Key Points
Differences in PTR indicate a potential advantage of using short-term stored PCs during inflammation.
Higher expression of dense granule metabolite components and lower CD62P and lactate levels were linked to improved PTR.
Visual Abstract
Platelet concentrates (PCs) are frequently used to prevent and treat bleeding in patients. However, their efficacy is reduced during inflammation as well as due to platelet storage lesion, including metabolomic shifts and changes in surface markers of stored PCs. This study aims to identify disparities between short- and long-term stored PCs during controlled inflammation, focusing on distinct metabolic pathways, alterations in surface markers and posttransfusion recovery (PTR). Twenty-four male participants received lipopolysaccharide or saline as control after an autologous transfusion of either short- (2 days) or long-term (7 days) stored PCs. Metabolomics and surface markers of these transfused PCs were assessed before transfusion using mass spectrometry and flow cytometry, respectively. Biotin-labeled platelets were used to assess surface markers after transfusion and determine PTR. Before transfusion, short-term stored PCs demonstrated increased glycolysis, pentose phosphate pathway activity, dense granule components (eg, serotonin, adenosine diphosphate, and epinephrine), and purine, arginine, and tryptophan metabolism. In contrast, long-term stored PCs exhibited elevated transsulfuration and taurine metabolism, along with higher levels of CD62P and CD63. During inflammation, a decreased PTR was found, particularly in long-term stored PCs. Higher expression of dense granule metabolite components and lower CD62P and lactate levels were correlated with improved PTR. Differences in metabolic pathways, surface markers, and PTR were identified between short- and long-term stored PCs in a controlled human experiment, suggesting a preference for the use of short-term stored PCs during inflammation. This trial was registered at the International Clinical Trials Registry Platform (https://trialsearch.who.int/) as #NL-OMON26852.
Introduction
Platelets, long recognized for their essential role in hemostasis, have emerged as active contributors to immune responses and key mediators at the crossroads of inflammation and coagulation.1-4 In the setting of inflammation, platelets become increasingly activated, aggregate more readily, and are consumed at a higher rate. This heightened activity and consumption, compounded by impaired mitochondrial function, leads to reduced platelet responsiveness and, ultimately, thrombocytopenia.5-10 To mitigate the risk of bleeding in these patients, platelet concentrates (PCs) are frequently transfused.11,12
The efficacy and safety of platelet transfusions remain debated, because not all recipients benefit, and some develop adverse effects, including immunomodulatory complications.12-17 Complicating this is the platelet storage lesion: from collection onward, PCs undergo biochemical and functional deterioration that reduces their therapeutic potential and mimics dysfunction seen in inflammatory conditions.5-10,18-26 Prolonged storage may also enhance immunomodulatory effects, with implications not yet fully understood.27 Despite advances in platelet biology, factors influencing transfusion effectiveness, particularly in inflammatory settings in which platelet function is already impaired, remain not yet clear.28 A better understanding of how inflammation and storage affect PC functionality is essential for improving quality assessment and transfusion strategies.
To address this gap, this study aimed to evaluate transfusion effectiveness by comparing posttransfusion recovery (PTR) and surface marker expression between short- and long-term stored PCs in a controlled inflammation model. In parallel, we analyzed metabolomic profiles after storage and explored correlations between PTR, surface marker expression, and metabolite levels to gain mechanistic insights into factors influencing transfusion outcomes.
Materials and methods
Participants and design
This study is part of a larger study on the influence of storage time of PCs in a 2-hit healthy volunteer model on transfusion-related acute lung injury; the results of this study must therefore be considered exploratory.29 The sample size was determined based on the primary outcome of the overall study as previously described.29 Male participants, aged 18 to 35 years, were allocated to participate in this part of the open-label randomized controlled study (supplemental Figure 1). Participants underwent a preparticipation screening process, which included a review of their medical history, assessment of vital signs, physical examinations, and laboratory tests encompassing blood and urine analysis. Participants were prohibited from engaging in any other intervention trials both 3 months before and after the study. Before enrollment, all volunteers provided written informed consent. The study procedures have been reviewed and approved by the Amsterdam UMC Medical Ethical Committee (2014_294#B2014961) and were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study is registered at the International Clinical Trials Registry Platform (NL-OMON26852).
Randomization and blood donation
The study was conducted in 2 phases. In phase 1, participants were allocated to receive Escherichia coli (O113) lipopolysaccharide (LPS; dose, 2 ng/kg; lot number 94332B1) to incite an inflammatory condition through toll-like receptor 4 stimulation. In phase 2, participants received an equal volume of physiological saline (0.9% [weight-to-volume ratio] sodium chloride) as control.30,31 Participants were scheduled to participate twice, once with LPS and once without, with a minimum interval of 1 year between sessions. All participants were randomly assigned to receive one of the following: short-term stored autologous PCs (2 days stored) or long-term stored autologous PCs (7 days stored). Participants who did not return for the second phase of the study were replaced by newly recruited individuals. Participants who completed both phases of the study received platelet transfusions with the same storage duration in each session. Treatment allocation concealment was achieved by generating a randomization sequence using R (version 4.3.2; Boston, MA). The sequence was placed in consecutively numbered opaque envelopes, which were opened only after each participant's inclusion. Depending on group allocation, the participants donated 1 unit (± 300 mL) of apheresis platelets 2 or 7 days before the study day at Sanquin Blood Bank. Apheresis PCs were obtained and stored in compliance with Dutch Blood Bank standards and preserved in 100% platelet additive solution E (PAS-E). The trial was conducted as an open-label randomized study because participants were required to donate either 2 or 7 days before the experiment.
