pairs. (B) CD14+ purified macrophages were cultured on transwell and in the bottom PBMCs were activated with 2 independent fit or senescent MSC pairs. 48 hours later, macrophages in the transwell were harvested and IL-10 mRNA was measured with GAPDH mRNA as endogenous housekeeping control. Fold induction of IL-10 mRNA was derived through δ-δ CT method. Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, supernatant was collected and human cytokine 30-plex panel Luminex assays were performed according to the manufacturer’s instructions. Fit and senescent MSC pairs from 4 independent donors were tested against different PBMC donors. Cytokine levels were shown in pg/mL. F, fit MSCs and activated PBMCs; N, no MSCs and activated PBMCs; S, senescent MSCs and activated PBMCs. (C) IL-1ra, IFNγ, IL-2r, CCL4, TNFα, IL-5, and IL-10 were decreased efficiently by fit but not senescent MSCs. (D) VEGF, GCSF, CXCL10, and CCL2 were upregulated by fit MSCs cocultured with activated PBMCs and the senescent MSCs display attenuated upregulation. (E) FGF-basic was substantially increased by senescent MSCs compared with fit MSCs. (F) Control, CXCL9, CXCL10, CXCL11, GCSF, CCL2, and IDO siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates for 3 to 4 days. Expression level of appropriate silenced mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of silenced gene over the control siRNA-transfected MSCs. (G) CXCL9, CXCL10, CXCL11, GCSF, CCL2, IDO, and control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. Proliferation of T cells (CD3+Ki67+) in the presence and absence of siRNA transfected MSCs is shown. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (H) VEGF and FGF were added exogenously to fit MSCs cocultured with activated PBMCs at indicated ratios. Four days later, CD3+ T-cell proliferation was measured by Ki67 intracellular staining. Similar results were obtained in a repeat experiment with an additional independent MSC donor.
Senescent MSCs display a defect in kynurenine production
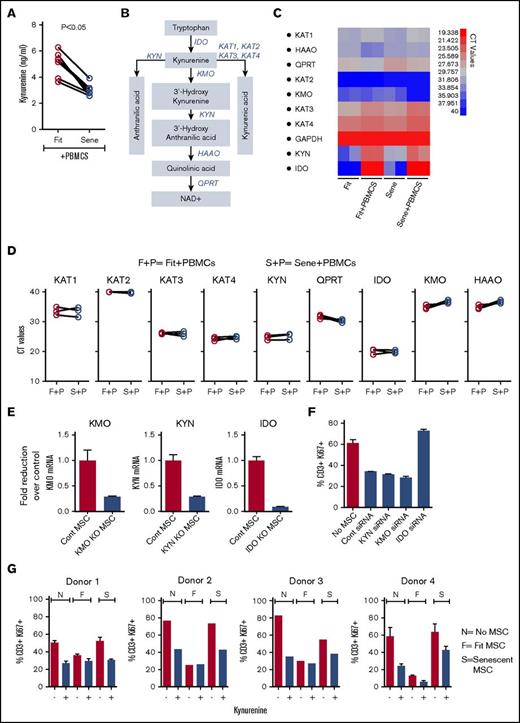
To test the cell biochemical activity of IDO in MSCs, we measured the levels of kynurenine accumulation in the culture supernatant of fit and senescent MSCs cocultured with activated PBMCs. Our results demonstrated that kynurenine levels were downregulated in senescent MSC cultures with PBMCs compared with fit MSC counterparts (Figure 5A). To further decipher the role of catabolic pathway of tryptophan in senescent MSCs, we investigated the levels of RNA encoding for enzymes downstream of tryptophan catabolism in fit and senescent MSCs cocultured with activated PBMCs30 (Figure 5B). Our results demonstrated that KYN, a major enzyme in kynurenine degradation pathway, is substantially upregulated on MSCs upon culture with PBMCs and clustered with IDO in grouping analysis (Figure 5C; supplemental Figure 2). However, KYN and the RNA of other enzymes involved in kynurenine degradation pathway are not differentially expressed in senescent MSCs compared with fit MSCs (Figure 5D). To define the role of KYN in MSCs’ immunobiology, we performed an siRNA knockdown approach (Figure 5E). We also silenced kynurenine 3-monooxygenase (KMO), which is an additional primary kynurenine-metabolizing enzyme, along with IDO as controls (Figure 5E). Our results demonstrated that, in contrast to IDO, both KYN and KMO–silenced MSCs inhibit T-cell proliferation as efficiently as control siRNA–transfected MSCs (Figure 5F). To test whether add-back of kynurenine to senescent MSC restores inhibitory properties, we added kynurenine exogenously in senescent MSC coculture with PBMCs. Our results demonstrated that PBMCs in the coculture of senescent MSCs were efficiently inhibited by exogenous kynurenine (Figure 5G).
