Key Points
EBV DNA in cell-free blood in patients with Hodgkin lymphoma correlated with the presence of virus in tumor.
Persistence of EBV DNA in cell-free blood 1 week after initiation of therapy predicted inferior event-free survival.
Abstract
Assay of cell-free DNA in blood offers an approach to assessment of tumor DNA. We sought to determine whether Epstein-Barr virus (EBV) DNA in cell-free blood is also a good surrogate for the presence of tumor DNA in children with Hodgkin lymphoma, as it is in adults, and whether it correlates with pediatric outcomes. Pediatric patients enrolled in a Children’s Oncology Group trial (AHOD0031) were studied at baseline and at 8 days after the initiation of treatment. At baseline, EBV DNA in cell-free blood correlated with the presence of EBV in tumor, and EBV DNA 8 days after the initiation of therapy predicted inferior event-free survival. EBV DNA in cell-free blood warrants further investigation as a marker of inadequate tumor response in Hodgkin lymphoma. This trial was registered at www.clinicaltrials.gov as #NCT00025259.
Introduction
Assay of circulating cell-free DNA in blood offers a convenient, noninvasive, and repeatable approach to assessment of tumor DNA.1,2 Circulating tumor DNA in patients with a variety of cancers has been detected by the presence of characteristic point mutations or patterns of cytosine guanine dinucleotide methylation. In Hodgkin lymphoma (HL), although an increase in circulating cell-free DNA in blood is well established,3 assays that detect tumor DNA by point mutations or abnormal patterns of methylation in cell-free blood have yet to be documented. This in part reflects the difficulties in molecular characterization of HL associated with the paucity of tumor cells relative to infiltrating cells in tumor masses. However, the association of Epstein-Barr virus (EBV) with tumor cells in ∼20% of patients suggests the possibility that tumor DNA that includes EBV sequences is one of the sources of EBV DNA in cell-free blood and may serve as a surrogate for tumor DNA.4-6
In a previous study, we investigated the relationship between EBV DNA in cell-free blood and the presence of EBV in tumor cells.4 In adults with advanced-stage HL, the presence of EBV DNA at copy number >60 per 100 µL of plasma correlates with tumor in situ hybridization (ISH), and detection of EBV DNA at 6 months after the initiation of therapy predicts inferior failure-free survival, consistent with the interpretation that EBV DNA above this threshold in patients with HL is indicative of the presence of tumor.4 We were interested in whether the approach would be applicable to children with HL. Primary EBV infection commonly occurs in childhood and adolescence and might confound the relationship between EBV DNA measurements in cell-free blood and EBV-associated tumor. We also sought to determine whether the presence of EBV cell-free DNA after the initiation of therapy would have prognostic value.4,7-9 To address these questions, we used specimens collected at baseline and at 8 days after the initiation of chemotherapy in a subset of patients with intermediate-risk classical HL enrolled in Children’s Oncology Group (COG) AHOD0031.
Methods
The COG AHOD0031 study evaluated dose-dense response-based therapy for patients <22 years of age with newly diagnosed intermediate-risk HL.10 The study enrolled 1712 eligible patients from 2002 to 2009. Early response was initially measured after 3 cycles of chemotherapy, but in 2003, the study was amended to assess early response to chemotherapy after 2 cycles. The present EBV analysis includes only patients with classical HL enrolled from 2003 to 2005 (after the amendment). Patients received chemotherapy consisting of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide for 2 cycles, followed by computed tomography response assessment, which guided further therapy. Institutional review board approval was obtained, and participants or their legal guardians provided informed consent.
EBV tumor status was determined by ISH of the tumor tissue.11-14 Sections from formalin-fixed, paraffin-embedded tissue blocks were stained with EBER probes (ISH iVIEW Blue detection kit; Ventana Medical Systems Inc., Oro Valley, AZ). Serum specimens collected at baseline (pretreatment) and at day 8 were assayed. DNA was isolated from 250 µL of serum using the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). Assays were carried out as previously described.4
The χ2 test was used to examine the association between presence of EBV at >60 per 100 µL in cell-free blood and presence of EBV in tumor. Event-free survival (EFS) was defined as time from study enrollment to disease relapse or progression, second malignancy, or death. Kaplan-Meier curves were constructed to estimate EFS. The log-rank test was used to compare EFS outcomes between groups.15 The Cox proportional hazards model was used to estimate the EFS hazard ratio between groups.16 All statistical tests performed were two sided, and P values <.05 were considered statistically significant. Statistical analyses were performed using Stata software (version 12; StataCorp LP, College Station, TX).
Results
Among 144 patients with paired serum specimens available from pretreatment and 8 days after the initiation of treatment, there were 133 with paraffin-embedded, formalin-fixed specimens suitable for ISH to determine the presence of EBV in tumor cells. ISH demonstrated the presence of EBV in 29 (22%) diagnostic tumor specimens. We evaluated the previously determined EBV DNA copy number threshold of >60 per 100 µL4 to determine whether this cutoff would appropriately classify patients as having EBV-associated tumors (Table 1). The results show a high concordance rate with ISH (88%). The sensitivity was 72%, and the specificity was 92%. This result suggests that although primary EBV infection usually occurs in childhood or adolescence, the presence of EBV in cell-free blood at concentrations >60 per 100 µL serves as a reliable indicator of the presence of EBV in HL in children.
