Key Points
Older MSDs or young HIDs lead to similar survival in older patients with AML.
Young HID is associated with less relapse, whereas older MSD is associated with less treatment-related mortality.
Visual Abstract
Selection of a suitable donor for allogeneic hematopoietic stem cell transplantation (allo-HCT) has mainly relied on HLA matching and, to date, a matched sibling donor (MSD) remains the first choice. However, patients with acute myeloid leukemia (AML) are older and therefore tend to have older siblings. Haploidentical donors (HIDs) are easily available, and offspring are younger than siblings. As donor age has been associated with worse outcomes, a younger HID might be a better choice than an older MSD for older patients with AML who receive transplantation in first complete remission (CR1). From the European Society for Blood and Marrow Transplantation registry database, we selected patients with AML aged ≥60 years who received transplantation in CR1, either from MSD aged ≥50 years or HID ≤40 years. HIDs received posttransplant cyclophosphamide as graft-versus-host disease (GVHD) prophylaxis, and MSDs received in vivo T-cell depletion. A total of 1247 patients were identified, including 721 MSDs and 526 HIDs. In univariate analysis, HID was associated with lower relapse incidence (P = .01), higher nonrelapse mortality (NRM; P = .01). The 2-year probability of overall survival (OS), leukemia-free survival (LFS), and GVHD-free and relapse-free survival (GRFS) were 62.5%, 56%, and 47%, respectively for the all population. In multivariate analysis, we confirmed that HID was associated with less relapse but more NRM, which translated into similar OS, LFS, and GRFS. Based on this retrospective study, young HIDs led to less relapse but higher NRM than older MSDs after allo-HCT in an older population with AML in CR1.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) remains, to date, the best consolidation strategy for patients with adverse-risk acute myeloid leukemia (AML) and potentially for those with intermediate-risk AML.1,2 A matched sibling donor (MSD) remains the first choice, with lower nonrelapse mortality (NRM) and a lower rate of acute graft-versus-host disease (GVHD).3 AML incidence increases with age, and with the advances in conditioning regimens and GVHD prophylaxis, allo-HCT is currently routinely performed in an older population with improved outcomes.4 However, older patients have older sibling donors, and donor age has been associated with higher NRM due to increased incidence of GVHD and/or slower immune recovery.5,6 Therefore, an MSD aged >67 years should be avoided.7 This statement, however, is not always the case, and in a retrospective European Society for Blood and Marrow Transplantation (EBMT) study in older patients with AML harboring FLT3-ITD, the impact of donor type was stronger than donor age. In other words, an MSD should not be excluded up front based on age.8 If a young matched unrelated donor (MUD) has been favored in multiple studies,5,6,9 it is not easily available when allo-HCT is urgent, and we know that delay in donor selection affect survival.10 On the contrary, a haploidentical donor (HID) is readily available and has become more frequently used with the emergence of posttransplant cyclophosphamide (PTCy) as GVHD prophylaxis. Outcomes using HIDs appear similar to other alternative donor types, such as mismatched unrelated11 or cord blood.12 Different studies have explored comparative analyses of outcomes between MSD, MUD, and HID showing similar outcomes13,14 or better survival with MSD, confirming the importance of HLA matching even in the PTCy era.15,16 However, the impact of donor age has also been demonstrated with an HID17,18 and a lower NRM is observed with a young HID compared with an older one. Nevertheless, young HID results in higher levels of NRM than with young MUD.19 Moreover, this detrimental effect of donor age is mainly observed in older patients20,21 and, for older patients, young offspring might be recommended over older sibling donors.21 To elucidate the best donor option in an urgent setting, we compared outcomes of young HIDs vs older MSDs in a homogeneous population of older patients with AML who underwent transplantation in first complete remission (CR1).
Methods
Patient selection and data collection
This is a retrospective EBMT registry-based analysis. The EBMT is a nonprofit, scientific society representing >600 transplant centers mainly in Europe, that are required to report all consecutive stem cell transplantations and their follow-up once a year. Data are entered, managed, and maintained in a central database with Internet access; each EBMT center is represented in this database. Audits are routinely performed to determine the accuracy of the data. Patients or legal guardians provide informed consent authorizing the use of their personal information for research purposes. The study was approved by the Acute Leukemia Working Party review board.