Exogenous addition of kynurenine corrects senescence-associated impaired immunosuppressive properties of MSCs. (A) Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, supernatant was collected and kynurenine levels were performed according to the manufacturer’s instructions. Cumulative of 6 donor pairs were shown. (B) Catabolism of kynurenine pathway and the enzymes that facilitate this cascade is shown with a cartoon adapted from reference 30. IDO, kynurenine aminotransferases (KAT), kynurenine 3-monooxygenase (KMO), KYN, 3-hydroxyanthranilate 3,4-dioxygenase (HAAO), and quinolinate phosphoribosyl transferase (QPRT). Fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates. Four days later, MSCs were harvested and the expression level of mRNA of kynurenine catabolic enzymes relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. (C) Representative heat map with CT values (red = high expression, blue = low expression) and (D) cumulative comparison between fit and senescent MSC pairs from 3 unique donors is shown. F+P, fit MSCs + activated PBMCs; S+P, senescent MSCs + activated PBMCs. (E) Control, KYN, KMO, and IDO siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates for 3 to 4 days. Expression level of appropriate silenced mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of silenced gene over the control siRNA-transfected MSCs. KYN, KMO, and IDO, and control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (F) Proliferation of T cells (CD3+Ki67+) in the presence and absence of siRNA transfected MSCs is shown. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (G) Kynurenine (500 uM) was added exogenously in the coculture of αCD3αCD28 Dynabeads-stimulated PBMCs with and without fit and senescent MSCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. F, fit MSCs + activated PBMCs; N, no MSCs + activated PBMCs; S, senescent MSCs + activated PBMCs. Kynurenine’s effect on T-cell proliferation with 4 independent fit and senescent MSC donor pairs is shown.
Exogenous addition of kynurenine corrects senescence-associated impaired immunosuppressive properties of MSCs. (A) Replicative fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, supernatant was collected and kynurenine levels were performed according to the manufacturer’s instructions. Cumulative of 6 donor pairs were shown. (B) Catabolism of kynurenine pathway and the enzymes that facilitate this cascade is shown with a cartoon adapted from reference 30. IDO, kynurenine aminotransferases (KAT), kynurenine 3-monooxygenase (KMO), KYN, 3-hydroxyanthranilate 3,4-dioxygenase (HAAO), and quinolinate phosphoribosyl transferase (QPRT). Fit and senescent MSCs were cultured in the presence and absence of αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates. Four days later, MSCs were harvested and the expression level of mRNA of kynurenine catabolic enzymes relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. (C) Representative heat map with CT values (red = high expression, blue = low expression) and (D) cumulative comparison between fit and senescent MSC pairs from 3 unique donors is shown. F+P, fit MSCs + activated PBMCs; S+P, senescent MSCs + activated PBMCs. (E) Control, KYN, KMO, and IDO siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs in 2-chamber transwell plates for 3 to 4 days. Expression level of appropriate silenced mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of silenced gene over the control siRNA-transfected MSCs. KYN, KMO, and IDO, and control siRNA-transfected MSCs were cultured with αCD3αCD28 Dynabeads-stimulated PBMCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (F) Proliferation of T cells (CD3+Ki67+) in the presence and absence of siRNA transfected MSCs is shown. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (G) Kynurenine (500 uM) was added exogenously in the coculture of αCD3αCD28 Dynabeads-stimulated PBMCs with and without fit and senescent MSCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. F, fit MSCs + activated PBMCs; N, no MSCs + activated PBMCs; S, senescent MSCs + activated PBMCs. Kynurenine’s effect on T-cell proliferation with 4 independent fit and senescent MSC donor pairs is shown.
