Key Points
PTCy+Aba arm is associated with less chronic GVHD and a favorable GRFS compared with methotrexate/tacrolimus.
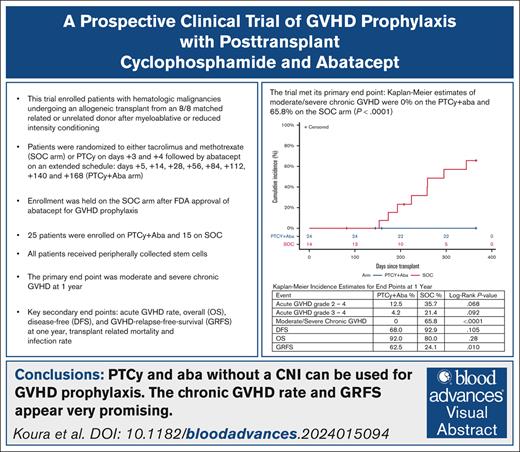
PTCy and Aba without a calcineurin inhibitor can be used for GVHD prophylaxis.
Visual Abstract
We conducted a prospective randomized clinical trial to investigate the combination of posttransplant cyclophosphamide (PTCy) and abatacept (Aba) for graft-versus-host disease (GVHD) prophylaxis. Patients with hematologic malignancies undergoing an allogeneic transplant from an 8/8 matched related or unrelated donor were randomized 1:1 to tacrolimus and methotrexate (standard-of-care arm [SOC]) or PTCy on days +3 and +4, followed by Aba on an extended schedule: days +5, +14, and +28, and every 4 weeks up to day +168 (PTCy+Aba). All patients received peripherally collected stem cells. The primary end point was moderate and severe chronic GVHD at 1 year. Following US Food and Drug Administration approval of Aba for GVHD prophylaxis leading to change in institutional SOC, the trial was amended to enroll only on the PTCy+Aba arm. A total of 25 patients enrolled on PTCy+Aba, and 15 on SOC. The trial met its primary end point: Kaplan-Meier estimates of moderate and severe chronic GVHD were 0% on the PTCy+Aba and 65.8% on the SOC arm (P < .0001). GVHD-free, relapse-free survival (GRFS) was 62.5% on PTCy+Aba and 24.1% on SOC (P = .010). There were no treatment-related deaths on PTCy+Aba and 2 on SOC. Overall survival (PTCy+Aba, 92%; SOC, 80%; P = .28), disease-free survival (PCTy+Aba, 68%; SOC, 92.9%; P = .105), and infection rates at 1 year were similar. Grade 3/4 acute GVHD rate was 4.2% on PTCy+Aba and 21.4% on SOC (P = .092). PTCy+Aba preserved regulatory T-cell proliferation and increased CD16+CD56dim cytotoxic natural killer cells. In conclusion, PTCy+Aba is well tolerated and associated with reduced chronic GVHD and improved GRFS. This trial was registered at www.ClinicalTrials.gov as #NCT03680092.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative option for most hematologic malignancies, but it is associated with significant complications, notably acute and chronic graft-versus-host disease (GVHD). Chronic GVHD is the main cause of late posttransplant mortality and also has a significant impact on the quality of life of survivors.1 Therefore, preventing GVHD has been one of the main objectives of transplant immunology.
For many years the combination of a calcineurin inhibitor (CNI; cyclosporine or tacrolimus) with a short course of methotrexate was the primary GVHD prophylaxis regimen.2 However, this combination was recently shown to be inferior to other approaches. In the Blood and Marrow Transplant Clinical Trials Network 1703 trial, patients who received posttransplant cyclophosphamide (PTCy), tacrolimus, and mycophenolate mofetil had a better GVHD-free, relapse-free survival (GRFS) at 1 year compared with patients receiving tacrolimus and methotrexate.3 In the ABA2 trial, patients who received abatacept (Aba) in addition to methotrexate and tacrolimus had less acute GVHD and improved severe-acute GVHD-free survival.4,5 Together, these studies suggest theoretical potential for immunological synergy between PTCy and Aba in GVHD prophylaxis, potentially even obviating the need for traditional prophylactic immunosuppression with agents including tacrolimus, methotrexate, and mycophenolate mofetil.
