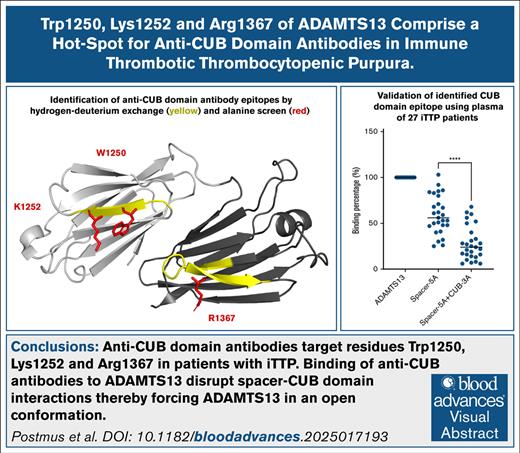
Key Points
Anti–CUB domain antibodies target residues Trp1250, Lys1252, and Arg1367 in patients with iTTP.
Binding of anti-CUB antibodies to ADAMTS13 disrupts spacer-CUB domain interactions, thereby forcing ADAMTS13 in an open conformation.
Visual Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare life-threatening thrombotic disorder, which results from the development of autoantibodies targeting ADAMTS13. Most patients (>90%) with iTTP display antibodies against a shared epitope in the spacer domain of ADAMTS13. Nevertheless, a smaller population of patients (20%-40%) also has antibodies directed toward the CUB (complement C1r/C1s, Uegf, Bmp1) domains of ADAMTS13. Here, we explored whether anti-CUB antibodies have a shared epitope located on CUB domains of ADAMTS13 that overlaps with the spacer-CUB domain interface. Hydrogen-deuterium exchange mass spectrometry revealed that a panel of patient-derived human monoclonal anti–CUB domain antibodies specifically targeted peptides 1248-1253 and 1359-1377 in the CUB1 and CUB2 domains, respectively. A parallel alanine screen showed that residues W1250, K1252, and R1367 are crucially involved in binding of anti–CUB domain antibodies. A triple alanine variant containing W1250A/K1252A/R1367A ameliorated the binding of all patient-derived monoclonal antibodies. This triple-alanine variant also showed greatly reduced binding of anti-CUB antibodies upon analysis of plasma samples of a panel of 27 patients with iTTP. Functional analysis of the anti-CUB antibodies showed that all antibodies were able to induce an open conformation but did not inhibit activity toward either peptide substrates or von Willebrand factor multimers under flow conditions. Collectively, our findings show that anti-CUB antibodies target residues W1250/K1252 and R1367. Binding of pathogenic antibodies disrupt the spacer-CUB domain interface, thereby inducing an open conformation in ADAMTS13.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening bleeding disorder with an estimated prevalence of 2 to 6 cases per million per year.1 Patients with iTTP develop antibodies against ADAMTS13 that either inhibit its function or enhance its clearance from circulation.2-4 The lack of functional ADAMTS13 activity results in the accumulation of ultralarge von Willebrand factor (VWF) multimers, which leads to the formation of platelet-rich microthrombi.5,6
It is well established that most patients (>90%) with iTTP display antibodies against a shared epitope in the spacer domain of ADAMTS13, consisting of residues R568, F592, R660, Y661, and Y665.3,4,7-9 Modification of these residues to alanine variants efficiently prevents binding of the antispacer antibodies.3,7,9 A proportion of patients (20%-40%) also develops antibodies against the CUB (complement C1r/C1s, Uegf, Bmp1) domains of ADAMTS13.2-4,10,11 Unlike antispacer antibodies, anti-CUB antibodies do not inhibit ADAMTS13 activity, but, instead, have been suggested to enhance clearance of ADAMTS13 from the circulation.2,12,13 Additionally, anti-CUB antibodies, similarly to antispacer antibodies, can induce an open conformation.14-20 Interestingly, an open conformation of ADAMTS13 has been identified as a prognostic biomarker for relapse.16,18 Induction of an open conformation suggested that anti-CUB antibodies target residues located within the spacer-CUB interface of ADAMTS13.20 A recent study revealed that a patient-derived anti-CUB single-chain variable fragment (designated 4-41) bound to the CUB2 domain of ADAMTS13, specifically in the 1358-1377 region.19
Here, we explore whether anti-CUB antibodies have a shared epitope located on the CUB domains that overlaps with a previously identified spacer-CUB domain interface.21 Detailed information on the binding sites of anti–CUB domain antibodies advances our understanding of the pathogenic immune response in iTTP. Additionally, identification of CUB domain epitopes may guide the generation of antibody resistant variants of ADAMTS13. To identify these epitopes we generated, based on previously published sequences,22,23 7 patient-derived monoclonal antibodies (mAbs) that target the CUB domains, and 1 anti–spacer domain antibody. Functional characteristics of the patient-derived human mAbs were determined with regard to conformation and activity of ADAMTS13, either toward a peptide substrate or VWF multimer under flow conditions. Binding profiles were determined by using hydrogen-deuterium exchange mass spectrometry (HDX-MS) and an extensive alanine screen of the CUB domains. This allowed for the identification of an extended antigenic surface in the CUB1 and CUB2 domains of ADAMTS13. Subsequent analysis of a panel of 27 patient samples showed that this antigenic surface was also targeted by anti–CUB domain antibodies that develop in patients with iTTP, indicating that this is a shared epitope among patients.