Biotinylation of platelets
On the day of transfusion, platelets were biotinylated according to Good Clinical Practice as previously described.32 In summary, 50 mL of PC was biotinylated with 5 μg/mL of N-hydroxysuccinimidobiotin (NHS-biotin, EZ-Link; Thermo Fisher Scientific, Waltham, MA) and returned to the storage bag.
Experiment
Participants were admitted for 1 day to the research unit of the department of intensive care of the Amsterdam University Medical Center, the Netherlands. Before the study procedures, a research physician assessed the participants, and blood was crossmatched as an additional safety procedure. A peripheral venous catheter (18G; BD, Plymouth, United Kingdom) was inserted to administer 1 L of normal saline for prehydration 1 hour before the experiment began. Maintenance fluids were then set at 1.5 L of physiological saline per 24 hours until the conclusion of the experiment. An arterial catheter (20G; BD) was inserted using ultrasound into the radial artery. During admission, participants underwent continuous monitoring, including blood pressure, temperature, heart rate, and respiratory rate. Blood samples were collected from the PC storage bag before and after biotinylation for metabolomic and flow cytometry analyses as described below. After the completion of safety checks and sample collection, participants were administered either LPS or control. Two hours later, a 300-mL autologous PC (including 50-mL biotinylated platelets) was infused over exactly 50 minutes before the infusion set was locked, and the posttransfusion period commenced.
Ten minutes, 30 minutes, and 1, 2, 3, 4, and 6 hours after transfusion, blood samples were drawn from an arterial line and were further processed and analyzed as described below (Figure 1). Additionally, platelet counts were measured before and after transfusion using a hematology analyzer (XN-9000; Sysmex, Norderstedt, Germany). On a return visit, 48 hours after transfusion, the final blood samples were collected using venipuncture. Whole blood samples were drawn using citrate tubes (BD; Figure 1).
Overview of the study. The study's time points are centrally presented, encompassing PTR and surface marker determination at the top, and metabolomic analyses at the bottom. BSA, bovine serum albumin; PBS, phosphate-buffered saline.
Overview of the study. The study's time points are centrally presented, encompassing PTR and surface marker determination at the top, and metabolomic analyses at the bottom. BSA, bovine serum albumin; PBS, phosphate-buffered saline.
Flow cytometry
Platelet samples and citrated blood (5 μL) were incubated with fluorescently labeled antibodies to assess activation markers and identify biotinylated platelets, both with and without thrombin receptor agonist peptide (TRAP) stimulation. After fixation, samples were analyzed by flow cytometry. Platelet survival was determined as the percentage of circulating biotinylated platelets, and PTR was calculated at 10 minutes and 1 hour after transfusion, following standard protocols. Detailed procedures are described in supplemental Methods 1.33,34
Liquid chromatography–mass spectrometry metabolomics
Pretransfusion samples of PCs before and after biotinylation were collected, and the cell pellets were stored at –80 °C before shipment to the University of Colorado Denver Anschutz Medical Campus for metabolomics testing. Metabolomics were performed as described in previous studies and in supplemental Methods 2.35
Statistical analysis
Data were assessed for normality, homogeneity of variance, and sphericity both visually and using quantile-quantile plots, as well as using the Shapiro-Wilk test, Levene test, and Mauchly test, respectively. Normally distributed and nonnormally distributed data were reported as mean (± standard deviation) or median (first to third quartile [interquartile range]), respectively. Normally distributed data were analyzed using the Student t test or analysis of variance, and nonparametric data were analyzed with the Wilcoxon rank-sum or Kruskal-Wallis test, as appropriate.
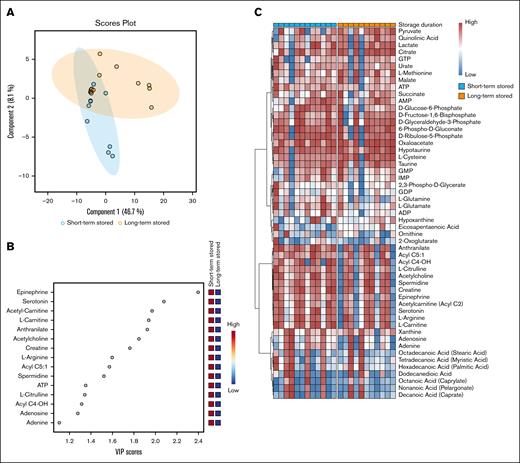
Pretransfusion data for short- and long-term stored PCs, before and after labeling, were compared. Pretransfusion metabolomics analysis including energy and redox metabolism–related metabolites. Partial least squares discriminant analysis was used to assess whether storage duration led to a significant shift in the metabolic profiles. The variable importance in projection score was calculated to determine the contribution of each metabolite to the separation between short- and long-term stored PCs. Metabolites exhibiting the most significant changes were scaled per metabolite (z score) and identified by heat maps and using hierarchical clustering to group metabolites. For a schematic representation of metabolomics, the effect size (Hedges g) for each metabolite was calculated between short- and long-term stored PCs.