Murine studies postulate that PTCy depletes donor alloreactive T cells and promotes donor regulatory T-cell development, while sparing T-cell populations with immunity against tumor antigens, thus preserving graft-versus-tumor (GVT) effect.6,7 Subsequent in-human studies support PTCy influences alloimmune response through regulatory T-cell sparing, the induction of hyporesponsive effector T-cell phenotypes, and the promotion of natural killer (NK) cell populations.8,9
Aba is a CTLA4 immunoglobulin modified antibody that binds to CD80 and CD86 on antigen-presenting cells, preventing interaction with CTLA-4 and CD28 on T cells.10 This costimulation blockade presumably leads to altered gene expression and limited effector memory T-cell expansion.4,11,12 In addition, Aba directly activates NK cells and may enhance the GVT effect.13,14 Belatacept, a similar CTLA4 immunoglobulin molecule, can be used instead of cyclosporine for the prevention of rejection in kidney transplantation.15 Aba is also effective for the treatment of steroid-refractory chronic GVHD.16
The optimal duration of Aba for GVHD prophylaxis is undetermined; however, previous work established that higher Aba concentration trough was associated with a favorable acute GVHD risk, without any observed exposure-toxicity relationships.5 In addition, other studies have demonstrated improved GVHD prophylaxis with 4 vs 3 doses,17 yet no impact on chronic GVHD with 4 doses,4 suggesting a potential role for an extended dosing schedule.
We hypothesized that PTCy followed by Aba on an extended administration schedule would prevent the development of chronic GVHD. Patients on the experimental arm did not receive a CNI because it has potential to interfere with CTLA4 immunoglobulin.18 Aba was started after PTCy administration to further avoid any interference with the action of high-dose cyclophosphamide.
Methods
Trial design
This randomized phase 2, open label, single-center trial compared 2 GVHD prophylaxis regimens: PTCy followed by Aba for 6 months (experimental or PTCy+Aba arm) vs methotrexate and tacrolimus (standard-of-care [SOC] arm) in patients receiving a peripheral blood allogeneic HSCT from a matched donor for a high-risk hematologic malignancy with either myeloablative (MA) or reduced-intensity conditioning (RIC). The primary objective was to compare the incidence of moderate/severe chronic GVHD at 1 year.
The protocol was approved by the University of California San Diego Institutional Review Board. All patients provided written informed consent. After the US Food and Drug Administration (FDA) approval of Aba for GVHD prophylaxis leading to change in institutional SOC, we amended our trial and subsequently only enrolled onto the PTCy+Aba arm.
Patients
We planned to enroll 50 eligible patients (25 per arm) who were at least 18 years of age and were undergoing their first allogeneic transplantation with the use of peripheral blood grafts from an 8/8 matched unrelated donor or matched related donor (matched at HLA-A, HLA-B, HLA-C, and HLA-DRB1 at high resolution on DNA-based typing). Eligible diagnoses included patients with high-risk or relapsed acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia in accelerated or blast phase, myelodysplastic/myeloproliferative neoplasms, non-Hodgkin Lymphoma, Hodgkin lymphoma, and multiple myeloma. All patients were determined clinically to require an allogeneic HSCT with a curative intent.
Treatment
Patients received conditioning as per institutional protocol. Options included either MA (busulfan 130 mg/m2 IV and fludarabine 40 mg/m2 IV for 4 days on days −6 to −3; or cyclophosphamide 60 mg/kg IV daily on days −5 and −4 with mesna and total body irradiation 2.0 Gy twice a day on days −3, −2, −1 for a total of 12.0 Gy; or RIC [fludarabine 30 mg/m2 daily for 4 days on days −6 to −3 and melphalan 140 mg/m2 on day −2]). Pretransplant cyclophosphamide was reduced to 50 mg/kg for patients who also received PTCy.