IFNγ stimulation restores senescent MSCs’ immunosuppressive properties
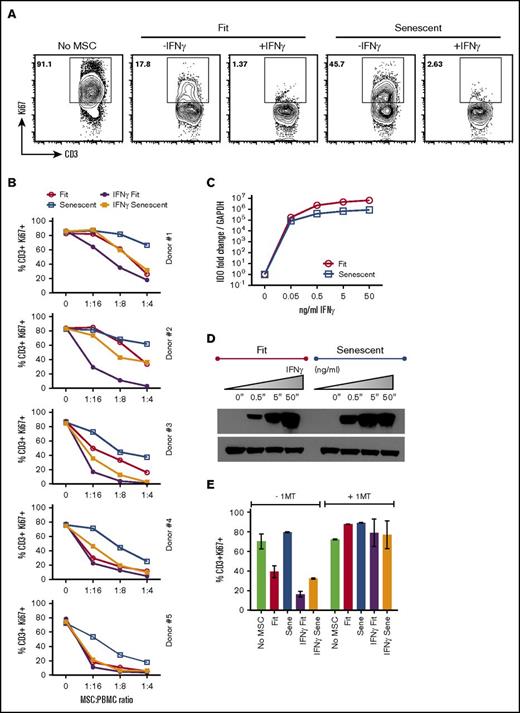
We have published a quantitative PCR array platform of >40 genes informed by published reports of MSCs’ functional responsiveness to IFNγ.20 Our results are consistent with our previous observation20 and demonstrate that IFNγ upregulates IDO, CXCL9, CXCL10, CXCL11, PD-L1, and ICAM-1 on MSCs. In addition, we observed that there is no significant difference in the global pattern of RNA transcriptional response to IFNγ observed between fit and senescent MSCs other than HLA-DR (Figure 6). These results suggested that IFNγ stimulation would correct the defective immunosuppressive properties of replicative senescent MSCs, consistent with published data showing that IFNγ stimulation enhances MSCs’ immunosuppressive properties.20,22,31,32 We tested whether IFNγ activation rescues senescent MSCs’ immunosuppressive properties and demonstrated that IFNγ-stimulated senescent MSCs inhibit T-cell proliferation as efficiently as actively growing fit MSCs (Figure 7A-B). However, IFNγ prelicensed fit MSCs exhibit significantly superior T-cell suppressor activity than all the MSC populations tested. Next we investigated the IDO response of fit and senescent MSCs. Our results demonstrated that IFNγ stimulation upregulates IDO RNA and protein on fit and senescent MSCs at comparable levels (Figure 7C-D). Blocking of IDO catalytic activity with 1-methyl tryptophan (1MT) negates the suppressive effect of IFNγ prelicensed senescent MSCs on T-cell proliferation (Figure 7E), suggesting that IDO plays a central role in conferring IFNγ rescue effects on the immunosuppressive properties of senescent MSCs.
Molecular genetic responses of fit and senescent MSCs to IFNγ. Fit and senescent MSC pairs derived from 2 independent donors were stimulated with 20 ng/mL IFNγ for 48 hours, and total cDNA were generated from RNA. Transcriptional profiles of >40 genes were investigated in Fluidigm nanoscale qPCR 48*48 array plates (n = 3/sample). Heat map of fit (F) and senescent (S) MSCs stimulated with ±20 ng/mL IFNγ showing the expression genes (red = high expression, blue = low expression). Heat map was generated using JMP software.
Molecular genetic responses of fit and senescent MSCs to IFNγ. Fit and senescent MSC pairs derived from 2 independent donors were stimulated with 20 ng/mL IFNγ for 48 hours, and total cDNA were generated from RNA. Transcriptional profiles of >40 genes were investigated in Fluidigm nanoscale qPCR 48*48 array plates (n = 3/sample). Heat map of fit (F) and senescent (S) MSCs stimulated with ±20 ng/mL IFNγ showing the expression genes (red = high expression, blue = low expression). Heat map was generated using JMP software.
IFNγ prelicensed senescent MSCs display enhanced immunosuppressive activity through IDO. αCD3αCD28 Dynabeads-stimulated PBMCs were cocultured in the presence and absence ± IFNγ prelicensed (20 ng/mL for 48 hours) fit and senescent MSCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Representative FACS plot and (B) dose-dependent T-cell inhibitory effect of ± IFNγ prelicensed fit and senescent MSC pairs from 5 independent donors is shown. (C) Fit and senescent MSCs were stimulated with IFNγ for 48 hours. Expression level of IDO mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of IDO over the unstimulated control. (D) IDO expression at protein level is shown by western blot analysis, and actin was used as an internal control. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (E) ± IFNγ prelicensed fit and senescent MSCs were cocultured with activated PBMCs in the presence and absence of IDO blocker, 1MT. Four days after, T-cell proliferation was measured by flow cytometry. Similar results were obtained in a repeat experiment with another MSC donor pair.