After transfusion, comparisons were conducted at each time point and over time between short- and long-term stored PCs in both without and during inflammation. Survival, PTR, and surface markers over time between short- and long-term stored PCs were evaluated using 2-way repeated measures analysis of variance, incorporating interaction terms for posttransfusion time and storage duration (short-term vs long-term stored PCs). Greenhouse-Geisser corrections were applied to adjust for violations of sphericity. Pearson correlation coefficients were calculated for surface markers and metabolic parameters associated with PTR to identify biomarkers and pathways for future research. A P value <.05 was considered to be statistically significant. Data were presented and analyzed using R (version 4.3.2)36 or MetaboAnalyst (version 6.0, Edmonton, Canada).37
Results
Between April 2018 and July 2023, a total of 12 participants were enrolled in phase 1 of the study, which involved the inflammatory condition. Of these, 9 also participated in phase 2, which involved the noninflammatory condition. All participants who received short-term stored PCs in phase 1 (n = 6) also received short-term stored PCs in phase 2, whereas 3 individuals who received long-term stored PCs in phase 1 did not continue to phase 2, necessitating the recruitment of 3 additional individuals during phase 2. In total, 23 of 24 participants were included for further analysis (supplemental Figure 1). One participant was excluded due to a protocol violation: a platelet unit stored for 7 days was inadvertently irradiated, in accordance with the standard procedure for allogeneic apheresis PCs at the blood bank. Participant characteristics were similar for all groups and are presented in Table 1. As previously described, LPS infusion in our study resulted in significant increases in leukocyte count and inflammatory markers, including interleukin-6, C-X-C motif ligand 8, and tumor necrosis factor α.29 Participants also exhibited a significant increased temperature, heart rate, and respiratory rate over time compared to participants not receiving LPS (supplemental Figure 2).
Baseline characteristics of participating participants
| Variables . | Control . | LPS . | P value . | ||
|---|---|---|---|---|---|
| Short-term stored PCs n = 6 . | Long-term stored PCs n = 5 . | Short-term stored PCs n = 6 . | Long-term stored PCs n = 6 . | ||
| Age, y | 27 (4.3) | 25 (4.9) | 25.3 (4.8) | 27.0 (3.1) | .75 |
| BMI, kg/m2 | 24.3 (2.6) | 24.7 (2.6) | 23.3 (0.8) | 25.8 (2.8) | .33 |
| Laboratory values at screening, platelet count, ×109/L | 295 (83) | 291 (41) | 295 (83) | 261 (42) | .79 |
| Baseline laboratory values | |||||
| Blood group∗ | .40 | ||||
| 0+ | 3 (50) | 4 (80) | 3 (50) | 4 (67) | |
| A+ | 3 (50) | 0 (0) | 3 (50) | 1 (17) | |
| B– | 0 (0) | 1 (20) | 0 (0) | 1 (17) | |
| Hemoglobin, g/dL | 13.2 (12.3-14.3) | 13.3 (13.0-14.0) | 13.4 (13.2-13.5) | 12.7 (12.4-13.5) | .89 |
| Platelet count, ×109/L | 206 (44.6) | 216 (21.8) | 201 (57.2) | 181.2 (40.4) | .60 |
| Leucocytes, ×109/L | 5.7 (1.4) | 5.4 (0.5) | 5.8 (1.2) | 4.6 (1.2) | .26 |
| Baseline physical examination | |||||
| Systolic blood pressure, mm Hg | 138 (14) | 133 (5) | 136 (11) | 138 (15) | .90 |
| Diastolic blood pressure, mm Hg | 70 (66-74) | 62 (61-62) | 70 (61-77) | 67 (61-71) | .49 |
| Mean arterial pressure, mm Hg | 92 (8) | 86 (3) | 92 (9) | 90 (8) | .61 |
| Heart rate, beats per minute | 64 (14) | 59 (8) | 72 (16) | 59.8 (11) | .30 |
| Saturation, % | 100 (99-100) | 100 (100-100) | 100 (99-100) | 100 (100-100) | .52 |
| Respiration rate, breaths per minute | 14 (13-15) | 14 (13-15) | 16 (14-16) | 17 (15-19) | .15 |
| Body temperature, °C | 36.9 (36.7-37.2) | 36.8 (36.8-36.9) | 36.8 (36.3-37.0) | 37 (36.3-36.8) | .44 |
| Variables . | Control . | LPS . | P value . | ||
|---|---|---|---|---|---|
| Short-term stored PCs n = 6 . | Long-term stored PCs n = 5 . | Short-term stored PCs n = 6 . | Long-term stored PCs n = 6 . | ||
| Age, y | 27 (4.3) | 25 (4.9) | 25.3 (4.8) | 27.0 (3.1) | .75 |
| BMI, kg/m2 | 24.3 (2.6) | 24.7 (2.6) | 23.3 (0.8) | 25.8 (2.8) | .33 |
| Laboratory values at screening, platelet count, ×109/L | 295 (83) | 291 (41) | 295 (83) | 261 (42) | .79 |
| Baseline laboratory values | |||||
| Blood group∗ | .40 | ||||
| 0+ | 3 (50) | 4 (80) | 3 (50) | 4 (67) | |
| A+ | 3 (50) | 0 (0) | 3 (50) | 1 (17) | |
| B– | 0 (0) | 1 (20) | 0 (0) | 1 (17) | |
| Hemoglobin, g/dL | 13.2 (12.3-14.3) | 13.3 (13.0-14.0) | 13.4 (13.2-13.5) | 12.7 (12.4-13.5) | .89 |
| Platelet count, ×109/L | 206 (44.6) | 216 (21.8) | 201 (57.2) | 181.2 (40.4) | .60 |
| Leucocytes, ×109/L | 5.7 (1.4) | 5.4 (0.5) | 5.8 (1.2) | 4.6 (1.2) | .26 |
| Baseline physical examination | |||||
| Systolic blood pressure, mm Hg | 138 (14) | 133 (5) | 136 (11) | 138 (15) | .90 |
| Diastolic blood pressure, mm Hg | 70 (66-74) | 62 (61-62) | 70 (61-77) | 67 (61-71) | .49 |
| Mean arterial pressure, mm Hg | 92 (8) | 86 (3) | 92 (9) | 90 (8) | .61 |
| Heart rate, beats per minute | 64 (14) | 59 (8) | 72 (16) | 59.8 (11) | .30 |
| Saturation, % | 100 (99-100) | 100 (100-100) | 100 (99-100) | 100 (100-100) | .52 |
| Respiration rate, breaths per minute | 14 (13-15) | 14 (13-15) | 16 (14-16) | 17 (15-19) | .15 |
| Body temperature, °C | 36.9 (36.7-37.2) | 36.8 (36.8-36.9) | 36.8 (36.3-37.0) | 37 (36.3-36.8) | .44 |
All variables are displayed as follows: count (%) for categorical variables; median (first to third quartile) for nonparametric variables; and mean (standard deviation) for parametric variables.