The experimental GVHD prophylaxis regimen (PTCy+Aba) consisted of the following: cyclophosphamide (50 mg/kg per day on days +3 and +4 after transplantation) with mesna and hydration; Aba (10 mg/kg IV based on actual body weight, with maximum dose of 1000 mg/d, on days +5, +14, +28, +56, +85, +112, +140, and +168 after transplantation). The SOC regimen consisted of tacrolimus (0.022 mg/kg per day using ideal body weight, starting on day −2 before transplant; adjusted for trough of 5-15 ng/mL); methotrexate (for fludarabine-based regimens: 5 mg/m2 on days +1,+3, +6, and +11 after transplant; and for total body irradiation/Cy, 15 mg/m2 on day +1, then 10 mg/m2 on days +3, +6, and +11 after transplant; methotrexate could be adjusted or held at the investigator’s discretion). Use of antithymocyte globulin was allowed only on the SOC arm, but no patients received antithymocyte globulin. Growth factors were allowed at the investigator’s discretion, 24 to 48 hours after the completion of the chemotherapy (PTCy on the experimental arm, and methotrexate on the SOC arm). Posttransplant supportive care and infection prophylaxis were otherwise provided according to institutional standards. Posttransplant maintenance therapies to prevent relapse were allowed except in cases of a known GVHD prophylactic effect.
End points
The primary end point was moderate/severe chronic GVHD at 1 year after transplant. Diagnosis and staging of chronic GVHD was based on the National Institutes of Health consensus criteria.19 Secondary end points were: GRFS and chronic GVHD and relapse-free survival (CRFS) at 1 year; overall chronic GVHD (including mild), grade 2 to 4 and 3 to 4 acute GVHD rate; overall survival (OS) and disease-free survival at 1 year; transplant-related mortality; grade ≥3infections or other treatment-related toxicities; and cognitive impairment (defined as change in mini-mental state examination between baseline, days +100 and +180, and 1 year after transplant). Acute GVHD was diagnosed and graded according to the Glucksberg criteria.20 GFRS was defined as the absence of acute GVHD grade 3 or 4 or moderate/severe chronic GVHD or relapse/nonrelapse mortality and CRFS as the absence of moderate/severe chronic GVHD or relapse or nonrelapse mortality by 1 year after transplant. GFRS and CFRS were key secondary end points.
Statistical analysis
The primary statistical hypothesis was that PTCy+Aba would reduce the risk of moderate and severe chronic GVHD by 1 year compared with GVHD prophylaxis with methotrexate and CNI.
Patients were randomly assigned in a 1:1 ratio with the use of a computer-generated random listing of the 2 treatment assignments to the experimental-prophylaxis arm or to the standard-prophylaxis arm after enrollment and before initiating the conditioning regimen. RANDI2 software was used for the randomization.21 Randomization was stratified for conditioning regimen and donor type but not disease type or risk. The study was powered for a large difference in the primary end point. At a type I error rate of 0.05, using a 2-sided test on the treatment effect from Cox regression, and assuming that all strata had similar odds ratios, we estimated 82% power to detect a significant difference in the primary outcome at 1 year (assuming an incidence of 10% in the experimental arm and 50% in the control arm). Estimated study duration was 2 years. After the change in SOC on 15 December 2021 with the FDA approval of Aba for GVHD prophylaxis in combination with methotrexate and a CNI, the trial was amended, and patients were no longer enrolled in the SOC arm. The first 30 patients were enrolled before amendment.
Numerical variables were summarized by mean and standard deviation. Categorical variables were summarized by frequency and percentage. Time-to-event was estimated by the Kaplan-Meier method and was compared across study arms by the log-rank test. Cox proportional hazard models were used to obtain hazard ratios and their 95% confidence intervals. The proportional hazards assumption was checked using the supremum test. All significance tests were 2-sided and used the 0.05 significance level. SAS version 9.4 (SAS Institute, Cary, NC) was used for statistical calculations.