Eligibility criteria for the study included all patients ≥60 years with de novo or secondary AML who received transplantation between 1 January 2012 and 31 December 2022, from either an MSD aged ≥50 years or an HID ≤40 years. Those cutoffs have been chosen to artificially select matched sibling and haploidentical offspring. Patients undergoing a second transplantation were excluded. We further selected patients with intermediate- or adverse-risk cytogenetics according to the European LeukemiaNet 2022 recommendations.1 All recipients of allo-HCT from MSDs received GVHD prophylaxis based on in vivo T-cell depletion (TCD) with either antithymocyte globulin (ATG) or alemtuzumab. All recipients of allo-HCT from HIDs received PTCy-based GVHD prophylaxis with or without additional ATG.
A total of 1247 patients from 209 centers met the study inclusion criteria and were selected for further analysis. Myeloablative conditioning was defined as a regimen containing either total body irradiation with a dose >6 Gy, a total dose of oral busulfan (Bu) >8 mg/kg, or a total dose of intravenous Bu >6.4 mg/kg. All other regimens were defined as reduced-intensity conditioning.22 Conditioning regimens were subsequently stratified according to the transplant conditioning intensity score as previously described.23
The following variables were included in the analysis: year of transplantation, patient age at HCT, sex, white blood cell count at diagnosis, de novo or secondary AML diagnosis, number of induction courses to achieve CR, time from diagnosis to allo-HCT, type of conditioning regimen, use of total body irradiation, in vivo TCD (including both ATG and alemtuzumab), use of PTCy, cytomegalovirus status of donor and recipient, donor type, donor age, source of stem cells, Karnofsky performance status score at transplantation, engraftment, presence of acute and chronic GVHD, grade of acute GVHD, and severity of chronic GVHD. Secondary AML has been defined as AML with a previous diagnosis of myelodysplastic syndromes or myeloproliferative neoplasms, or AML secondary to cytotoxic therapies. Molecular data were unfortunately missing for many patients, and we therefore did not include those data in our final analysis.
Statistical analysis and end points definitions
The primary end point was GVHD-free and relapse-free survival (GRFS). Secondary end points included relapse incidence (RI), NRM, leukemia-free survival (LFS), overall survival (OS), and acute and chronic GVHD incidence. All outcomes were measured from the time of transplantation. GRFS was defined as survival without grade 3 to 4 acute GVHD, extensive chronic GVHD, relapse, or death.24 NRM was defined as death without previous relapse. LFS was defined as survival without relapse; patients alive without relapse were censored at the time of last contact. OS was based on death from any cause. The probabilities of GRFS, LFS, and OS were calculated by the Kaplan-Meier method, and those of acute and chronic GVHD, NRM, and RI by the cumulative incidence function to accommodate competing risks. For NRM, relapse was the competing risk, and for relapse, the competing risk was NRM. For acute and chronic GVHD, death without the event or relapse were the competing risks. Univariate comparisons between groups were performed using the χ2 and Fisher exact test for categorical variables, and the Mann-Whitney U test for continuous variables, the Gray statistic for cumulative incidence functions (GVHD, NRM, and RI), and the log-rank test for survival outcomes (OS, LFS, and GRFS). For all univariate analyses, continuous variables were categorized, and the median value was used as a cutoff point. A Cox proportional hazards model was used for multivariate regression, including factors differing in distribution between the groups or those considered to be conceptually important. As there was a collinearity between in vivo TCD and donor type, we excluded in vivo TCD from the multivariate analysis. To test for a center effect, we introduced a random effect or frailty for each center into the model. Proportional hazards assumptions were checked systematically using the Grambsch-Therneau residual–based test. Results were expressed as hazard ratio (HR) with 95% confidence interval (CI). Statistical analyses were performed with SPSS 25.0 (SPSS Inc, Chicago, IL) and R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Results
Patient characteristics
Table 1 summarizes patient characteristics according to donor type. A total of 1247 patients were identified, including 721 MSDs and 526 HIDs. The median patient age was 65 years (interquartile range [IQR], 62-67), and the median follow-up was 24 months (IQR, 22-26), significantly longer for MSDs than HIDs. The median age of MSDs was 61 years (IQR, 63-67), and the median age of HIDs was 33 years (IQR, 29-37). No difference was observed between groups with respect to cytogenetic risk category, or diagnosis of de novo or secondary AML. We found a higher frequency of female donor to male recipient combination in MSD allo-HCT and more recipient cytomegalovirus positivity in HID allo-HCT. The time from diagnosis to allo-HCT was significantly longer in HID compared with MSD grafts (P < .0001). Bone marrow as the stem cell source was used in 3% of transplants from MSDs and 20% of transplants from HIDs (P < .0001). A myeloablative conditioning regimen was administered to 18% and 22% of recipients of transplants from MSDs and HIDs, respectively. ATG use was significantly higher in MSD patients (83%) than in HID patients (8%; P < .001). The main conditioning regimens were fludarabine and Bu in MSD allo-HCT, and thiotepa, Bu and fludarabine in HID allo-HCT (results not shown).