Methods
Patient sample selection
All 27 citrated plasma samples have been obtained from the French thrombotic microangiopathies (TMA) Registry (principal investigator: P.C. and A.V.). Samples exhibit high titers of anti-ADAMTS13 antibodies (>50 U/mL), and display ADAMTS13 activity of <10 IU/dL, as determined by Technozym ADAMTS13-INH enzyme-linked immunosorbent assay (ELISA; Technoclone, Vienna, Austria) and the Fluorescence Resonance Energy Transfer Substrate (FRETS)-VWF73 method (Peptide Institute Inc, Osaka, Japan), as described previously.24 Informed consent according to the Declaration of Helsinki was obtained for each patient. The study was approved by the ethics committee of Hospital Pitié-Salpêtrière and Hospital Saint-Antoine Assistance Publique.
Production of antibodies and ADAMTS13 variants
Antibody amino acid sequences of the variable regions were obtained from a patent application (US2019/0031771 A1), ordered as synthetic gene fragments (GeneWiz), and cloned into a full-length immunoglobulin G1 (IgG1) construct. After sequencing, all antibodies were produced in Expi293 and purified by protein A. Synthetic gene fragments of the CUB-ALA variants were ordered at GeneWiz and cloned into the previously described wild-type ADAMTS13 (wtADAMTS13) or 5ALA construct using MreI and XhoI.25,26 All ADAMTS13 variants were expressed using the ExpiCHO system and purified by Twin-StrepTag. Additional information with regard to cloning and purification can be found in the supplemental Methods.
Binding ELISA optimal dilution of mAbs and patient samples
Alanine screening enzyme-linked immunosorbent assays (ELISAs) were performed to assess the binding of the previously mentioned antibodies (1-403, 1-407, 1-410, 1-416, 1-440, 1-441, z1-201, and z1-303) as well as antibodies present in the plasma of 27 patients with iTTP, as previously described.9,25-27 In short, ADAMTS13 (5.86 nM in 1% bovine serum albumin) and variants were captured on antimetalloprotease mAb 3H9–coated 96-well plates for 1 hour at 37°C.28 The II-1 antibody, which targets a cryptic site on the spacer domain, was used for a calibration curve ranging from 250 ng/μL to 0.11 ng/μL.29 mAbs were added at 0.033 nM, with exception of antibody 1-403, which was added at a final concentration of 0.165 nM. Patient samples were diluted according to the optimal dilution for each patient (25-200 fold). Antibodies or diluted patient samples were incubated for 1 hour at 37°C. Anti-IgG horseradish peroxidase was used for detection (1:10 000) and incubated for 1 hour at 37°C. Signal was developed 10 minutes using a TMB (3,3',5,5'-tetramethylbenzidine) mixture (0.42 mM TMB, 5% dimethyl sulfoxide, 110 mM sodium acetate [pH 5.5], and 0.015% H2O2,). The reaction was stopped using 1 M H2SO4 and the absorption at 450 nm was measured using the SpectraMax PLUS 384 (Molecular Devices). To determine binding relative to wtADAMTS13, background was subtracted, and optical density values were converted to relative binding usin g the II-1 calibration curve.
HDX-MS peptide database generation
For HDX-MS experiments, first a peptide database of ADAMTS13 was generated. For this, 30 pmol of ADAMTS13 was diluted in a volume of 6 μL of HDX-MS dilution buffer (25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl, 5 mM CaCl2; pH 6.0). Afterward, samples were handled by an autosampler (CTC PAL HTX-XT Dual Head Autosampler, Leap Technologies). The autosampler further diluted ADAMTS13 1:5 in HDX-MS dilution buffer to a final concentration of 0.83 μM. The samples were 1:1 diluted in quench buffer (8 M urea, 1 M TCEP [Tris(2-carboxyethyl)phosphine]; pH 3.0) and incubated for 2 minutes at 4°C. Samples were digested using an in-line 2.3 × 30 mm immobilized protease type XIII/pepsin column (weight-to-weight ratio, 1:2) (NBA2014005) for 2 minutes at 200 μL/min flow using isocratic flow buffer (0.1% formic acid in H2O). Peptides were captured and desalted using a C8 trap column (POROS R2 AL media) and separated on a C18 analytical column (POROS 20 AL media) using a 16-minute 5%-to-50% buffer B gradient by combining high-performance liquid chromatography buffer A (0.1% formic acid in H2O) and high-performance liquid chromatography buffer B (0.1% formic acid in 80% acetonitrile). MS1 and MS2 spectra were obtained at mass-to-charge ratio of 300 to 4000 (Thermo Fisher LTQ Orbitrap XL). All peptides were identified using PEAKS software (−logP > 15). Precursor mass, charge, and retention time were exported into HDExaminer and for MS1 identification of peptides for deuterium uptake. Coverage of the CUB domains (residue 1192-1427) was 82% (supplemental Figure 1).