There was a continuously monitored safety stopping rule applied to deaths related to the experimental arm. The rule used an exact sequential Pockock boundary. It was applied sequentially as patients enrolled in the experimental arm of the study. The stopping rule was assessed after each patient had passed 100 days after transplant. The stopping rule was designed to stop early with probability 60% if 100-day experimental-treatment–related mortality is ≥10%.
Correlative studies
Peripheral blood samples were collected at days +28, +100, +180, and +365 after transplant for analysis of lymphocyte populations. Flow cytometry was performed to identify conventional T cells, regulatory T cells (Tregs), and NK cells, and evaluate their proliferative activity. Peripheral blood mononuclear cells from the patients were incubated with Zombie Yellow (BioLegend) viability dye before labeling with surface antibodies (CD4-APC-Cy7; CD8a-PerCP-Cy5.5; CD25-PE-Cy7; CD127-APC, CTLA-4-BV42, HLA-DR-FITC, CD56-Pacific Blue, CD44-AF700, CD3-BV711, CD16-BV650; BioLegend). Cells were then fixed and permeabilized with True Nuclear Transcription Factor Buffer Set (BioLegend) per manufacturer’s instructions before intracellular labeling with Foxp3-PB (BioLegend) antibodies. Samples were run with color and fluorescence minus one controls. Flow cytometry analyses were acquired on Bio-Rad ZE5 Cell Analyzer. Data were analyzed using FlowJo version 10 software. All data were analyzed using 2-way analysis of variance with correction for repeated measures and multiple comparisons to examine treatment groups and identify time-dependent effects. Corrected P values <.05 were considered statistically significant.
Immunogenicity studies
Blood samples for determination of antibodies to Aba were collected at baseline and on days +28, +56, +84, +112, +140, and +168. Samples were assayed for presence of Aba-specific antibodies by ICON Clinical Laboratories. Antibodies against Aba, a CTLA4 immunoglobulin fusion protein were measured in human serum using an electrochemiluminescent immunoassay method using Meso Scale Discovery technology, which uses a ruthenium metal chelate (SulfoTag) as the electrochemiluminescent label.
Results
Patients
Between 28 January 2020 and 26 May 2022, a total of 43 patients were enrolled in the trial at the University of California, San Diego. Three patients did not receive treatment after enrollment: 2 patients were randomized to the SOC arm and their insurance did not allow participation, and the third patient withdrew consent before randomization. Initially, patients were randomized between the SOC and the experimental arms and stratified by donor type (matched related donor vs matched unrelated donor) and by conditioning (MA vs RIC). However, in December 2021, the FDA approved Aba for GVHD prophylaxis, and our institutional SOC changed to include Aba in combination with methotrexate and tacrolimus. We amended the protocol and stopped enrolling on the SOC arm. All subsequent patients were treated on the PTCy+Aba arm. Patient baseline characteristics are shown in Table 1. Patients were followed for 1 year after transplant. Most patients had AML and ALL. No patients with myeloma were enrolled. No patients with lymphoma were on the PTCy+Aba arm. Notably more patients on the PTCy+Aba arm had more advanced disease (transplant in second complete remission or stable disease; Table 1; supplemental Table 1).
Primary end point
The trial met its primary end point: Kaplan-Meier estimates of moderate/severe chronic GVHD by 1 year after transplant were 0% on the PTCy+Aba arm and 65.8% (8 patients) on the SOC arm (P < .0001; Figure 1).
Main outcomes. Kaplan-Meier curves for incidence of moderate/severe chronic GVHD in first year (A), GRFS (B), freedom from relapse in first year (C), and OS (D).
Main outcomes. Kaplan-Meier curves for incidence of moderate/severe chronic GVHD in first year (A), GRFS (B), freedom from relapse in first year (C), and OS (D).
Secondary end points
Table 2 summarizes the Kaplan-Meier estimates for all the end points at 1 year. Although all patients had donor engraftment by day +28, 1 patient on each arm had secondary graft failure. These 2 patients were included in analysis for OS and relapse-free survival but excluded from analysis pertaining to GVHD because both underwent a second allogeneic HSCT. The patient on the SOC arm subsequently died of infectious complications (toxoplasmosis), whereas the patient on the PTCy+Aba arm underwent a successful salvage haploidentical HSCT and was alive through the duration of the study.