Patient characteristics (N = 1247)
| . | MSD age ≥50 years (n = 721) . | HID age ≤40 years (n = 526) . | P value . |
|---|---|---|---|
| Median follow-up (IQR), mo | 37 (31-42) | 24 (22-26) | <.0001 |
| Patient age, median (range), y | 65 (60-75) | 65 (60-82) | .17 |
| Donor age, median (range), y | 61 (58-65) | 33 (18-40) | <.0001 |
| Year of allo-HCT, median (range) | 2018 (2012-2022) | 2020 (2012-2022) | <.0001 |
| De novo/sAML (%) | 83/17 | 85/15 | .47 |
| Cytogenetics risk, Int/Adv (%) | 70/30 | 72/28 | .39 |
| Sex, M/F (%) | 58/42 | 68/32 | .0006 |
| F donor to M recipient (%) | 33 | 21 | <.0001 |
| CMV+ recipients (%) | 67 | 79 | .0003 |
| Diagnosis to allo-HCT (range), mo | 4.7 (2-22) | 5.3 (2-23) | <.0001 |
| PB/BM (%) | 97/3 | 80/20 | <.0001 |
| MAC/RIC (%) | 18/82 | 22/78 | .06 |
| TCI 1-2/2.5-3.5/4-6 (%) | 56/41/3 | 61/36/2 | .2 |
| ATG/alemtuzumab (%) | 83/17 | 8/0 | <.0001 |
| KPS ≥90 (%) | 67 | 71 | .06 |
| . | MSD age ≥50 years (n = 721) . | HID age ≤40 years (n = 526) . | P value . |
|---|---|---|---|
| Median follow-up (IQR), mo | 37 (31-42) | 24 (22-26) | <.0001 |
| Patient age, median (range), y | 65 (60-75) | 65 (60-82) | .17 |
| Donor age, median (range), y | 61 (58-65) | 33 (18-40) | <.0001 |
| Year of allo-HCT, median (range) | 2018 (2012-2022) | 2020 (2012-2022) | <.0001 |
| De novo/sAML (%) | 83/17 | 85/15 | .47 |
| Cytogenetics risk, Int/Adv (%) | 70/30 | 72/28 | .39 |
| Sex, M/F (%) | 58/42 | 68/32 | .0006 |
| F donor to M recipient (%) | 33 | 21 | <.0001 |
| CMV+ recipients (%) | 67 | 79 | .0003 |
| Diagnosis to allo-HCT (range), mo | 4.7 (2-22) | 5.3 (2-23) | <.0001 |
| PB/BM (%) | 97/3 | 80/20 | <.0001 |
| MAC/RIC (%) | 18/82 | 22/78 | .06 |
| TCI 1-2/2.5-3.5/4-6 (%) | 56/41/3 | 61/36/2 | .2 |
| ATG/alemtuzumab (%) | 83/17 | 8/0 | <.0001 |
| KPS ≥90 (%) | 67 | 71 | .06 |
Adv, adverse; BM, bone marrow; CMV, cytomegalovirus; F, female; Int, intermediate; KPS, Karnofsky performance status; M, male; MAC, myeloablative conditioning; PB, peripheral blood; RIC, reduced-intensity conditioning; sAML, secondary AML; TCI, transplant conditioning intensity.
Univariate analysis of transplant outcomes
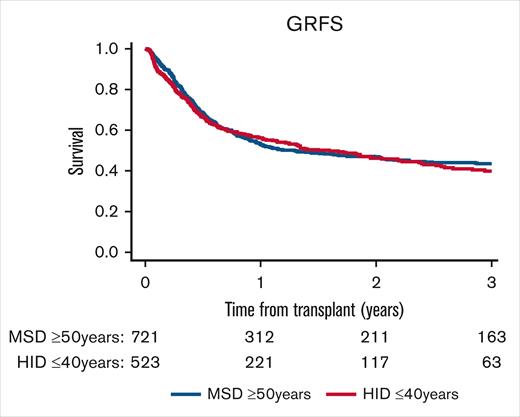
The 2-year probability of GRFS was 46.8% (95% CI, 42.8-50.7) for MSDs and 46.2% (95% CI, 41.2-51) for HIDs (P = .76), as illustrated in Figure 1. The 2-year RI was 28.6% (95% CI, 25.1-32.2) for MSDs, significantly higher than for HIDs (20.1% [95% CI, 16.4-24.2]; P < .001; Figure 2A). This significant effect did not translate to a better 2-year probability of LFS for HIDs, as shown in Figure 2C, with 56.7% (95% CI, 52.7-60.5) for MSDs vs 55.5% (95% CI, 50.5-60.3) for HIDs (P = .73). The 2-year cumulative incidence of NRM was lower for MSDs (14.7% [95% CI, 12-17.6]) than for HIDs (24.4% [95% CI, 20.4-28.6]; P < .001), as illustrated in Figure 2B. However, the 2-year probability of OS was similar in the 2 donor groups, 62.9% (95% CI, 58.9-66.7) for MSDs vs 62% (95% CI, 57-66.6) for HIDs (P = .29; Figure 2D).