HDX-MS deuterium uptake experiments
For deuterium uptake experiments, 30 pmol ADAMTS13 was incubated in a 1:1 ratio with the antibodies in 6 μL of H2O dilution buffer and incubated for 1 hour at room temperature. Samples were diluted 1:5 in D2O dilution buffer (25 mM HEPES, 150 mM NaCl, 5 mM CaCl2, 94.5% D2O; pH 6.0) and incubated for 0, 10, 100, or 1000 seconds at 24°C. Afterward, the samples were 1:1 diluted in quench buffer and incubated for 2 minutes at 4°C. To account for back-exchange, 100% deuterated states were determined experimentally by overnight incubation of ADAMTS13 in a D2O dilution buffer. Peptides were generated and separated in the same manner as described for the baseline measurements. MS1 spectra were obtained using the LTQ Orbitrap XL, deuterium uptake (%) was determined using HDExaminer. Uptake graphs were plotted using GraphPad Prism 10.2.3.
Functional characterization of anti-CUB antibodies
ADAMTS13 activity assays (and the conformational ELISA) were performed as previously reported.25 Detailed description of these assays as well as the surface plasmon resonance (SPR) method can be found in the supplemental Methods.
Results
Anti-CUB antibodies display high affinity toward ADAMTS13
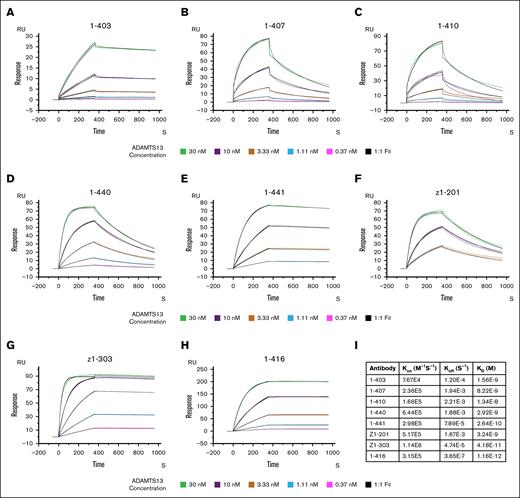
All patient-derived mAbs were screened for their binding affinities to ADAMTS13 using SPR (Figure 1A-H). The antibodies were immobilized on a protein G chip, after wtADAMTS13 was added at concentrations ranging from 0.37 nM to 30 nM. Interestingly, the binding curves of wtADAMTS to 1-407 and 1-410 did not fit a 1:1 binding model with full-length ADAMTS13 but only when using a TSP2-CUB variant lacking the MDTCS domains (supplemental Figure 2). All anti-CUB displayed binding affinities ranging from 1.6 nM to 42 pM (Figure 1I). Associations constants (Kon) values of anti-CUB antibodies were similar to that of the antispacer antibody (1-416). Marked differences in the rate of dissociation (Koff) were observed for the different antibodies, with lower relative Koff values for 1-403, 1-441, Z1-303, and 1-416 when compared with 1-407, 1-410, 1-440, and Z1-101. Overall, these data show that human mAbs bound with high affinity to ADAMTS13.
Anti-ADAMTS13 antibodies display high affinity for ADAMTS13. (A-H) Binding between 7 patient-derived anti-CUB antibodies and 1 antispacer antibody (1-416), and wtADAMTS13 as determined by SPR (n = 2). Figures display association (first 360 seconds) and dissociation (rest of the chromatogram) curves upon flowing of various concentration of ADAMTS13 across a chip coated with the respective antibody, the binding that occurs is expressed in response units. (I) Table of binding constants as determined by fitting of the experimental data using a 1:1 binding model. Green, 30 nM ADAMTS13; purple, 10 nM ADAMTS13; gold, 3.33 nM ADAMTS13; light blue, 1.11 nM ADAMTS13; and magenta, 0.37 nM ADAMTS13. Black, fit according to 1:1 binding model.
Anti-ADAMTS13 antibodies display high affinity for ADAMTS13. (A-H) Binding between 7 patient-derived anti-CUB antibodies and 1 antispacer antibody (1-416), and wtADAMTS13 as determined by SPR (n = 2). Figures display association (first 360 seconds) and dissociation (rest of the chromatogram) curves upon flowing of various concentration of ADAMTS13 across a chip coated with the respective antibody, the binding that occurs is expressed in response units. (I) Table of binding constants as determined by fitting of the experimental data using a 1:1 binding model. Green, 30 nM ADAMTS13; purple, 10 nM ADAMTS13; gold, 3.33 nM ADAMTS13; light blue, 1.11 nM ADAMTS13; and magenta, 0.37 nM ADAMTS13. Black, fit according to 1:1 binding model.