The key secondary end point of GRFS was 62.5% in the PTCy+Aba and 24.1% in the SOC arm and was statistically significant (P = .010; Figure 1). CRFS was 66.7% in the PTCy+Aba arm and 28.6% in the SOC arm (P = .022; supplemental Figure 1).
At 1 year there were 2 deaths on the PTCy+Aba arm, both due to relapse; and 3 on the SOC arm (1 due to relapse). There were no treatment-related deaths on the PTCy+Aba arm. On the SOC arm, 1 patient died of idiopathic pneumonia syndrome and 1 patient died of infectious complications (toxoplasmosis) who had also had secondary graft failure. There was no statistically significant difference in the rates of OS at 1 year (PTCy+Aba, 92%;SOC, 80%; P = .28). There were 9 relapses on the PTCy+Aba arm and 2 relapses on the SOC arm. Of patients with relapse after transplant on the PTCy+Aba arm, 4 patients had undergone transplant in second complete remission (CR2) for AML, 3 patients had AML in first complete remission (CR1) with measurable residual disease at time of transplant, 1 patient had ALL in CR2 and myelodysplastic syndrome, and 1 had myelofibrosis. There was no statistically significant difference in the disease-free survival at 1 year (PTCy+Aba, 68.0%; SOC, 92.9%; P = .105; Figure 1; supplemental Figure 1).
Grade 3/4 acute GVHD rate was 4.2% on the PTCy+Aba arm (n = 1) and 21.4% (n = 3) on the SOC arm (P = .092), whereas grade 2 to 4 acute GVHD occurred in 12.5% on PTCy+Aba and 35.7% on SOC (P = .068; supplemental Figure 1). There were no cases of acute GVHD of the gut or liver on the PTCy+Aba arm. There were 2 cases of mild chronic GVHD on the PTCy+Aba arm. Overall chronic GVHD rate (including mild) was 9.1% on PTCy+Aba and 74% on SOC (P < .0001).
Safety
Adverse events (AEs) are summarized in Table 3. The most common AE in both arms was infection, with comparable incidence between arms. One patient on the SOC arm died of an infection. One patient on the SOC arm developed posttransplant lymphoproliferative disorder. AEs noted in the SOC but not PTCy+Aba arm were: thrombotic microangiopathy, nephrotoxicity, hypertension, electrolyte derangement, and neurotoxicity. Such toxicities are commonly caused by tacrolimus. There were no noninfectious severe AEs (grade ≥3) in the experimental arm, whereas 2 occurred in the SOC arm (nephrotoxicity and thrombotic microangiopathy). Mini-mental state examinations at baseline and days +100, +180, and 1 year after transplant did not reveal any significant cognitive impairment related to either the experimental or the SOC arm.
Immunogenicity
Among 259 primary samples and 259 duplicate (backup) samples received, a total of 253 study samples were successfully analyzed for anti-Aba antibodies using the validated method. Of these samples, 8 were confirmed positive with Aba during confirmatory analyses; 4 patients (3 on PTCy+Aba and 1 on SOC) had a positive result only at baseline that turned negative after the transplant.