Only blood groups of participating participants are presented.
Before transfusion
Metabolomics
Using global metabolomics data acquisition with targeted data analysis focusing on energy and redox metabolism, the collected data encompassing >5000 measurements across various metabolic pathways. The partial least squares discriminant analysis indicated the 2 largest components and are presented in Figure 2A. The variable importance in projection scores identified the most influential metabolites distinguishing short-term and long-term stored PCs (Figure 2B). Hierarchical clustering further revealed differences between storage conditions despite donor variability (Figure 2C). The metabolomic schematic overview based on effect size further revealed that glycolysis intermediates, such as glucose-6-phosphate and 2,3-phosphoglycerate, were more abundant in short-term stored PCs, whereas glycolysis end products, such as pyruvate, were elevated in long-term stored PCs (supplemental Figures 3 and 4). These findings suggest that glycolytic intermediates are progressively consumed over time and that long-term stored PCs may not effectively proceed into the tricarboxylic acid cycle. Short-term stored PCs also exhibited higher levels of dense granule components, such as serotonin and adenosine diphosphate (ADP). Additionally, metabolites from the pentose phosphate pathway (PPP), purine synthesis, and arginine metabolism were more abundant in short-term stored PCs, whereas metabolites associated with transsulfuration and taurine synthesis, as well as tryptophan metabolite end products, were elevated in long-term stored PCs. Acetyl-carnitines and fatty acids were in general also higher in short-term stored PCs, whereas eicosapentaenoic acid, in particular, was elevated in long-term stored PCs (supplemental Figures 4 and 5). Noteworthy, the labeling process had minor impact on metabolites in both short- and long-term stored PCs (supplemental Figure 6).
Overview of PC metabolomics. (A) The partial least squares discriminant analysis (PLS-DA) scores plots of short and long-term stored PCs using components 1 and 2. The clustering pattern highlights distinct metabolic profiles influenced by storage duration, indicating potential biochemical shifts over time. (B) The variable importance in projection (VIP) scores from the PLS-DA model, highlighting the metabolites that had the greatest influence on the separation between short- and long-term stored samples. Higher VIP scores indicate metabolites that contributed most to the observed metabolic differences, potentially identifying key biomarkers affected by storage duration. (C) Pretransfusion heat map of all included metabolites. All data were categorized by storage duration (short- or long-term stored). Hierarchical clustering was used to group metabolites in clusters. AMP, adenosine 5′-monophosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; IMP, inosine monophosphate.
Overview of PC metabolomics. (A) The partial least squares discriminant analysis (PLS-DA) scores plots of short and long-term stored PCs using components 1 and 2. The clustering pattern highlights distinct metabolic profiles influenced by storage duration, indicating potential biochemical shifts over time. (B) The variable importance in projection (VIP) scores from the PLS-DA model, highlighting the metabolites that had the greatest influence on the separation between short- and long-term stored samples. Higher VIP scores indicate metabolites that contributed most to the observed metabolic differences, potentially identifying key biomarkers affected by storage duration. (C) Pretransfusion heat map of all included metabolites. All data were categorized by storage duration (short- or long-term stored). Hierarchical clustering was used to group metabolites in clusters. AMP, adenosine 5′-monophosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; IMP, inosine monophosphate.