Effect of anti-CUB antibodies on proteolytic activity and ADAMTS13 conformation
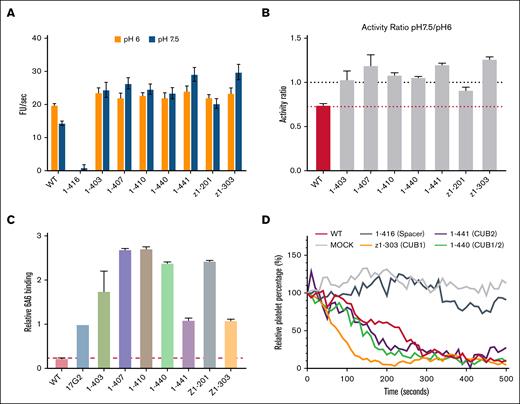
We characterized the effect of anti-CUB antibodies on ADAMTS13 activity using the FRETS-VWF73 substrate. Results obtained showed that, unlike the antispacer antibody (1-416), anti-CUB antibodies did not impair ADAMTS13 activity at either pH 6.0 or pH 7.5 (Figure 2A). Instead, the anti-CUB antibodies were able to increase activity at pH 7.5, shifting the ratio between the 2 activities to 1 (Figure 2B). Conformation of full-length ADAMTS13 is dependent on pH. ADAMTS13 is in a closed (less active) conformation at pH 7.5 and in an open (more active) conformation at pH 6. The increased activity observed at pH 7.5 suggests that the anti-CUB antibodies induce an open conformation. We used a previously established assay to monitor conformational changes within the protease domain using mAb 6A6, which only binds ADAMTS13 in an open conformation.15 As a control we used mAb 17G2 directed toward the CUB1 domain, which induces an open conformation of ADAMTS13.16-18,20 Human mAbs 1-441 and z1-303 induced an open conformation of ADAMTS13 in a manner similar to 17G2 (Figure 2C). Incubation of the other CUB domain antibodies 1-403, 1-407, 1-410, 1-440, and Z1-201 resulted in enhanced binding of 6A6, suggesting that the cryptic epitope recognized by 6A6 is more fully exposed upon incubation with these anti-CUB antibodies (Figure 2C).
Anti-CUB antibodies induce an open conformation and do not inhibit ADAMTS13 activity. (A) Activity of wtADAMTS13 in absence or presence of antibodies toward the FRETS-VWF73 substrate at pH 6 or pH 7.5 (n = 3). (B) Ratio of activity at pH 7.5 divided by activity at pH 6, as in indicator of conformation (n = 3). (C) Binding of wtADAMTS13 in the presence or absence of anti-CUB antibodies to the 6A6 antibody (n = 3). All binding is relative to wtADAMTS13 in the presence of the activating 17G2 antibody. (D) Activity of wtADAMTS13, in presence or absence of antispacer or anti-CUB antibodies, toward platelet-bound VWF multimers under flow (n = 2). Platelets bound to VWF multimers are counted over time and expressed as a percentage relative to the starting frame. Loss of platelets is the result of cleavage of the VWF multimer strings. FU,fluorescent unit; WT, wild-type ADAMTS13.
Anti-CUB antibodies induce an open conformation and do not inhibit ADAMTS13 activity. (A) Activity of wtADAMTS13 in absence or presence of antibodies toward the FRETS-VWF73 substrate at pH 6 or pH 7.5 (n = 3). (B) Ratio of activity at pH 7.5 divided by activity at pH 6, as in indicator of conformation (n = 3). (C) Binding of wtADAMTS13 in the presence or absence of anti-CUB antibodies to the 6A6 antibody (n = 3). All binding is relative to wtADAMTS13 in the presence of the activating 17G2 antibody. (D) Activity of wtADAMTS13, in presence or absence of antispacer or anti-CUB antibodies, toward platelet-bound VWF multimers under flow (n = 2). Platelets bound to VWF multimers are counted over time and expressed as a percentage relative to the starting frame. Loss of platelets is the result of cleavage of the VWF multimer strings. FU,fluorescent unit; WT, wild-type ADAMTS13.
In physiological conditions, it has been shown that the CUB domains bind the D4 domain of VWF.30-32 Loss of the CUB domains, also resulted in loss of activity toward the full-length VWF multimers.32-35 Therefore, the activity of ADAMTS13 was studied in the absence or presence of antibodies in the flow assay, in which VWF multimers are secreted from HUVECs under flow (Figure 2D; supplemental Figure 3). The antispacer antibody 1-416 strongly inhibited cleavage of the VWF multimers. In contrast, in the presence of anti-CUB antibodies, targeting either CUB1 (z1-303), CUB2 (1-441), or an overlapping CUB1/2 epitope (1-440), ADAMTS13 remained able to efficiently cleave the VWF multimers.
HDX reveals key binding sites for anti-CUB antibodies
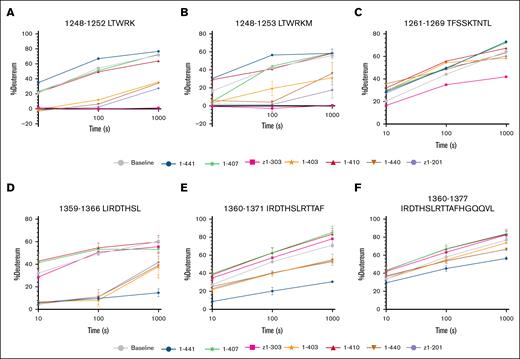
To determine anti-CUB antibody binding regions, we used HDX-MS to identify peptide sequences that show reduced deuterium uptake in the presence of anti-CUB antibodies. HDX-MS measurements revealed strong binding of 1-403, 1-440, z1-201, and z1-303 in the CUB1 domain to peptides 1248-1252 and 1248-1253, as indicated by the strongly reduced deuterium uptake (Figure 3A-B). In contrast, 1-441 appeared to slightly enhance deuterium exchange by these peptides. Additionally, a minor decrease in deuterium uptake was observed for z1-303 on peptide 1261-1269 (Figure 3C).
Deuterium uptake plots of CUB domain peptides with reduced uptake. (A-C) Peptides located within the CUB1 domain of ADAMTS13. (D-F) Peptides located within the CUB2 domain of ADAMTS13. Uptake of ADAMTS13 in absence of antibodies is colored in grey. All uptake experiments were performed in triplicate.