Correlative studies
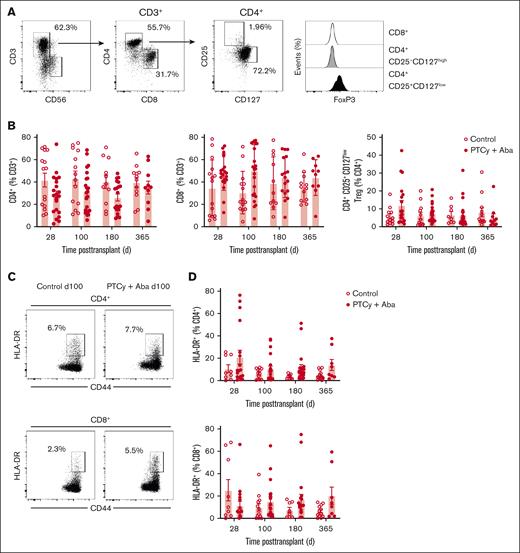
CD4+, CD8+, and CD4+CD25+CD127lowFoxP3+ Tregs were identified from the peripheral blood by flow cytometry (Figure 2A). Although FoxP3 expression correlated with CD25+CD127low phenotype CD4+ T cells in all samples, CD25+CD127low was a more consistent measure of Tregs (as evidenced by control samples) and used as the primary measure of Treg frequency. Patients in the SOC and PTCy+Aba arms had similar proportions of CD4+, CD8+, and Tregs (Figure 2B). Patients in both arms had similar inhibition of T-cell activation as measured by HLA-DR upregulation (Figure 2C-D) of conventional effector CD4+ and CD8+ T cells.
Peripheral blood T-cell analysis. T cells in the peripheral blood were analyzed by flow cytometry at days 28, 100, 180, and 365 after transplant. (A) Representative gating strategy. (B) Comparison of CD4+, CD8+, and CD4+CD25+CD127low Tregs in SOC and PTCy+Aba treatment groups across time points. (C) T-cell activation was assessed by measuring HLA-DR upregulation. Representative examples shown for 1 patient each in the SOC and PTCy+Aba groups at day 100 after transplant. (D) Comparison of T-cell activation as indicated by HLA-DR expression in SOC and PTCy+Aba treatment groups across time points.
Peripheral blood T-cell analysis. T cells in the peripheral blood were analyzed by flow cytometry at days 28, 100, 180, and 365 after transplant. (A) Representative gating strategy. (B) Comparison of CD4+, CD8+, and CD4+CD25+CD127low Tregs in SOC and PTCy+Aba treatment groups across time points. (C) T-cell activation was assessed by measuring HLA-DR upregulation. Representative examples shown for 1 patient each in the SOC and PTCy+Aba groups at day 100 after transplant. (D) Comparison of T-cell activation as indicated by HLA-DR expression in SOC and PTCy+Aba treatment groups across time points.
Total NK cell populations were similar between study arms. However, time-dependent effects were observed in the PTCy+Aba treatment arm, with increased proportions of CD16+CD56dim cytotoxic NK cells (P < .001) and decreased proportions of CD16−CD56bright NK cells (P < .001).22
Discussion
There remains a significant need for well-tolerated GVHD prophylaxis regimens that effectively prevent chronic GVHD. The modern GVHD prophylactic regimen comprising PTCy, tacrolimus, and mycophenolate mofetil (MMF) has improved the chronic GVHD rate at 1 year, which, although reduced compared with tacrolimus methotrexate alone, still stands at 21.9%.3 Our trial, however, uniquely investigates a CNI-free GVHD prophylaxis approach, combining PTCy with an extended course of Aba. This randomized trial met its primary end point of moderate and severe chronic GVHD at 1 year as well as its key secondary end point of GFRS at 1 year. These findings make it an important addition to the growing body of literature of PTCy+Aba GVHD prophylaxis studies. The reduction in chronic GVHD was mainly because of the strikingly low number of chronic GVHD cases seen in the PTCy+Aba experimental arm. The study was powered for a large difference in the primary end point at 1 year, further study results showed large differences for both the primary end point and key secondary end point GFRS, therefore these results remain highly promising despite the midstudy amendment altering randomization to the experimental arm only.