GRFS by donor type. The 2-year probability of GRFS was 46.8% (95% CI, 42.8-50.7) for older MSDs and 46.2% (95% CI, 41.2-51) for young HIDs (P = .76).
GRFS by donor type. The 2-year probability of GRFS was 46.8% (95% CI, 42.8-50.7) for older MSDs and 46.2% (95% CI, 41.2-51) for young HIDs (P = .76).
RI, NRM, LFS, and OS by donor type. The 2-year RI was 28.6% (95% CI, 25.1-32.2) for older MSDs, significantly higher than for young HIDs (20.1% [95% CI, 16.4-24.2]; P < .001) (A). The 2-year cumulative incidence of NRM was lower for older MSDs (14.7% [95% CI, 12-17.6]) than for young HIDs (24.4% [95% CI, 20.4-28.6]; P < .001) (B). The 2-year probability of LFS was 56.7% (95% CI, 52.7-60.5) for older MSDs vs 55.5% (95% CI, 50.5-60.3) for young HIDs (P = .73) (C). The 2-year probability of OS was 62.9% (95% CI, 58.9-66.7) for older MSDs vs 62% (95% CI, 57-66.6) for young HIDs (P = .29) (D).
RI, NRM, LFS, and OS by donor type. The 2-year RI was 28.6% (95% CI, 25.1-32.2) for older MSDs, significantly higher than for young HIDs (20.1% [95% CI, 16.4-24.2]; P < .001) (A). The 2-year cumulative incidence of NRM was lower for older MSDs (14.7% [95% CI, 12-17.6]) than for young HIDs (24.4% [95% CI, 20.4-28.6]; P < .001) (B). The 2-year probability of LFS was 56.7% (95% CI, 52.7-60.5) for older MSDs vs 55.5% (95% CI, 50.5-60.3) for young HIDs (P = .73) (C). The 2-year probability of OS was 62.9% (95% CI, 58.9-66.7) for older MSDs vs 62% (95% CI, 57-66.6) for young HIDs (P = .29) (D).
The overall cumulative incidence of grade 2 to 4 acute GVHD was significantly lower for MSDs (12.9% [95% CI, 10.6-15.5]) than for HIDs (24.2% [95% CI, 20.5-28]; P < .001). However, the cumulative incidence of grade 3 to 4 acute GVHD was not statistically different, being 4.6% (95% CI, 3.2-6.3) for MSDs vs 6.6% (95% CI, 4.6-9) for HIDs (P = .12). The 2-year cumulative incidence of chronic GVHD was similar in the 2 donor groups: 30.2% (95% CI, 26.5-33.9) for MSDs vs 30.8% (95% CI, 26.3-35.4) for HIDs (P = .9). The 2-year cumulative incidence of extensive chronic GVHD was also similar in the 2 donor groups, with 13.5% (95% CI, 10.9-16.4) for MSDs vs 11.7% (95% CI, 8.7-15.2) for HIDs (P = .26). The rate of graft failure was significantly higher in HID than in MSD grafts (5.3% vs 1.1%; P < .0001; results not shown). In both donor groups, the main cause of death was disease relapse, followed by infections (results not shown).