Deuterium uptake plots of CUB domain peptides with reduced uptake. (A-C) Peptides located within the CUB1 domain of ADAMTS13. (D-F) Peptides located within the CUB2 domain of ADAMTS13. Uptake of ADAMTS13 in absence of antibodies is colored in grey. All uptake experiments were performed in triplicate.
Also, several peptides located in the CUB2 domain of ADAMTS13 were targeted. In the presence of 1-403, 1-440, 1-441, and z1-201, reduced deuterium exchange on peptides 1359-1366 and 1360-1371 was observed (Figure 3D-E). For antibodies 1-403, 1-440, and z1-201, the largest effects were observed for peptide 1359-1366. Antibody 1-441 induced a similar reduction in deuterium exchange for both peptides, suggesting that contact residues for this antibody were shared between both of these peptides. Additionally, peptide 1360-1377 showed a minor reduction in deuterium uptake for 1-441 (Figure 3F). For antibody 1-407 and 1-410, no peptides were observed with reduced deuterium exchange uptake.
Binding of antibodies to CUB domain single-alanine variants
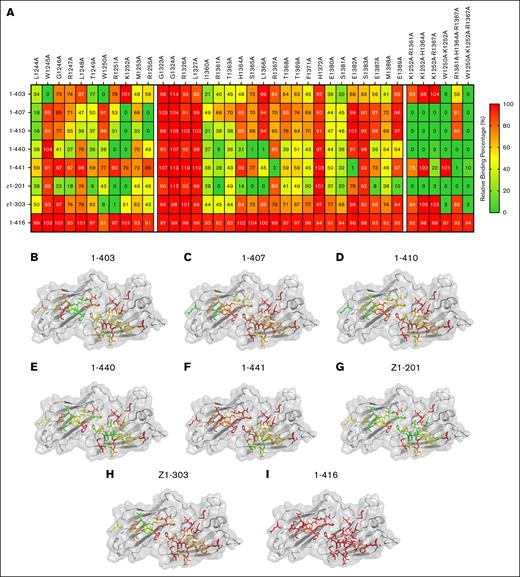
To confirm our observations with HDX-MS, an alanine screen of the CUB-domains was performed. Specific residues were selected according to the following criteria: firstly, mutated residues were those that showed reduced deuterium uptake, as well as extensions of these peptides (residues 1244-1255 and 1360-1372); secondly, we included residues that had previously been suggested to be involved in the spacer-CUB domain interactions (residues 1323-1327, 1379-1383, and 1387-1390).21 In total, 38 single-alanine substitution variants were designed, of which 33 were successfully expressed. C1254A, C1325A, D1362, W1379A, and F1390A did not show any protein expression (data not shown). Of the expressed variants, 11 were in the CUB1 domains, and 22 were in the CUB2 domain. The effect of each of the mutations on the binding of the patient-derived mAbs was explored by ELISA (Figure 4A). Binding values were then mapped onto the structure of the CUB domains (PDB: 7B01) for each of the antibodies (Figure 4B-I). The binding data from the alanine screen revealed that 1-403, 1-407, 1-410, and z1-303 predominantly bound to amino acids located in the CUB1 domain of ADAMTS13. Hydrophobic residues W1245 and W1250 were crucial for the binding of 1-403 to the CUB1 domain. For 1-407 and 1-410, K1252 and R1255 were identified as key binding residues (Figure 4A). Binding of z1-303 was critically dependent on W1250 and R1251. Interestingly, binding of antibody 1-440 and z1-201 was dependent on residues R1251 and K1252 in the CUB1 domain as well as on residues R1361, S1365, and L1366 in the CUB2 domain. These findings show that residues from both CUB1 and CUB2 domain contribute to the antigen surface of 1-440 and z1-201. Binding of antibody 1-441 was critically dependent on the presence of R1367 and E1382 in the CUB2 domain; changes in the CUB1 domain did not affect the binding of 1-441 to ADAMTS13. Overall, these data suggest the presence of an extended antigenic surface composed of residues derived from both the CUB1 and CUB2 domains.
Binding of patient-derived antibodies to single or combination alanine variants. (A) Heat map of the average binding of patient-derived antibodies to various CUB domain alanine variants, expressed as a percentage relative to wtADAMTS13 binding (n = 3). (B-H) Relative binding percentage of 7 patient-derived anti-CUB antibodies against single-alanine variants displayed on the structure of the CUB domains of ADAMTS13. (I) Patient-derived antispacer antibody (1-416) does not show any alterations in binding toward CUB domain alanine variants.
Binding of patient-derived antibodies to single or combination alanine variants. (A) Heat map of the average binding of patient-derived antibodies to various CUB domain alanine variants, expressed as a percentage relative to wtADAMTS13 binding (n = 3). (B-H) Relative binding percentage of 7 patient-derived anti-CUB antibodies against single-alanine variants displayed on the structure of the CUB domains of ADAMTS13. (I) Patient-derived antispacer antibody (1-416) does not show any alterations in binding toward CUB domain alanine variants.