Platelet surface markers
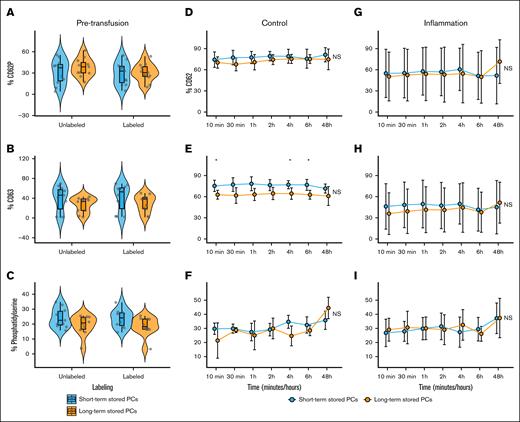
Mean CD62P expression before transfusion was significantly higher in long-term stored PCs (8.62% ± 2.97%; n = 12) than short-term stored PCs (4.21% ± 3.85%; n = 11; P < .01; Figure 3A). Furthermore, mean CD63 expression was significantly higher in long-term stored PCs (3.84% ± 2.26%) than short-term stored PCs (1.51% ± 0.87%; P < .01; Figure 3B). Short-term stored PCs exhibited greater resistance to activation during biotinylation than long-term stored PCs (Figure 3A). After TRAP stimulation, there were no differences in CD62P, CD63, and phosphatidylserine expression between short- and long-term stored PCs in the storage bag and after labeling (Figure 4A-C).
Pretransfusion (labeled or unlabeled) and posttransfusion (control or during inflammation) platelet surface markers as determined by flow cytometry. (A) Mean CD62P expression before transfusion was significantly higher in long-term stored platelets (8.62% ± 2.97%) than short-term stored PCs (4.21% ± 3.85%; P < .01). After biotinylation, long-term stored PCs exhibited a significantly higher expression of CD62P (14.10% ± 5.99%) than nonbiotinylated long-term stored PCs (P < .05), whereas the biotinylation of short-term stored PCs did not lead to a significant increase in CD62P expression (4.92% ± 2.13%). (B) Mean CD63 expression was significantly higher in long-term stored PCs (3.84% ± 2.26%) than short-term stored platelets (1.51% ± 0.87%; P < .01). The biotinylation process did not affect CD63 expression for both short- and long-term stored PCs. (C) Phosphatidylserine levels between short- and long-term stored PCs was not significantly different. (D-I) The line plot displays the mean and standard deviation of surface markers over time. Statistical comparisons were performed at each time point using an unpaired t test, alongside a 2-way repeated measures analysis of variance (ANOVA) to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
Pretransfusion (labeled or unlabeled) and posttransfusion (control or during inflammation) platelet surface markers as determined by flow cytometry. (A) Mean CD62P expression before transfusion was significantly higher in long-term stored platelets (8.62% ± 2.97%) than short-term stored PCs (4.21% ± 3.85%; P < .01). After biotinylation, long-term stored PCs exhibited a significantly higher expression of CD62P (14.10% ± 5.99%) than nonbiotinylated long-term stored PCs (P < .05), whereas the biotinylation of short-term stored PCs did not lead to a significant increase in CD62P expression (4.92% ± 2.13%). (B) Mean CD63 expression was significantly higher in long-term stored PCs (3.84% ± 2.26%) than short-term stored platelets (1.51% ± 0.87%; P < .01). The biotinylation process did not affect CD63 expression for both short- and long-term stored PCs. (C) Phosphatidylserine levels between short- and long-term stored PCs was not significantly different. (D-I) The line plot displays the mean and standard deviation of surface markers over time. Statistical comparisons were performed at each time point using an unpaired t test, alongside a 2-way repeated measures analysis of variance (ANOVA) to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
Pretransfusion (labeled or unlabeled) and posttransfusion (control or during inflammation) platelet surface markers stimulated with TRAP as determined by flow cytometry. (A-C) Surface markers were similar for both short- and long-term stored PCs after stimulation with TRAP. (D-I) The line plots show the mean and standard deviations of the percentage of activation and prophagocytic markers over time of transfused PCs up to 48 hours after transfusion. No major differences were found between short- and long-term stored PCs after stimulation with TRAP. At a few time points, CD63 expression was higher in short-term stored PCs (panel E). The line plot displays the mean and standard deviation of surface markers over time. Statistical comparisons were performed at each timepoint using an unpaired t test, alongside a 2-way repeated measures ANOVA to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
Pretransfusion (labeled or unlabeled) and posttransfusion (control or during inflammation) platelet surface markers stimulated with TRAP as determined by flow cytometry. (A-C) Surface markers were similar for both short- and long-term stored PCs after stimulation with TRAP. (D-I) The line plots show the mean and standard deviations of the percentage of activation and prophagocytic markers over time of transfused PCs up to 48 hours after transfusion. No major differences were found between short- and long-term stored PCs after stimulation with TRAP. At a few time points, CD63 expression was higher in short-term stored PCs (panel E). The line plot displays the mean and standard deviation of surface markers over time. Statistical comparisons were performed at each timepoint using an unpaired t test, alongside a 2-way repeated measures ANOVA to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
After transfusion
After transfusion, the mean platelet count increment was 28 × 109/L (± 23 × 109/L; supplemental Figure 7). No significant differences between CD62P and CD63 and phosphatidylserine were found over time between the PCs (Figure 3D-I; supplemental Table 1). Although no significant time-dependent or interaction effects were found for CD63 expression, storage duration showed a significant main effect, with long-term stored platelets exhibiting reduced CD63 expression after TRAP stimulation (Figure 4E; supplemental Table 2). During inflammation, transfused platelets showed elevated CD62P expression 10 minutes after transfusion, followed by a decline over time, whereas under healthy conditions, CD62P levels progressively increased.