Multivariate analysis
Results of the multivariate analysis are shown in Table 2. The purpose of this study was to compare older MSDs with young HIDs in older patients with AML who underwent transplantation in CR1. We confirmed in this multivariate analysis that young HIDs were associated with significantly less relapse and more NRM, which translated into similar GRFS, LFS, and OS. Young HIDs were associated with significantly more grade 2 to 4 acute GVHD (HR, 2.67; 95% CI, 1.86-3.81; P < .0001) but a similar rate of grade 3 to 4 (HR, 1.49; 95% CI, 0.84-2.63; P = .17) compared with an older MSD. No impact of donor type was observed on the 2-year cumulative incidence of chronic GVHD (HR, 1.05; 95% CI, 0.78-1.42; P = .74) and extensive chronic GVHD (HR, 0.78; 95% CI, 0.49-1.25; P = .3; data not shown). The main prognostic factors associated with higher risk of worse GRFS were older patient age, secondary AML, adverse cytogenetics, and a lower performance status (Karnofsky performance status score <90). Increasing patient age was also associated with lower OS. Secondary AML was associated with higher risk of RI and NRM, which translated into worse LFS and OS. Adverse cytogenetics correlated not surprisingly with higher risk of RI and decreased LFS and OS. A lower performance status at the time of allo-HCT translated into higher NRM, and worse LFS and OS. Finally, the use of peripheral blood stem cells (PBSCs) as stem cell source was associated with lower risk of RI and NRM, which translated into improved LFS and OS compared with bone marrow. The cumulative incidence of grade 3 to 4 acute GVHD was strongly associated with increased patient age (HR, 2.4; 95% CI, 1.14-5.08; P = .022), and the risk of extensive chronic GVHD was lower among patients with better performance status at the time of allo-HCT (HR, 0.54; 95% CI, 0.36-0.82; P = .004; data not shown).
Multivariate analysis of outcomes GRFS, RI, NRM, LFS, and OS using a Cox proportional hazards model for the entire cohort
| . | P value . | HR . | 95% CI . |
|---|---|---|---|
| GRFS | |||
| HID vs MSD | .9 | 0.99 | 0.9-1.21 |
| Age (per 10 years) | .015 | 1.4 | 1.07-1.83 |
| Secondary AML | <.0001 | 1.57 | 1.27-1.94 |
| Adverse cytogenetics | <.0001 | 1.58 | 1.32-1.9 |
| KPS ≥90 | .002 | 0.75 | 0.62-0.9 |
| Year allo-HCT | 1.00 | 1.00 | 0.96-1.04 |
| Diagnosis to allo-HCT | .07 | 1.03 | 1.00-1.06 |
| RIC vs MAC | .49 | 1.09 | 0.86-1.37 |
| Female to male | .053 | 1.21 | 1.00-1.46 |
| CMV+ patients | .95 | 0.99 | 0.8-1.23 |
| PB vs BM | .095 | 0.78 | 0.58-1.04 |
| RI | |||
| HID vs MSD | .003 | 0.62 | 0.45-0.86 |
| Age (per 10 years) | .42 | 1.18 | 0.79-1.78 |
| Secondary AML | .0007 | 1.7 | 1.25-2.3 |
| Adverse cytogenetics | <.0001 | 2.24 | 1.72-2.91 |
| KPS ≥90 | .089 | 0.79 | 0.59-1.04 |
| Year allo-HCT | .66 | 1.01 | 0.96-1.07 |
| Diagnosis to allo-HCT | .33 | 1.02 | 0.98-1.08 |
| RIC vs MAC | .12 | 1.34 | 0.93-1.93 |
| Female to male | .53 | 1.09 | 0.82-1.45 |
| CMV+ patients | .3 | 0.85 | 0.63-1.15 |
| PB vs BM | .04 | 0.62 | 0.4-0.98 |
| NRM | |||
| HID vs MSD | .012 | 1.54 | 1.1-2.17 |
| Age (per 10 years) | .079 | 1.48 | 0.95-2.31 |
| Secondary AML | .003 | 1.72 | 1.2-2.46 |
| Adverse cytogenetics | .2 | 1.24 | 0.9-1.71 |
| KPS ≥90 | .028 | 0.7 | 0.51-0.96 |
| Year allo-HCT | .48 | 1.02 | 0.96-1.09 |
| Diagnosis to allo-HCT | .57 | 1.01 | 0.96-1.07 |
| RIC vs MAC | .79 | 1.05 | 0.72-1.55 |
| Female to male | .4 | 1.15 | 0.83-1.59 |
| CMV+ patients | .11 | 1.37 | 0.93-2.01 |
| PB vs BM | .034 | 0.63 | 0.41-0.97 |
| LFS | |||
| HID vs MSD | .54 | 0.93 | 0.75-1.16 |
| Age (per 10 years) | .092 | 1.29 | 0.96-1.73 |
| Secondary AML | <.0001 | 1.69 | 1.35-2.13 |
| Adverse cytogenetics | <.0001 | 1.72 | 1.41-2.1 |
| KPS ≥90 | .008 | 0.76 | 0.62-0.93 |
| Year allo-HCT | .52 | 1.01 | 0.97-1.05 |
| Diagnosis to allo-HCT | .29 | 1.02 | 0.98-1.06 |
| RIC vs MAC | .23 | 1.17 | 0.9-1.51 |