IFNγ prelicensed senescent MSCs display enhanced immunosuppressive activity through IDO. αCD3αCD28 Dynabeads-stimulated PBMCs were cocultured in the presence and absence ± IFNγ prelicensed (20 ng/mL for 48 hours) fit and senescent MSCs. Four days post culture, T-cell proliferation was measured by Ki67 intracellular staining. (A) Representative FACS plot and (B) dose-dependent T-cell inhibitory effect of ± IFNγ prelicensed fit and senescent MSC pairs from 5 independent donors is shown. (C) Fit and senescent MSCs were stimulated with IFNγ for 48 hours. Expression level of IDO mRNA relative to GAPDH was evaluated by quantitative SYBR green real-time PCR. δ-δ CT method was applied to calculate the fold induction of IDO over the unstimulated control. (D) IDO expression at protein level is shown by western blot analysis, and actin was used as an internal control. Similar results were obtained in a repeat experiment with an additional independent MSC donor. (E) ± IFNγ prelicensed fit and senescent MSCs were cocultured with activated PBMCs in the presence and absence of IDO blocker, 1MT. Four days after, T-cell proliferation was measured by flow cytometry. Similar results were obtained in a repeat experiment with another MSC donor pair.
Discussion
Several industry-sponsored advanced clinical trials have investigated the clinical utility of bulk-produced culture-expanded random donor allogeneic MSCs for the treatment of various immune and tissue injury disorders. Considering that large-scale culture expansion invariably leads to some measure of replicative senescence in a final cell product, we investigated whether senescent MSCs deploy comparable potency and functionalities germane to immune suppression to their early passage, “fit” counterpart. Using the ISCT guidelines,33 we have shown that senescent MSCs are plastic-adherent, despite displaying cell-cycle arrest in standard culture conditions, and express surface molecules such as CD105, CD90, CD73, and CD44, and lack expression of hematopoietic markers akin to and indistinguishable from fit MSCs. However, replication-exhausted MSCs display classical senescence-associated protein and genetic markers such as β-galactosidase and P16INK4A, respectively.13,17 Consistent with previous reports,13 we have observed hypertrophic phenotype changes in senescent MSCs, which have been shown to be a major limitation for the use of MSC transplantation for cartilage repair.34-37
Considering that IFNγ plays a dominant role in activation of MSCs,38,39 analysis of MSCs’ responsiveness to IFNγ is evolving commonly used method to define their immune plasticity.40,41 Here, we have shown that senescent MSCs are responsive to IFNγ as defined by STAT1 phosphorylation. However, downstream effector pathways display significant variations as IFNγ upregulates MHC class I, PDL-1, and PDL-2, but not HLA-DR in senescent MSCs. Indeed, a senescence-associated defect in MHC class II upregulation by IFNγ and impaired GM-CSF–dependent proliferation was demonstrated in macrophages derived from aged mice.42-44 In addition, upregulation of MHC class II is also affected in cell-cycle–arrested macrophages, which corroborates with our findings with growth-arrested senescent MSCs.45 These results suggest that MHC class II induction defect is a common feature of telomere-shortened senescent cells of both hematopoietic and nonhematopoietic origins. Although we demonstrate that the role of HLA-DR in MSCs in inhibiting T-cell proliferation is dispensable, IFNγ-induced defect in HLA-DR expression could serve as a surrogate marker for loss of MSCs’ inhibitory potency and replication exhaustion.