The mean percentage of labeled platelets without inflammation increased at 10 minutes after transfusion to 1.74% (± 0.47%; n = 6) in short-term and to 1.45% (± 0.18%; n = 5) in long-term stored PCs, respectively. These percentages continued to rise, reaching a peak 4 and 6 hours after transfusion: 1.90% (± 0.39%) in the short-term stored PCs and 1.83% (± 0.40%) in long-term stored PCs (Figure 5A), respectively. After 48 hours, the percentage of biotin-labeled platelets had decreased to 1.38% (± 0.58%) in short-term and 1.19% (± 0.28%) in long-term stored PCs. During inflammation, the mean percentage of biotin-labeled platelets increased 10 minutes after transfusion to 1.58% (± 0.44%; n = 6) in the short-term and 1.40% (± 0.49%; n = 6) in long-term stored PCs (Figure 5D). The peak values were reached 2 and 4 hours after transfusion, with percentages of 1.65% (± 0.55%) and 1.68% (± 0.64%) for short-term and long-term stored PCs, respectively. After 48 hours, the percentage of biotin-labeled platelets decreased to 1.31% (± 0.46%) in short-term stored PCs and 1.03% (± 0.49%) in long-term stored PCs.
Survival and PTR of biotinylated platelets without and during inflammation. (A,D) Percentage of biotinylated platelets in circulation. (B,E) Percentage of biotinylated platelets related to the 10-minute measurement after transfusion. (C,F) Percentage of biotinylated platelets related to the 1-hour measurement after transfusion. The line plot displays the mean and standard deviation of recovery or PTR over time. Statistical comparisons were performed at each time point using an unpaired t test, alongside a 2-way repeated measures ANOVA to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
Survival and PTR of biotinylated platelets without and during inflammation. (A,D) Percentage of biotinylated platelets in circulation. (B,E) Percentage of biotinylated platelets related to the 10-minute measurement after transfusion. (C,F) Percentage of biotinylated platelets related to the 1-hour measurement after transfusion. The line plot displays the mean and standard deviation of recovery or PTR over time. Statistical comparisons were performed at each time point using an unpaired t test, alongside a 2-way repeated measures ANOVA to assess changes over time and between conditions. Significance levels are indicated as follows: ∗P < .05; ∗∗P < .01; ∗∗P < .001; ∗∗∗∗P < .0001; NS, not statistically significant.
PTR, calculated as the percentage of biotin-labeled platelets at each time point relative to the measurement 10 minutes or 1 hour after transfusion, showed no significant differences between short- and long-term stored PCs in the control group (P = .21 and .46, respectively; Figure 5B-C). Long-term stored PCs took longer to reach peak levels and showed a steeper decrease between 6 and 48 hours after transfusion (Figure 5B-C,E-F). PTR analysis related to both 10-minute and 1-hour measurements revealed significant interactions (10 minutes, F(6,54) = 3.14 [P < .05]; 1 hour, F(4,36) = 4.30 [P < .01]), indicating that the kinetics of platelet recovery differed by storage duration. Although storage duration alone had no significant main effect, the effect of time was highly significant at both time points (P < .0001), reflecting distinct recovery and clearance profiles over time (Figure 5E-F; supplemental Table 3). The 48-hour PTR related to the 1-hour measurement during inflammation was significantly higher for short-term stored PCs (80.1% ± 12.2%) than long-term stored PCs (63.6% ± 4.4%; P < .05). However, the 48-hour PTR related to 10-minute measurement did not show a significant difference (81.6% ± 10.2% vs 71.8% ± 11.7%; P = .16).
Correlation between surface markers or metabolites and PTR
The correlation matrix of all metabolites and surface markers is presented in Figure 6. Additionally, supplemental Figure 8 highlights the top 25 surface markers and metabolites of platelets correlated with 48-hour PTR relative to the 1-hour measurement. Lactate, hypotaurine, cysteine, and CD62P exhibited a negative correlation with PTR, with the latter suggesting that increased platelet activation before transfusion may impair platelet recovery. Although small, a positive correlation was found between dense granule components, such as serotonin and epinephrine, and PTR.
Metabolite and surface marker correlations in platelets. Correlation plot displaying all metabolites and surface markers of platelets from the PCs and 48-hour PTR (related to 1 hour). Red boxes indicate positive correlations, whereas blue boxes indicate negative correlations. AMP, adenosine 5′-monophosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; IMP, inosine monophosphate.
Metabolite and surface marker correlations in platelets. Correlation plot displaying all metabolites and surface markers of platelets from the PCs and 48-hour PTR (related to 1 hour). Red boxes indicate positive correlations, whereas blue boxes indicate negative correlations. AMP, adenosine 5′-monophosphate; ATP, adenosine triphosphate; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; IMP, inosine monophosphate.
Discussion
In this open-label randomized controlled study, we report the following relevant findings regarding platelet surface markers, intracellular metabolism, and PTR during controlled inflammation. First, short-term stored PCs exhibited significantly lower expression of CD62P and CD63 than long-term stored PCs and were less affected by the biotinylation process. Short-term stored PCs exhibited higher adenosine triphosphate and dense granule components, along with increased activity in glycolysis, the PPP, and purine, arginine, and tryptophan metabolism. In contrast, long-term stored PCs showed elevated end metabolites of glycolysis and tryptophan metabolism, as well as enhanced transsulfuration and taurine metabolism. These findings indicate a metabolic shift over the storage period, reflecting a transition from anabolic and signaling processes in short-term storage toward stress response and catabolic pathways in long-term stored PCs. Third, after transfusion, no differences in CD62P, CD63, and phosphatidylserine expression were observed between short- and long-term stored PCs. However, during inflammation, long-term stored PCs took longer to reach peak levels and were cleared from circulation more rapidly. Higher levels of dense granule components were correlated with increased PTR, whereas elevated levels of CD62P and lactate may be associated with reduced PTR.
