Key points
ASCT for AL amyloidosis decreased significantly (71% average annual reduction) with the introduction of Dara-CyBorD.
ASCT now targets patients with suboptimal response, relapsed/refractory disease, lymphoplasmacytic clone, or higher plasma cell burden.
Visual Abstract
This retrospective analysis examined 15 years of autologous stem cell transplantation (ASCT) for light chain (AL) amyloidosis at the Mayo Clinic (2010-2024). We aimed to assess ASCT utilization trends, factors influencing practice changes, and current indications amid newer therapies. Four hundred and forty-one ASCTs were divided into cohort 1 (2010-2019) and cohort 2 (2020-2024), revealing a significant ASCT reduction in cohort 2 (385 vs 56, average 71% annual decrease). Cohort 2 patients were older, more likely to have relapsed/refractory disease, and had higher baseline bone marrow plasma cell burden compared to cohort 1. Pre-ASCT induction was more frequent in cohort 2 (89.3% vs 56.4%), with daratumumab (Dara)–cyclophosphamide-bortezomib-dexamethasone (CyBorD) replacing CyBorD as the predominant induction regimen. Lymphoma-based regimens were also more common in cohort 2 (15.1% vs 5.3%, P = .02). Day-100 satisfactory hematological response improved in cohort 2 (91.1% vs 72.7%, P = .001), although hematological complete response rates did not significantly differ (50.9% vs 38.8%, P = .09). In summary, there is a decrease in the utilization of ASCT in AL amyloidosis. This procedure is primarily reserved for patients with suboptimal responses, relapsed/refractory disease, lymphoplasmacytic clones (predominantly immunoglobulin M amyloidosis), or high bone marrow plasma cell burden (myeloma phenotype). This study underscores a significant shift in ASCT practice, driven by novel therapies, emphasizing more personalized management in AL amyloidosis.
Introduction
Autologous stem cell transplantation (ASCT) has historically served as a cornerstone in the management of light chain (AL) amyloidosis, offering the potential for deep and durable remissions, particularly in patients with good performance status and limited organ involvement.1,2 However, the landscape of ASCT for AL amyloidosis has undergone significant changes over the past 15 to 20 years. The introduction of induction therapy prior to ASCT became increasingly common, aiming to reduce the clonal burden and improve patient tolerance to high-dose melphalan conditioning.3-5 Furthermore, the emergence of effective standard-intensity regimens, capable of achieving deep remissions with lower toxicity, such as bortezomib- and daratumumab (Dara)-based therapies, has provided alternative treatment strategies.6,7 These newer regimens have demonstrated remarkable efficacy in inducing hematological responses, potentially reducing the need for ASCT. This evolution necessitates a reassessment of the role of ASCT in the contemporary management of AL amyloidosis.
Methods
This retrospective study investigated the 15-year application of ASCT for AL amyloidosis in our center. The study aimed to analyze trends in ASCT utilization, determine factors influencing practice changes, and define trends in ASCT indication in the context of newer therapies. Data were collected from all ASCTs performed for AL amyloidosis between 1 January 2010 and 31 December 2024. The institutional review board approved the study. Patients were categorized as having received induction therapy if they underwent pre-ASCT treatment to reduce tumor burden, irrespective of response. Relapsed or relapsed-refractory patients were defined as those exhibiting a rise in monoclonal protein and/or involved free light chain prompting a change in therapy, even without meeting hematological progression criteria. Hematological response was assessed using consensus criteria.8 Patients with a baseline difference between involved and uninvolved light chains (dFLC) of 20 to 50 mg/L were considered to have achieved a dFLC-partial response if post-treatment dFLC was <10 mg/L.9,10 A satisfactory hematological response was defined as complete response (CR), very good partial response, or dFLC-partial response. For comparative analysis, the study period was divided into 2 cohorts: cohort 1 (2010-2019) and cohort 2 (2020-2024), based on the introduction of Dara–cyclophosphamide-bortezomib-dexamethasone (CyBorD) as a routine frontline therapy regimen beginning in 2020. Cardiac staging was performed using the Mayo 2004 classification incorporating the European modification.11,12 Conversion between the various cardiac biomarkers utilized in this staging system was conducted as previously described.13
Results
ASCT utilization for AL amyloidosis significantly declined from cohort 1 (385 transplants, 2010-2019) to cohort 2 (56 transplants, 2020-2024), a 71% reduction (Figure 1). The baseline characteristics of both cohorts are detailed in the Table 1. Patients in cohort 2 were older, more likely to undergo ASCT >12 months after diagnosis (33.4% vs 14%, P < .001), and had higher bone marrow plasma cell burden at diagnosis (median 13% vs 8%, P = .002). There were no significant differences between the cohorts in organ involvement pattern, light chain isotype, baseline dFLC, or baseline and pre-ASCT cardiac stage. Across both cohorts, patients with pre-ASCT cardiac stage 3A (n = 43) typically had cardiac biomarker levels within the accepted threshold for safe transplant (troponin T ≤0.06 μg/L or high sensitivity troponin T ≤75 ng/L in 88.4% of patients; N-terminal pro–B-type natriuretic peptide ≤5000 ng/L in 93% of patients). Patients with cardiac stage 3B (n = 14) across both cohorts commonly presented with severely reduced glomerular filtration rates (median estimated glomerular filtration rate, 8 mL/min per 1.73 m2; interquartile range, 6-11), with (n = 10) or without (n = 4) heart involvement. Most patients in both cohorts received ASCT for newly-diagnosed disease, while a larger proportion of cohort 2 patients underwent ASCT for relapsed/refractory disease compared to cohort 1 (26.9% vs 8.8%, P < .001).