Binding of antibodies to CUB domain combination variants
Various combinations of key residues (W1250, K1252, R1361, H1364, and R1367) were explored. Combination variants including the R1361A mutation showed reduced expression levels (data not shown). A W1250A/K1252A/R1361A/H1364A/R3167A variant containing a combination of all 5 alanines was also not released into the medium, most likely due to misfolding (data not shown). We therefore excluded this 5-alanine CUB domain variant from our panel of CUB domain variants. Combination variants of 1252A with 1361A or 1364A showed binding profiles for the human anti-CUB antibodies similar to that of 1252A by itself, indicating no additional benefit (Figure 4A; right panel). In contrast, addition of 1367A prevented binding of human mAb 1-441. Combination of 1250A with 1252A, both located in the CUB1 domain, prevented binding of 6 of 7 anti-CUB domain antibodies. Binding of antibody 1-441, which was dependent on CUB2 domain residues, was not reduced by replacing W1250 and K1252 by an alanine (Figure 4A; right panel). A combination variant, consisting of only mutants located in the CUB2 domain (1361A/1364A/1367A), reduced binding of 1-440, 1-441, and Z1-201, and to a lesser extent binding of 1-403. A triple variant, comprising substitutions in the CUB1 and CUB2 domain (W1250A, K1252A, and R1367A), was capable of preventing binding of all human anti-CUB domain antibodies (Figure 4A; right panel). Our results suggest that 2 key residues in the CUB1 domain, and 1 key CUB2 domain residue provide an antigenic hot spot for the binding of patient-derived anti-CUB domain antibodies (Figure 4A).
Binding of patient plasma-derived anti-ADAMTS13 antibodies to the CUB domain variants
To understand whether the epitope that was identified by HDX-MS and the alanine substitutions was present in the broader iTTP population, we screened a subset of the CUB domain variants using the plasmas of 27 patients with iTTP. These patients were selected based on the presence of a subset of antibodies that did not target the spacer domain.26 To further characterize this cohort, we assessed the binding of patient-derived antibodies to wtADAMTS13 and a previously described spacer domain variant in which residues 568, 592, 660, 661, and 665, which comprise an immunodominant epitope in the spacer domain, were replaced by alanine residues (5ALA). The inclusion criteria for the plasma samples in this panel was that at least 25% of the binding was accounted for by nonspacer antibodies as reflected by the residual binding of >25% to the 5ALA spacer domain variant (Figure 5A). CUB-ALA variants were then combined with the 5ALA variant to explore their effect on the binding on nonspacer antibodies (Figure 5A). Average binding of the patient samples to the 5ALA variant was 59% compared with wtADAMTS13 (Figure 5B). All CUB-ALA variants further reduced the binding of iTTP antibodies (Figure 5B). Binding of the antibodies present in the patient samples to the single CUB-ALA variants, 5ALA-1250A, 5ALA-1252A, and 5ALA-1367A, was 40%, 42%, and 49%, respectively (Figure 5B). The combination variant 5ALA-1250A-1252A was more effective than single variants, with 33% binding compared with wtADAMTS13, a reduction of 26% compared with the 5ALA variant (Figure 5B-C). Moreover, a triple mutant, consisting of 1250A, 1252A, and 1367A, was the most effective, with a total binding of 28%, or 31% less than that of 5ALA (Figure 5B-C). The benefit of the addition of 1367A to the 1250A-1252A variants was variable for different patient samples analyzed (Figure 5A,D). For 21 of 27 patient samples, a clear reduction in binding was observed upon combining the triple CUB-ALA variant and the 5ALA variant. Six patients (rAD13-064, rAD13-071, rAD13-088, rAD13-091, LRB20, and LRB32) with high levels of nonspacer antibodies still displayed relatively high levels of binding (ranging from 50%-68% residual binding when compared with wtADAMTS13), even after introduction of the triple CUB-ALA variant.
Binding of antibodies present in plasma from 27 different patients with iTTP against CUB domain alanine variants. (A) Heat map of the average binding of antibodies present in plasma of patients with iTTP to various CUB domain and spacer domain alanine combination variants, expressed as a percentage relative to wtADAMTS13 binding (n = 3). (B) Average (± standard deviation) binding of patient samples to all tested ADAMTS13 variants, relative to wtADAMTS13. Significance was determined using a paired repeated-measures 1-way analysis of variance (ANOVA), with Geisser-Greenhouse correction, followed by a post hoc Tukey multiple comparisons test. (C) The relative reduction of antibody binding to CUB domain alanine variants combined with spacer 5ALA, as compared with binding to the 5ALA variants. (D) Linked scatterplot of binding of patient samples to 5ALA, 5ALA-1250A-1252A, and 5ALA-1250A-1252A-1367A, showing the effect per patient. Significance was determined using a paired repeated-measures 1-way ANOVA, with Geisser-Greenhouse correction, followed by a post hoc Tukey multiple comparisons test. ∗∗∗∗, P < .0001; ∗∗∗, P < .001; WT, wild-type.
Binding of antibodies present in plasma from 27 different patients with iTTP against CUB domain alanine variants. (A) Heat map of the average binding of antibodies present in plasma of patients with iTTP to various CUB domain and spacer domain alanine combination variants, expressed as a percentage relative to wtADAMTS13 binding (n = 3). (B) Average (± standard deviation) binding of patient samples to all tested ADAMTS13 variants, relative to wtADAMTS13. Significance was determined using a paired repeated-measures 1-way analysis of variance (ANOVA), with Geisser-Greenhouse correction, followed by a post hoc Tukey multiple comparisons test. (C) The relative reduction of antibody binding to CUB domain alanine variants combined with spacer 5ALA, as compared with binding to the 5ALA variants. (D) Linked scatterplot of binding of patient samples to 5ALA, 5ALA-1250A-1252A, and 5ALA-1250A-1252A-1367A, showing the effect per patient. Significance was determined using a paired repeated-measures 1-way ANOVA, with Geisser-Greenhouse correction, followed by a post hoc Tukey multiple comparisons test. ∗∗∗∗, P < .0001; ∗∗∗, P < .001; WT, wild-type.