Metabolomics
The metabolomic findings of this study are supported by a previous study on platelet metabolism during storage.38 The intracellular and extracellular metabolomic levels were daily evaluated in apheresis PCs stored for 10 days; the first few days of storage (0-3 days) were associated with active glycolysis and PPP, whereas medium-term stored PCs (4-6 days) were associated with a more active tricarboxylic acid cycle.38 Our comparison focused on metabolites in PCs stored for 2 and 7 days, rather than daily. Our short-term stored PCs exhibited higher metabolite levels associated with glycolysis and the PPP, similar to the previous study, whereas our long-term stored PCs showed elevated metabolites related to transsulfuration and taurine synthesis, as well as increased end products of glycolysis and tryptophan metabolism. Although pyruvate levels were highly elevated in long-term stored PCs, intracellular lactate levels did not show a significant increase. We assume that platelets release lactate into the storage solution to maintain intracellular homeostasis, a phenomenon also observed previously, during which extracellular lactate levels increased during storage while intracellular levels remained stable.38
Moreover, other studies have shown that metabolic differences during storage can be explained by variations in collection methods, such as apheresis vs buffy coat, or by changes in storage temperature.39-41 Additionally, these changes could be influenced by various factors, including the specific characteristics of individual blood donors and storage conditions such as the type of plasticizer and storage solution.42-44 We also observed interdonor variability, suggesting that both donor- and storage-dependent factors may influence platelet metabolism over the course of storage; however, this lies beyond the scope of this analysis.
Interestingly, we found elevated levels of dense granule components and spermidine in short-term stored PCs, both of which may be relevant to coagulation and fibrinolysis.45-47 Serotonin and ADP are typically secreted by platelets to induce vasoconstriction and activate nearby platelets, respectively. In contrast, polyamines including spermidine may serve as substrates for crosslinking enzymes in the coagulation cascade (eg, factor XIIIa).48 However, the precise role of spermidine and other metabolites in platelets and coagulation remains unclear and is a topic of interest.
Survival, PTR, and surface markers
Our findings contribute to the existing literature by demonstrating that long-term stored PCs are especially cleared more rapidly during inflammation than short-term stored PCs. Notably, long-term stored PCs exhibited a slower rise to peak levels, followed by a more rapid decline within 48 hours after transfusion. It is generally assumed that the peak concentration of labeled platelets from the PC in circulation occurs between 10 minutes and 1 hour after transfusion. However, our findings suggest that this peak may occur later, depending of the clinical scenario and storage duration of the PC. This may be explained by the initial sequestration of transfused platelets by the spleen and liver, with their delayed return to the circulation several hours later.49,50
In interpreting PTR, it is important to differentiate between platelet clearance (eg, hepatic removal of aged or activated platelets) and platelet consumption (eg, use in active hemostasis). Although both result in platelet removal, they involve distinct physiological processes. The observed decrease in CD62P expression in the first hours during inflammation may reflect the selective consumption of activated, CD62P-expressing platelets, leaving behind a population of quiescent transfused platelets. The higher PTR observed at 48 hours during inflammation for short-term stored PCs may therefore reflect reduced posttransfusion activation, making these platelets less likely to participate in inflammatory hemostasis. This interpretation highlights the complex interplay between platelet activation state, storage duration, and the inflammatory condition. Another important factor is that short-term stored PCs have been shown to be more resistant to activation during labeling, which may influence PTR because activated platelets are typically cleared more rapidly from the circulation. This difference in activation susceptibility could partly explain the more rapid clearance observed for long-term stored PCs. However, it is noteworthy that we observed differences in PTR only under inflammatory conditions and not in healthy volunteers, suggesting that inflammation plays a role in modulating these effects.
Although not directly compared, transfused platelets under inflammatory conditions appear less responsive to TRAP stimulation than under healthy conditions, as suggested by lower mean activation levels over time and greater interindividual variability. This may indicate that systemic inflammation reduces platelet responsiveness to stimulation, a hypothesis that warrants further investigation in future studies.