The reduction in chronic GVHD observed in our study may be attributed to the addition of PTCy and the extended duration of Aba, which was given for a total of 6 months. Previous studies have reported similar findings. For instance, long-term follow-up of a phase 2 study in pediatric patients undergoing haploidentical HSCT for nonmalignant diseases showed reduced chronic GVHD when PTCy was combined with extended-dose Aba (starting day 0 and continued until 6 months after HSCT) and sirolimus.23 Similarly, a phase 1b-2 study investigated GVHD prophylaxis after peripheral blood haploidentical HSCT with PTCy, a short-course Aba (4 doses starting on day +5) and short-course tacrolimus, reporting a 15.9% chronic GVHD rate.17
In contrast, the ABA2 trial, which used a short-course Aba without PTCy, did not show a reduction in chronic GVHD.4 These differences suggest that the combination of PTCy with extended-dose Aba may play a key role in chronic GVHD prevention. Further studies are needed to clarify the impact of PTCy combined with extended-dose Aba. In addition, the impact of CNIs needs to be further investigated, because these may blunt the effects of Aba in chronic GVHD prevention.
The study was not powered to detect a significant difference in acute GVHD. However, acute GVHD rate and severity appeared to be quite low with PTCy+Aba. There was only 1 case of grade 3/4 acute GVHD (of the skin only). Some patients developed grade 1/2 acute GVHD of the skin but there were no cases of acute GVHD of the liver or gut. The low rate of acute GVHD is comparable with ABA2 trial results and other studies investigating Aba as part of GVHD prophylaxis regimens.4,5,17,23-25 PTCy has generally not decreased mild acute GVHD, however it has been shown to decrease grade 3/4 GVHD when compared with tacrolimus and methotrexate prophylaxis without PTCy.3
The combination PTCy+Aba was easy to administer because it does not require monitoring of levels and strict twice a day adherence to an oral medication. It was also safe, with a low number of associated grade ≥3 AEs that were all expected. Patients on the experimental arm did not develop the toxicities that are frequently caused by CNIs, such as nephrotoxicity, thrombotic microangiopathy, hypertension, electrolyte imbalances, and neurologic complications. The rate and the severity of infection was within the expected range for allogeneic transplant recipients. There were no unusual infections and the rate of cytomegalovirus reactivation appeared to be similar in both arms. The only case of posttransplant lymphoproliferative disorder was in the tacrolimus and methotrexate arm. Patients engrafted well, and there was only 1 case of secondary graft failure.
Any attempt to better prevent GVHD can cause a higher incidence of relapse by also interfering with the GVT effect.26 This trial was not designed and did not have the power to detect differences in relapse rate. Relapse risk was not well balanced between the 2 arms and was higher in the PTCy+Aba arm, as 12 of 15 patients in the SOC arm were in CR1 but only 9 of 25 in the PTCy+Aba arm (Table 1; supplemental Table 1). The disease types that were treated also differed between the arms. Overall, the relapse rate appeared to be within expected after an allogeneic transplant, but the actual relative relapse risk associated with PTCy+Aba and the potential impact of this regimen on the GVT effect needs to be examined further in future studies. Previously published clinical studies using Aba as part of GVHD prophylaxis with CNIs and/or PTCy have not found increased risk of relapse in secondary and exploratory analyses, although these studies similarly were not designed to investigate relapse as a primary end point.4,5,17
Our study, in line with previous reports, suggests that, mechanistically, both PTCy and Aba have a profound impact on T cells.6,7,10 Specifically, our data show that the combination PTCy+Aba resulted in sustained inhibition of effector CD4+ and CD8+ T-cell activation (Figure 2).
PTCy has been shown previously to promote immature NK cell populations,8,9 meanwhile, CTLA4–immunoglobulin can stimulate NK cells.13,23,27 In our study, patients receiving the combination PTCy+Aba exhibited a preferential expansion of CD16+/CD56bright NK cells (Figure 3), which have a higher cytotoxic activity. Notably, this effect persisted well beyond the expected in vivo half-life of Aba,5 suggesting that the combination of PTCy+Aba can induce long-lasting changes to the immune system. Although the clinical implications of NK cell modulation remain unclear and warrant further investigation, this modulation may play an important role in GVT effect and immune defense against viral pathogens.