Discussion
Patients with iTTP develop pathogenic auto-antibodies against ADAMTS13. In ∼90% of patients, these antibodies target an epitope located within the spacer domain, consisting of 5 key residues, R568, F592, R660, Y660, and Y661.3,4,7-9 These antibodies strongly inhibit ADAMTS13 activity. A smaller subset of patients (20%-40%) display antibodies against other domains of ADAMTS13, including the CUB1 and CUB2 domain, which accelerate clearance of ADAMTS13 from the plasma.2-4,10-13 Here, we generated full-length IgG of 7, previously published,22 patient-derived anti-CUB domain antibodies. The impact of these antibodies on conformation and activity was studied. Additionally, using HDX-MS and alanine substitutions, we identified a distinct epitope composed of W1250, K1252, and R1367, which is targeted by these anti-CUB antibodies.
The HDX-MS data showed that antibodies targeted both CUB domains of ADAMTS13, as demonstrated by reduction in deuterium uptake on peptides 1248-1252 and 1248-1253 in the CUB1 domain, as well as 1359-1366 and 1360-1371 in the CUB2 domain. The alanine substitution data further refined, as well as expanded, these epitopes. Important residues within the CUB1 domain included, W1245, G1246, R1247, T1249, W1250, R1251, K1252, and R1255. Also, substitutions of R1361, H1364, S1365, L1366, R1367, S1381, E1382, E1387, and E1389 in the CUB2 domain resulted in reduced antibody binding. The combination of K1252A and W1250A reduced the binding of 6 of 7 anti-CUB antibodies, with only the CUB2-targeting 1-441 antibody still binding to that variant. Addition of R1367A to the double mutant abolished the binding of all patient-derived antibodies.
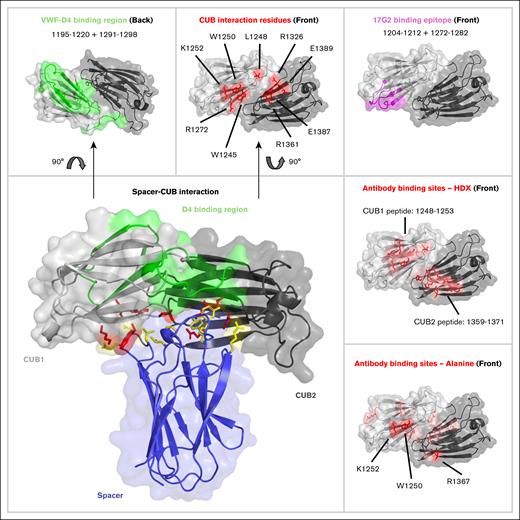
Both the HDX-MS data as well as the alanine substitutions showed that the binding site of the anti-CUB antibodies overlap with residues involved in the spacer-CUB interaction, as reported in literature (Figure 6). Key residues for this interaction include W1245, L1248, W1250, K1252, and R1272 in the CUB1 domain, and R1326, R1361, E1387, and E1389 in the CUB2 domain.21
Comparison of residues located on the CUB domain of ADAMTS13 that are involved in binding of the spacer domain, and residues that are targeted by anti-CUB antibodies. Antibody epitopes as identified by HDX-MS or an alanine scan of the CUB domains, reveal that the residues overlap with the previously identified residues for maintaining the spacer-CUB interaction, but not with residues involved in the binding of the D4 domain of VWF. CUB domain PDB:7B01. Spacer domain PDB:6QIG.
Comparison of residues located on the CUB domain of ADAMTS13 that are involved in binding of the spacer domain, and residues that are targeted by anti-CUB antibodies. Antibody epitopes as identified by HDX-MS or an alanine scan of the CUB domains, reveal that the residues overlap with the previously identified residues for maintaining the spacer-CUB interaction, but not with residues involved in the binding of the D4 domain of VWF. CUB domain PDB:7B01. Spacer domain PDB:6QIG.