Strengths and limitations
This study is, to our knowledge, the first to compare surface markers and metabolite levels between short- and long-term stored PCs related with PTR. Moreover, biotinylated platelets were only administered 2 decades ago to human volunteers.51 A strength of this study is the randomized and controlled setting, including endotoxemia, platelet products, and blood sampling. Another advantage was the use of autologous platelets in PAS-E, which effectively avoided the immunological effects of any antigen mismatch, allowing for us to focus solely on the impact of storage duration. This study’s broad range of outcome measures enabled a multifaceted comparison of short- and long-term stored PCs. Furthermore, this is, to our knowledge, the first trial since recent improvements in platelet-biotin labeling technique to show that biotinylated platelets can be safely transfused and remain in circulation for at least 48 hours after transfusion.52
There are some important limitations to the observations presented in this study. First, this study was part of a larger study examining the impact of platelet storage time, and no adjustments for multiplicity have been made; these findings should be regarded as exploratory. It is important to highlight that the research questions were established before the study, and all data were collected prospectively. Although baseline demographics were similar across all study groups, participants were not randomly assigned to receive either LPS or saline, which could potentially lead to unbalanced groups. This approach was chosen to prioritize investigating the study's primary research question. No data were obtained from the storage solution (ie, extracellular metabolism), which may have led to a more complete overview of metabolite levels and acidity between short- and long-term stored PCs. Due to frequent blood draws and continuous monitoring, we collected samples from an arterial catheter during the study and a venous sample during follow-up, which may have led to changes in activation at the final measurements. Our data were limited to a few key time points and were obtained from apheresis platelets stored in PAS-E. Consequently, it remains uncertain whether our results can be extrapolated to pooled platelet products or those stored in different solutions, such as plasma. We acknowledge the potential for survivorship bias in our study, because only the platelets recoverable through flow cytometry cell sorting are available for analysis after transfusion. Last, this study focused solely on intracellular metabolic activity, extracellular surface markers, and PTR, without assessing platelet functionality.
Clinical impact
From a clinical perspective, the outcomes suggest that short-term stored PCs are potentially better suited for transfused patients with thrombocytopenia during inflammation, with previous studies also showing poor platelet transfusion response after transfusion of longer stored PCs.14,22,24 For prophylactic purposes, such as before invasive procedures, this disparity is of lesser concern, particularly because the peak levels of both short- and long-term stored PCs occur at 4 and 6 hours after transfusion, respectively. Alternatively, in therapeutic platelet transfusions for bleeding, there is uncertainty regarding the use of short-term vs long-term stored PCs. Our observations of potential signs of enhanced coagulation effects in both short-term (eg, higher ADP and serotonin levels) and long-term stored PCs (eg, elevated CD62P levels) suggest they may accelerate their impact on coagulation, warranting further investigation in future studies.
Conclusions
This study showed that short-term stored PCs had more favorable metabolic and functional profiles than long-term stored PCs in an inflammatory model. Improved PTR was linked to higher dense granule components and lower activation markers. These findings suggest short-term stored PCs may be more effective during inflammation and warrant further study.
Acknowledgments
The authors express their appreciation to S.J. Raasveld and D.F.L. Filippini of the Amsterdam UMC, NL for providing backup supervision for the volunteers. The authors thank K.W. Tan for his support during the donation process. The authors thank D. de Korte for his contribution to the development of the design of the study. The authors specially thank A.F. Sufredini of the National Institutes of Health (NIH; Bethesda, MA) who kindly provided the endotoxin used in this study. The authors thank all Amsterdam UMC and Sanquin personnel who helped produce the autologous platelet concentrates.
This study is funded by Zorgonderzoek Medische Wetenschappen, part of the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (the Dutch Organization for Scientific Research, Den Haag, The Netherlands; project 09150172010047) and Sanquin PPO (18-06/L-number 2329). Furthermore, A.P.J.V. is supported by a personal grant of Landsteiner Stichting voor Bloedtransfusieresearch (LSBR; Dutch; project number 1931F).
The sponsors of this work were not involved in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
The figures in the visual abstract and schematical overview figures in the manuscript are developed using BioRender.com. Van Wonderen, S.F. (2025) https://BioRender.com/38yqf6j and https://BioRender.com/7i9wjcj Figure 1: Created in BioRender. Van Wonderen, S.F. (2025) https://BioRender.com/l56u148 Figure S4: Created in BioRender. Van Wonderen, S.F. (2025) https://BioRender.com/mlmgi37 Figure S5: Created in BioRender. Van Wonderen, S.F. (2025) https://BioRender.com/asiwvvx.
Authorship
Contribution: A.P., R.v.B., B.J.B., and A.P.J.V. contributed to study conception and design; S.F.v.W., F.L.F.v.B., S.d.B., and C.V. recruited participants and provided study materials; S.F.v.W., F.L.F.v.B., P.G.P., E.B.B., A.A., S.d.B., A.M.T.-d.B., C.A.P., R.B.E.v.A., E.H.T.L., J.A.R., A.D. and C.V. were involved in data collection; S.F.v.W., F.L.F.v.B., A.A., J.S., J.A.R., and A.D. were involved in the data analyses; S.F.v.W., F.L.F.v.B., M.C.A.M., T.R.L.K., A.D., and A.P.J.V. prepared the manuscript; and all authors read and approved of the final version before submission.
Conflict-of-interest disclosure: A.D. is a founder of Omix Technologies Inc, unrelated to the contents of the manuscript; and is a consultant for Hemanext Inc. The remaining authors declare no competing financial interests.
Correspondence: Alexander P. J. Vlaar, Intensive Care Medicine and Laboratory of Experimental Intensive Care and Anesthesiology, Amsterdam University Medical Center location University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; email: a.p.vlaar@amsterdamumc.nl.
References
Author notes
Metabolomics data for this study are available at the National Institutes of Health Common Fund's National Metabolomics Data Repository website (https://www.metabolomicsworkbench.org), the Metabolomics Workbench (study ID ST003937).
Original data are available on reasonable request from the corresponding author, Alexander P. J. Vlaar (a.p.vlaar@amsterdamumc.nl).
The full-text version of this article contains a data supplement.