| Female to male | .3 | 1.12 | 0.9-1.38 |
| CMV+ patients | .77 | 1.03 | 0.82-1.3 |
| PB vs BM | .002 | 0.62 | 0.46-0.84 |
| OS | |||
| HID vs MSD | .76 | 1.04 | 0.81-1.34 |
| Age (per 10 years) | .023 | 1.47 | 1.05-2.04 |
| Secondary AML | .0001 | 1.65 | 1.27-2.12 |
| Adverse cytogenetics | <.0001 | 1.75 | 1.4-2.19 |
| KPS ≥90 | .01 | 0.74 | 0.59-0.93 |
| Year allo-HCT | .88 | 1.00 | 0.96-1.05 |
| Diagnosis to allo-HCT | .75 | 1.01 | 0.97-1.05 |
| RIC vs MAC | .11 | 1.28 | 0.95-1.74 |
| Female to male | .17 | 1.18 | 0.93-1.49 |
| CMV+ patients | .58 | 1.08 | 0.83-1.4 |
| PB vs BM | .0009 | 0.56 | 0.4-0.79 |
| . | P value . | HR . | 95% CI . |
|---|---|---|---|
| GRFS | |||
| HID vs MSD | .9 | 0.99 | 0.9-1.21 |
| Age (per 10 years) | .015 | 1.4 | 1.07-1.83 |
| Secondary AML | <.0001 | 1.57 | 1.27-1.94 |
| Adverse cytogenetics | <.0001 | 1.58 | 1.32-1.9 |
| KPS ≥90 | .002 | 0.75 | 0.62-0.9 |
| Year allo-HCT | 1.00 | 1.00 | 0.96-1.04 |
| Diagnosis to allo-HCT | .07 | 1.03 | 1.00-1.06 |
| RIC vs MAC | .49 | 1.09 | 0.86-1.37 |
| Female to male | .053 | 1.21 | 1.00-1.46 |
| CMV+ patients | .95 | 0.99 | 0.8-1.23 |
| PB vs BM | .095 | 0.78 | 0.58-1.04 |
| RI | |||
| HID vs MSD | .003 | 0.62 | 0.45-0.86 |
| Age (per 10 years) | .42 | 1.18 | 0.79-1.78 |
| Secondary AML | .0007 | 1.7 | 1.25-2.3 |
| Adverse cytogenetics | <.0001 | 2.24 | 1.72-2.91 |
| KPS ≥90 | .089 | 0.79 | 0.59-1.04 |
| Year allo-HCT | .66 | 1.01 | 0.96-1.07 |
| Diagnosis to allo-HCT | .33 | 1.02 | 0.98-1.08 |
| RIC vs MAC | .12 | 1.34 | 0.93-1.93 |
| Female to male | .53 | 1.09 | 0.82-1.45 |
| CMV+ patients | .3 | 0.85 | 0.63-1.15 |
| PB vs BM | .04 | 0.62 | 0.4-0.98 |
| NRM | |||
| HID vs MSD | .012 | 1.54 | 1.1-2.17 |
| Age (per 10 years) | .079 | 1.48 | 0.95-2.31 |
| Secondary AML | .003 | 1.72 | 1.2-2.46 |
| Adverse cytogenetics | .2 | 1.24 | 0.9-1.71 |
| KPS ≥90 | .028 | 0.7 | 0.51-0.96 |
| Year allo-HCT | .48 | 1.02 | 0.96-1.09 |
| Diagnosis to allo-HCT | .57 | 1.01 | 0.96-1.07 |
| RIC vs MAC | .79 | 1.05 | 0.72-1.55 |
| Female to male | .4 | 1.15 | 0.83-1.59 |
| CMV+ patients | .11 | 1.37 | 0.93-2.01 |
| PB vs BM | .034 | 0.63 | 0.41-0.97 |
| LFS | |||
| HID vs MSD | .54 | 0.93 | 0.75-1.16 |
| Age (per 10 years) | .092 | 1.29 | 0.96-1.73 |
| Secondary AML | <.0001 | 1.69 | 1.35-2.13 |
| Adverse cytogenetics | <.0001 | 1.72 | 1.41-2.1 |
| KPS ≥90 | .008 | 0.76 | 0.62-0.93 |
| Year allo-HCT | .52 | 1.01 | 0.97-1.05 |
| Diagnosis to allo-HCT | .29 | 1.02 | 0.98-1.06 |
| RIC vs MAC | .23 | 1.17 | 0.9-1.51 |
| Female to male | .3 | 1.12 | 0.9-1.38 |
| CMV+ patients | .77 | 1.03 | 0.82-1.3 |
| PB vs BM | .002 | 0.62 | 0.46-0.84 |
| OS | |||
| HID vs MSD | .76 | 1.04 | 0.81-1.34 |
| Age (per 10 years) | .023 | 1.47 | 1.05-2.04 |
| Secondary AML | .0001 | 1.65 | 1.27-2.12 |
| Adverse cytogenetics | <.0001 | 1.75 | 1.4-2.19 |
| KPS ≥90 | .01 | 0.74 | 0.59-0.93 |
| Year allo-HCT | .88 | 1.00 | 0.96-1.05 |
| Diagnosis to allo-HCT | .75 | 1.01 | 0.97-1.05 |
| RIC vs MAC | .11 | 1.28 | 0.95-1.74 |
| Female to male | .17 | 1.18 | 0.93-1.49 |
| CMV+ patients | .58 | 1.08 | 0.83-1.4 |
| PB vs BM | .0009 | 0.56 | 0.4-0.79 |
Discussion
Allo-HCT remains the best consolidation strategy in high-risk AML and is commonly performed in patients aged >50 years.25 To date, an MSD remains the gold standard, because HLA factors remains the most important in affecting survival in allo-HCT.26 However, older patients with AML have older MSDs, and donor age has been postulated as the most important non-HLA factor to be considered when selecting a donor.27,28 The effect of donor age on survival after allo-HCT has been well documented with MUD.5,6,29 However, time from diagnosis to allo-HSCT is longer for MUD, which is not always suitable for patients with AML when the transplantation is urgent.10 Besides MSDs, HIDs with PTCy-based GVHD prophylaxis appear to be a valuable alternative, leading to similar outcomes compared with MUDs