EBER vs baseline cell-free EBV DNA
| . | Baseline EBV DNA− . | Baseline EBV DNA+ . | Total . |
|---|---|---|---|
| EBER− | 96 | 8 | 104 |
| EBER+ | 8 | 21 | 29 |
| Total | 104 | 29 | 133 |
| . | Baseline EBV DNA− . | Baseline EBV DNA+ . | Total . |
|---|---|---|---|
| EBER− | 96 | 8 | 104 |
| EBER+ | 8 | 21 | 29 |
| Total | 104 | 29 | 133 |
Cutoff of >60 vs ≤60 copies per 100 μL. P < .001.
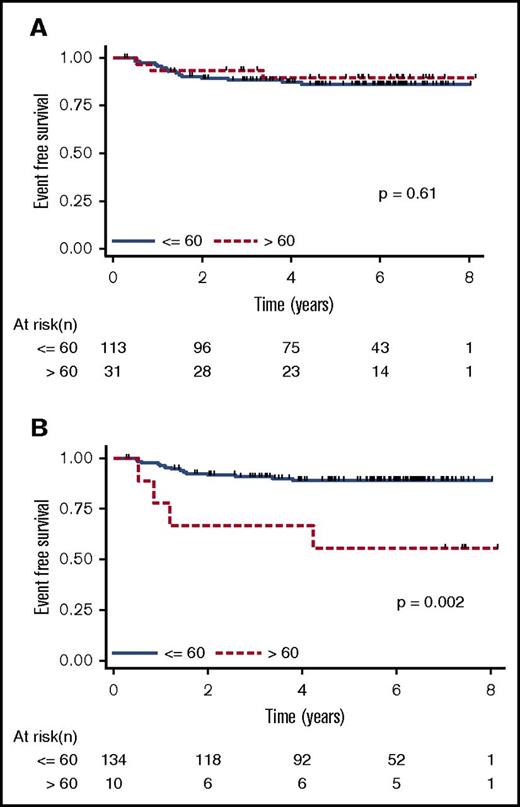
After the second cycle, patients were classed as rapid early responders versus slow early responders and subsequently randomly assigned to early-response–based therapy vs standard therapy. Rapid early response, assessed by computed tomography scan after 2 cycles of therapy, was more frequent in the serum EBV DNA+ group than in the serum EBV DNA− group (94% vs 73%; P = .014). However, comparison of patients identified as having EBV+ or EBV− tumors with regard to 4-year EFS did not identify any differences. The EFS for serum DNA EBV+ patients and serum DNA EBV− patients is shown in Figure 1A. Thus, at baseline, the presence of EBV DNA in cell-free blood reflects the presence of EBV in tumor cells but does not have any prognostic value per se (ie, children with EBV+ and those with EBV− tumors fare similarly with the treatment schema followed in this trial). These findings are consistent with previous reports that EBV+ HL in pediatric populations does not adversely affect EFS.17-19
Kaplan-Meier EFS estimates. EFS estimates stratified by serum EBV DNA status (≤60 copies per 100 µL serum compared with >60 copies per 100 µL serum) at (A) baseline and (B) day 8. Note that there were patients with serum EBV copy number determinations but no ISH, so the number of patients included is greater than that shown in Table 1.
Kaplan-Meier EFS estimates. EFS estimates stratified by serum EBV DNA status (≤60 copies per 100 µL serum compared with >60 copies per 100 µL serum) at (A) baseline and (B) day 8. Note that there were patients with serum EBV copy number determinations but no ISH, so the number of patients included is greater than that shown in Table 1.
In contrast to baseline, EBV DNA positivity at day 8 after the initiation of chemotherapy was associated with lower 4-year EFS (67% for serum EBV DNA+ vs 89% for serum EBV DNA− patients; P = .002; Figure 1B). Because all of the first events in this cohort were relapse or progressive disease, it seems that the presence of EBV DNA in cell-free blood 8 days after the initiation of therapy is associated with treatment failure.
Discussion
One of the concerns about the use of EBV DNA as a tumor marker is the possibility that viral DNA detected in cell-free blood might not be tumor derived but might instead be virion DNA. This possibility has been studied extensively in patients with nasopharyngeal carcinoma. By assaying susceptibility to nuclease digestion (reduced when viral DNA is protected by a viral capsid) and the ability to pellet virion particles by ultracentrifugation, several investigators have concluded that cell-free viral DNA is largely accounted for by DNA released from tumor cells.20,21 Similar but less extensive investigations suggest that EBV DNA in cell-free blood from patients with HL is also tumor derived.7
The results presented here suggest that cell-free EBV DNA in blood is a tumor marker in EBV-associated HL. At baseline, the presence of EBV in pediatric HL did not have prognostic significance, but the presence of EBV DNA at 8 days after the initiation of therapy seems to identify patients with an inadequate response to treatment. We note that the strength of our conclusions is limited by the small number of EBV DNA+ patients. However, this result suggests that persistence of EBV DNA in cell-free blood after the initiation of therapy may serve as a marker of inadequate tumor response and warrants further study.
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 3 June 2008.
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grants CA95423, P50CA96888, and R21CA188824 (R.F.A.), CA98543 (Children’s Oncology Group), and U24 CA114766.
Authorship
Contribution: M.H., L.C., C.L.S., and R.F.A. designed the correlative investigation; R.F.A.’s laboratory carried out virologic studies; S.B.K. oversaw ISH and pathology review; D.L.F., C.L.S., L.C., and R.E.H. designed and coordinated the clinical trial; L.C., J.J.G.W., C.L.S., J.A.K., M.H., A.B., and D.L.F. analyzed the data; J.J.G.W., C.L.S., L.C., J.A.K., and R.F.A. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard F. Ambinder, CRB1 Room 389, 1650 Orleans St, Baltimore, MD 21287; e-mail: rambind1@jhmi.edu.