Replicative senescent human fibroblasts and epithelial cells secrete proinflammatory cytokines such as IL-6, IL-8, and GM-CSF as part of the senescence-associated secretory phenotype (SASP).46 Using a 30-plex cytokine analysis, we did not observe an altered secretome by resting senescent MSCs (supplemental Figure 3). However, we have observed that human MSCs are exquisitely responsive to proximate activated PBMCs. We have shown here that relative to fit MSCs, their senescent counterpart cocultured with allogeneic immune responders PBMCs deployed a blunted upregulation MSC-sourced cytokines such as VEGF, GCSF, CXCL10, and CCL2 and were less effective at downregulation of PBMC-sourced cytokines such as IL-1ra, IFNγ, IL-2r, CCL4, TNFα, IL-5, and IL-10. Importantly, we have found that senescent MSCs deploy a blunted secretome response upon interaction with immune responders, with substantially reduced expression of VEGF, CCL2, GCSF, and CXCL10. To better understand the contribution of these and other effector molecules by fit MSCs, we knocked down CXCL9, CXCL10, CXCL11, GCSF, and CCL2 individually without any meaningful effect on veto properties of MSCs. In contrast, knockdown of IDO abolished MSC suppressor function. These data suggest that IDO remains the key effector pathway for blocking PBMC proliferation in vitro. However, we have found that senescent MSCs are defective in kynurenine production upon interaction with PBMCs. Considering that senescence-associated aging affects cellular amino acid metabolism, this may provide an explanation for defective tryptophan catabolism by senescent MSCs.47-52 It has also been demonstrated that IDO in senescent MSCs undergo proteasomal degradation, which could additionally account for defective kynurenine production and associated impaired immune suppression.53 Consistent with our previous study, we also show here that IL-10 expression is increased in macrophages cocultured with MSCs.54 We also show that senescent MSCs upregulate IL-10 production by macrophages like fit MSCs despite exhibiting poor IDO activity. Published data suggest that MSC and macrophage interactions are complex and involve additional effector pathways other than IDO.55
IFNγ induces cellular senescence in human endothelial cells through p53-dependent DNA damage.56 Although it is unknown whether such a mechanism occurs in MSCs, IFNγ leads to a cytostatic nonproliferative response and affects the differentiation potential of MSCs.20,57 Nevertheless, IFNγ rescue of senescent-associated defective immunosuppressive properties suggests that IFNγ prelicensing may serve as a remedy for MSCs’ utilization as cellular pharmaceutical for immune disorders. We also compared >40 transcripts before and after treatment with IFNγ that are important for MSCs’ immune function, homing, and regenerative properties in fit and senescent counterparts. The majority of the genes are highly upregulated both on fit and senescent MSCs, suggesting that IFNγ prelicensing can partially correct the defective immunosuppressive properties of senescent MSCs.
In most industrial clinical trials, cryobanked MSCs are used. High prefreeze senescence cell content has been shown to correlate with poor post-thaw function of MSCs,58 and thus both replicative senescence and thawing from cryopreservation may be additive detrimental determinants of MSC function and potency. In the present study, we have used MSCs that are cultured in fetal calf serum growth–supplemented media akin to the most prevalent culture methods in support of clinical trials. We have found that addition of human platelet lysate does not rescue proliferation of MSCs with pre-established senescence, suggesting that although replicative senescence can be postponed or prevented at some extent with the appropriate culture expansion medium, it cannot be reversed (supplemental Figure 4).19
Acquired senescence in culture-adapted MSCs may provide insight on why industry-sponsored clinical trials using heavily expanded MSCs do not show efficacy, yet meet standards of safety.59 Our data support the notion that replication fit MSCs are distinct and superior to senescent MSCs in their ability to suppress T-cell proliferation, and that culture conditions, including use of licensing cytokines, may provide a remedy to mitigate this defect.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Shala Yuan and Marco Garcia for technical assistance.
The study was supported by a grant from ACTSI/ImmunoEngineering Pilot Award. Research reported in this publication was supported in part by developmental funds from the Winship Cancer Institute of Emory University (R.C.). This work was directly supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases award R01DK109508.
Authorship
Contribution: R.C. designed the research plan, performed most experiments, analyzed results, and wrote the manuscript; D.R. and L.J.A. helped with cytokine multipex experiments; E.K.W. provided bone marrow from healthy individuals; S.N. performed western blot analysis for IDO; K.M. performed β-galactosidase assays; D.A. and G.G. helped with qPCR arrays; and J.G. designed the research plan, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacques Galipeau, Department of Medicine, University of Wisconsin Carbone Comprehensive Cancer Center, University of Wisconsin–Madison, 1111 Highland Ave, Madison, WI 53705; e-mail: jgalipeau@medicine.wisc.edu.