or MSDs.11,13 Today, we have multiple donor choices readily available for an older patient, and the question of the best suitable donor remains open. The purpose of the current study was to compare older MSDs with young HIDs in older patients with AML who underwent transplantation in CR1. We used the cutoffs of ≤40 years for HID and ≥50 years for MSD to virtually compare young offspring vs older siblings; however, the exact kinship was not analyzed in this study. By multivariate analysis, we found no difference between the 2 types of donor in terms of GRFS, LFS, and OS, but significantly less relapse and more treatment-related mortality with HIDs. An older MSD remains therefore safer in an older patient with more comorbidities. In contrast, HID was associated with significantly less relapse, suggesting a potential stronger graft-versus-leukemia (GVL) effect, but exposes older patients to higher NRM, secondary to a higher rate of acute GVHD and more infections. The lower relapse rate observed with HIDs has been demonstrated by others,30,31 but remains controversial.21,32
The detrimental effect of donor age on outcomes has mainly been related to a higher risk of acute GVHD,5,19,21,33 due to a different graft composition in terms of lymphocytes subsets. Aging has been associated with fewer regulatory T cells and increased memory T cells.34,35 Moreover, impaired thymic function in both older patients and older donors may further contribute to the risk of GVHD. Other factors include age-related epigenetic changes in the donor associated with risk of GVHD and survival.36,37 Older donors have a lower ability of mobilization leading to lower targeted CD34+ cell dose and delayed engraftment.28,38,39 Older donors also experienced longer recovery time from donation.40 Finally, aging has been associated with clonal hematopoiesis, which has been correlated with higher risk of hematological malignancies.41 Younger donors should therefore be favored, except perhaps for those with DNMT3A clonal hematopoiesis, which has been associated with better survival.42 Besides younger age, HIDs may offer opportunities to explore specific HLA factors in order to enhance the GVL effect, such as HLA-B leader matching43,44 and HLA-DRB1 mismatches.45 Based on those HLA factors, if several younger HIDs are available, better outcomes are expected with HLA-B leader–matched and HLA-DRB1–mismatched HIDs over older MSDs.27,46,47
In our study, we decided to include all MSDs with a standard calcineurin inhibitor-based GVHD prophylaxis with either ATG or alemtuzumab. All HIDs received PTCy-based GVHD prophylaxis. Due to the collinearity between GVHD prophylaxis and donor type, both in vivo TCD and PTCy have been excluded from the multivariate analysis. PTCy was first used for HIDs with impressive results in term of incidence of both acute and chronic GVHD,48 but is currently increasingly used in other settings, such as MSDs, MUDs, and mismatched unrelated donors.49 Prospective studies have even demonstrated the feasibility and efficacy of PTCy as GVHD prophylaxis in MSDs and MUDs.50,51 As PTCy may mitigate the detrimental effect of HLA disparities,52 our results might have been different with a homogeneous GVHD prophylaxis, and we may speculate on better results with MSDs. However, retrospective studies with PTCy as GVHD prophylaxis showed that HLA matching still matters,15 and that young MUD grafts lead to better outcomes than young HIDs.9,19,53 This study has several limitations due to its retrospective nature. First, the decision to choose 1 donor over another was not captured in the registry, which may lead to a selection bias. As mentioned above, the collinearity between GVHD prophylaxis and donor type does not allow to study independently the effect of GVHD prophylaxis. Finally, molecular data were missing for most of the patients, and were therefore not included in our analysis.