SPR binding analysis of the anti-CUB antibodies revealed high affinity binding to ADAMTS13 (42 pM-1.6 nM). Two antibodies, 1-407 and 1-410, did not bind wtADAMTS13 according to a 1:1 model but did for the TSP2-CUB2 variant. This suggests that the presence of the MDTCS region alters the binding kinetics of these antibodies to ADAMTS13. This could indicate that these antibodies target an epitope that contains residues both on the CUB as well as the spacer domain. Additional support for this hypothesis is present in the original publication of these antibodies, showing that 1-410 bound both TSP5-CUB2 (spanning from TSP5 until CUB2) as well as the CS fragment (containing only the cysteine-rich and spacer domain).22
Anti-CUB antibodies induced an open conformation, as shown by both the FRETS-VWF73 activity assay as well as the conformational ELISA using the 6A6 antibody. The anti-CUB antibodies increased binding to the 6A6 antibody to a larger extent than the murine mAb 17G2 (Figure 2C). Recently, it was shown that 17G2 targets residues 1203-1216 and 1270-1284 peptides in the CUB1 domain,20 which do not overlap with the antigenic hot spot (W1250, K1252, and R1367), as identified in this study (Figure 6). We propose that the elevated binding observed for the human mAbs used in this study is due to the overlap of their binding site with residues involved in spacer-CUB domain interactions.21
Our flow data showed that anti-CUB antibodies do not inhibit ADAMTS13 activity toward VWF, unlike antispacer antibodies (this study).7 This suggests that the binding site of the D4 domain of VWF does not overlap with the binding site of the anti-CUB antibodies identified in this study. This finding is supported by previous findings showing that peptide 1195-1220 and 1291-1298, which is located on the opposite side of the antigenic epitope on the CUB domains, are crucial for ADAMTS13 activity under flow (Figure 6).35
Screening the identified triple variant (W1250A-K1252A-R1367A) against a population of 27 patients with iTTP that contained non–spacer-targeting antibodies revealed that it was effective at reducing the binding of these antibodies, indicating this epitope is a common target of anti-CUB domain antibodies. Most of the effect of the triple mutant was driven by W1250A and K1252A, with the addition of R1367A only having a minor benefit over the double mutant. Additionally, binding of only 1 of 7 patient-derived antibodies was affected by this variant, indicating that the shared anti-CUB epitope primarily consists of the CUB1 domain residues. This is further supported by the relatively large distance (∼19.5 Å) between R1367 and W1250/K1252 (Figure 6).
Interestingly, 6 patients (rAD13-064, rAD13-071, rAD13-088, rAD13-091, LRB20, and LRB32) still had >50% of binding relative to wtADAMTS13, despite the introduction of the spacer 5ALA as well as the triple CUB-ALA mutations. This highlights the presence of an even smaller subset of antibodies with a distinct binding site in patients with iTTP. Patient LRB20 displayed 25% binding to the MDTCS-5ALA variants, indicating that part of its unidentified response is against the MDTCS region. However, for the other 5 patients, the remaining antibodies target either the TSP domains, or an area on the CUB domains that was not included in this study. Both antispacer and anti-CUB antibodies target epitopes on ADAMTS13 that are cryptic in a closed conformation.3,4,7-9 Therefore, it could be possible that these unidentified epitopes could adhere to the same principle. A potential binding site for this specific subset of patient-derived antibodies could be located within the TSP8 domain of ADAMTS13. SAXS data of ADAMTS13 have shown that TSP7 and TSP8 are in close proximity to the MDTCS region.36 Additionally, anti-TSP8 mouse antibodies were able to induce an open conformation of ADAMTS13 and increase activity.15 Together, this suggests that cryptic epitopes might be available on the TSP8 domains, which seems to be a prerequisite for antibody development.
In conclusion, our data show that anti-CUB antibodies target a cryptic epitope (consisting of W1250, K1252, and R1367) located in the CUB1 and CUB2 domain of ADAMTS13, that is otherwise concealed by the spacer domain, similar to how antispacer antibodies target an epitope concealed by the CUB domain. This CUB domain epitope was shared among multiple patients with iTTP. Binding of the anti-CUB antibodies to the CUB domain of ADAMTS13 induce an open conformation, most likely by competing for spacer domain binding. Binding of anti-CUB antibodies did not inhibit ADAMTS13 function, not in static nor in flow assays, consistent with a model in which VWF and anti-CUB domain antibodies bind to opposite sites on the spacer domain. The triple-alanine variant described in this study (W1250A/K1252A/R1367A) could potentially serve as an improved recombinant protein therapy for iTTP, because the antibodies epitopes are removed, thereby avoiding antibody-mediated clearance of ADAMTS13.
Acknowledgments
This study was financed by grants from the Landsteiner Foundation for Blood Transfusion Research (LSBR 2026).
The funding agencies had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Authorship
Contribution: T.P., G.N., and J.V. designed the study; T.P. and R.J. generated ADAMTS13 variants and patient-derived antibodies, and performed antibody and patient-derived antibody binding experiments; T.P. and P.K. performed surface plasmon resonance experiments; T.P., R.J., C.v.d.Z., F.v.A., and A.J.H. performed hydrogen-deuterium exchange mass spectrometry experiments; T.P., R.J., P.K., G.N., and J.V. analyzed and interpreted the data; B.S.J., A.V., and P.C. provided patient samples; K.V. provided mouse monoclonal antibodies that were used in this study; and T.P. and J.V. wrote the first draft of the manuscript.
Conflict-of-interest disclosure: T.P and J.V. are listed on a patent applications regarding ADAMTS13. P.C. is a member of the clinical advisory board for Alexion, Sanofi, Takeda, and Janssen. A.V. is a member of the advisory boards for Sanofi, Takeda, and LFB-Biomédicaments. B.S.J. received speaker fees from Sanofi, Takeda, and LFB-Biomédicaments. The remaining authors declare no competing financial interests.
Correspondence: Jan Voorberg, Department of Molecular Hematology, Sanquin-Amsterdam University Medical Center Landsteiner Laboratory, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; email: j.voorberg@sanquin.nl.
References
Author notes
Original data are available on request from the corresponding author, Jan Voorberg (j.voorberg@sanquin.nl).
The full-text version of this article contains a data supplement.