NK cells. Peripheral blood NK cell analysis. NK cells in the peripheral blood were analyzed by flow cytometry at days 28, 100, 180, and 365 after transplant. (A) Representative gating strategy to identify CD3−CD56+ NK cells, separated into cytotoxic CD16+CD56dim and CD16−CD56bright NK cells. Representative examples shown for 1 patient each in the SOC and PTCy+Aba groups at day 180 after transplant. (B) Comparison of total NK cells, CD16+CD56dim and CD16−CD56bright NK cells in the SOC and PTCy+Aba treatment groups across time points.
NK cells. Peripheral blood NK cell analysis. NK cells in the peripheral blood were analyzed by flow cytometry at days 28, 100, 180, and 365 after transplant. (A) Representative gating strategy to identify CD3−CD56+ NK cells, separated into cytotoxic CD16+CD56dim and CD16−CD56bright NK cells. Representative examples shown for 1 patient each in the SOC and PTCy+Aba groups at day 180 after transplant. (B) Comparison of total NK cells, CD16+CD56dim and CD16−CD56bright NK cells in the SOC and PTCy+Aba treatment groups across time points.
Antibody formation to Aba did not occur and the few patients who had detectable antibodies at baseline cleared those antibodies after transplant. This was expected as immunogenicity is severely impaired within the first 6 months after allogeneic HSCT.
This trial had several limitations. It was conducted in only 1 center and had a small sample size. The randomization stopped after Aba was approved in combination for GVHD prophylaxis and the institutional SOC changed, and therefore there was an uneven number of patients in each arm. The study was not stratified for risk of relapse, nor was it designed to assess risk of relapse between arms. The small number of patients might not have enabled us to detect relatively rare and unexpected side effects of the PTCy+Aba combination.
Importantly, the SOC in most institutions has changed since the publication of Blood and Marrow Transplant 1703 trial that showed that PTCy, tacrolimus, and MMF was associated with a superior GFRS compared with tacrolimus and methotrexate, at least in patients receiving RIC.3 Therefore, a major limitation of this study is that the comparator arm used in this trial is no longer SOC in most institutions.
Nevertheless, the study clearly showed that the combination PTCy+Aba for GVHD prophylaxis is feasible and associated with low rates of chronic GVHD and improved GFRS. It is a regimen that could be used in patients with classical hematologic disease in which there is no risk of relapse or in patients with comorbidities that do not allow them to tolerate a CNI, such as kidney dysfunction. It warrants further examination in a large phase 3 trial with PTCy, tacrolimus, and MMF as the comparator arm.
Acknowledgments
The authors thank Cursor.gr for their assistance with graphic design.
This investigator-initiated trial was supported by a Bristol Myers Squibb Research Grant.
Authorship
Contribution: D.T. designed the study; J.M. performed statistical analysis and helped with the interpretation of the results; D.T., D.K., and K.D. collected, analyzed, and interpreted the results, and wrote the manuscript; Q.Z., P.O., and G.P.M. performed the correlative studies; A.H., T.N.T., A.G., C.M., C.C., J.K.M., A.-R.J., and E.D.B. provided cases for the study; K.M. performed the randomizations and was the research pharmacist; J.J.M. and M.P. were the clinical trial coordinators; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: D.K. declares consultancy with, and research funding from, Bristol Myers Squibb (BMS). J.K.M. reports consultancy with Shoreline Bio and Autolus. A.G. declares consultancy with Seattle Genetics. T.N.T. declares consultancy with BMS, Gilead, Rigel, and Servier; and reports research funding from Function Oncology. G.P.M. reports research funding from ThermoFisher, CareDx, Werfen, Pirche Ag; and received travel support from ThermoFisher. D.T. declares consultancy with BMS and Veraxa; and received research funding from BMS. The remaining authors declare no competing financial interests.
Correspondence: Dimitrios Tzachanis, Moores Cancer Center, 3855 Health Sciences Dr, 0960, La Jolla, CA 92093-0960; email: dtzachanis@health.ucsd.edu.
References
Author notes
Data are available on request from the corresponding author, Dimitrios Tzachanis (dtzachanis@health.ucsd.edu).
The full-text version of this article contains a data supplement.