Interestingly, we found that PBSCs were associated with lower RI and NRM, which translated into better LFS and OS, suggesting a stronger GVL effect. However, a recent study from the EBMT showed no impact of stem cell source on NRM, LFS, and OS.54 Moreover, in older patients, the use of PBSCs in HID transplants has been associated with worse LFS and OS.55 Based on the retrospective nature of our study, we could not analyze further the impact of stem cell source, but PBSCs should not be excluded in the HID setting. The addition of ATG to PTCy, which was used in 8% of our patients, may in part explain our results.56
In conclusion, with the limitations of a retrospective study, we have shown that either an older MSD or a young HID led to similar survival in older patients with AML who received transplantation in CR1. However, a young offspring offers the opportunity of a stronger GVL, associated with less relapse, whereas an older matched sibling remains safer, associated with less treatment-related mortality. The influence of other HLA and non-HLA factors should be further investigated in the future to allow selection of the best donor for a transplant recipient.
Authorship
Contribution: X.P., A.N., J.S., S.P., M.M., and F.C. wrote the manuscript; X.P., M.L., J.S., S.P., M.M., and F.C. designed the study and analyzed the data; and E.P., D.B., P.C., J.M., N.K., C.B., S.N., C.C.-L., G.S., E.F., A.H., I.W.B., and A.N. provided the data and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xavier Poiré, Section of Hematology, Institut Roi Albert II, Cliniques Universitaires Saint-Luc, 10, Ave Hippocrate, 1200 Brussels, Belgium; email: xavier.poire@saintluc.uclouvain.be.
References
Author notes
Original data are available on request from the corresponding author, Xavier Poiré (xavier.poire@saintluc.uclouvain.be).


![RI, NRM, LFS, and OS by donor type. The 2-year RI was 28.6% (95% CI, 25.1-32.2) for older MSDs, significantly higher than for young HIDs (20.1% [95% CI, 16.4-24.2]; P < .001) (A). The 2-year cumulative incidence of NRM was lower for older MSDs (14.7% [95% CI, 12-17.6]) than for young HIDs (24.4% [95% CI, 20.4-28.6]; P < .001) (B). The 2-year probability of LFS was 56.7% (95% CI, 52.7-60.5) for older MSDs vs 55.5% (95% CI, 50.5-60.3) for young HIDs (P = .73) (C). The 2-year probability of OS was 62.9% (95% CI, 58.9-66.7) for older MSDs vs 62% (95% CI, 57-66.6) for young HIDs (P = .29) (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/20/10.1182_bloodadvances.2024015582/2/m_blooda_adv-2024-015582-gr2.jpeg?Expires=1769112064&Signature=H0T9gN~5v~YdeMOe2Oaf5pZqsdCSQ1f07OV-lRqU8OiLkEWPePl6d4fV9WS--gad1obDSOxvo2zsU3VvgXFYdIdGa78BQM4-Q8xG6Jjm6pQCSoKbBl9UWDeEFcKZ8QjPu57ERJI2vUUvp5cHTBzExXBymWkNCP5xrSVswMmYLtKGQeW~rLPyTF2Tl2SDMdXjeW4AZiaTvIsoBFfCv7WVimxg3ywxKKFjw8jlwNitIEBzLxgoa0-kBTMBzapoAy723n~EktyjbPY1LjpKrlJmDB5kfVngrpM2Vr0wmJB4nGQWXIkvpkHRAArNs51yar8O3hrkvqI~wsYHNHChhLwICQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)