Trends in ASCT for AL amyloidosis, 2010-2024, including the number of ASCTs performed following induction therapy.
Trends in ASCT for AL amyloidosis, 2010-2024, including the number of ASCTs performed following induction therapy.
Baseline characteristic of the study cohorts
| Characteristics . | Cohort 1 (n = 385) . | Cohort 2 (n = 56) . | P value . |
|---|---|---|---|
| Age at diagnosis, median (IQR) | 59 (55-65) | 62 (57-68) | .01 |
| Age at ASCT, median (IQR) | 60 (55-65) | 63 (58-68) | .009 |
| Time from diagnosis to ASCT, n (%) | |||
| >6 mo | 166 (43.1) | 39 (69.6) | <.001 |
| >12 mo | 54 (14) | 19 (33.4) | <.001 |
| Disease status at ASCT, n (%) | <.001 | ||
| Newly-diagnosed disease | 351 (91.2) | 41 (73.2) | |
| Relapsed/refractory disease | 34 (8.8) | 15 (26.9) | |
| Male gender, n (%) | 240 (62.3) | 29 (51.8) | .13 |
| Lambda light chain isotype, n (%) | 263 (68.3) | 41 (73.2) | .45 |
| Heavy chain isotype, n (%) | |||
| IgG | 149 (38.7) | 24 (42.9) | <.001 |
| IgA | 36 (9.4) | 11 (19.6) | |
| IgM | 19 (4.9) | 6 (10.7) | |
| IgD | 1 (0.3) | 2 (3.6) | |
| No heavy chain association | 180 (46.7) | 13 (23.2) | |
| No. of organs involved, n (%) | .78 | ||
| 1 | 200 (51.9) | 28 (50) | |
| >1 | 185 (48.1) | 28 (50) | |
| Involved organs, n (%) | |||
| Kidney involvement | 230 (59.7) | 30 (53.6) | .38 |
| Heart involvement | 184 (47.8) | 29 (52.8) | .57 |
| Gastrointestinal involvement | 61 (15.8) | 8 (14.6) | .8 |
| Peripheral nerve involvement | 47 (12.2) | 7 (12.5) | .95 |
| Autonomic nerve involvement | 42 (10.9) | 7 (12.5) | .72 |
| Liver involvement | 38 (9.9) | 2 (3.6) | .09 |
| Baseline dFLC (mg/L), median (IQR) | 165 (61-553) | 189 (85-604) | .72 |
| Baseline BMPCs (%), median (IQR) | 8 (5-15) | 14 (6-30) | .008 |
| Cardiac stage at baseline, 1/2/3A/3B, % | 44/44/10/2 | 40/38/16/6 | .28 |
| Cardiac stage at ASCT, 1/2/3A/3B, % | 43/45/9/3 | 37/45/14/4 | .64 |
| ECOG PS at ASCT, 0/1/2-3, % | 46/51/3 | 19/81/0 | .01 |
| Characteristics . | Cohort 1 (n = 385) . | Cohort 2 (n = 56) . | P value . |
|---|---|---|---|
| Age at diagnosis, median (IQR) | 59 (55-65) | 62 (57-68) | .01 |
| Age at ASCT, median (IQR) | 60 (55-65) | 63 (58-68) | .009 |
| Time from diagnosis to ASCT, n (%) | |||
| >6 mo | 166 (43.1) | 39 (69.6) | <.001 |
| >12 mo | 54 (14) | 19 (33.4) | <.001 |
| Disease status at ASCT, n (%) | <.001 | ||
| Newly-diagnosed disease | 351 (91.2) | 41 (73.2) | |
| Relapsed/refractory disease | 34 (8.8) | 15 (26.9) | |
| Male gender, n (%) | 240 (62.3) | 29 (51.8) | .13 |
| Lambda light chain isotype, n (%) | 263 (68.3) | 41 (73.2) | .45 |
| Heavy chain isotype, n (%) | |||
| IgG | 149 (38.7) | 24 (42.9) | <.001 |
| IgA | 36 (9.4) | 11 (19.6) | |
| IgM | 19 (4.9) | 6 (10.7) | |
| IgD | 1 (0.3) | 2 (3.6) | |
| No heavy chain association | 180 (46.7) | 13 (23.2) | |
| No. of organs involved, n (%) | .78 | ||
| 1 | 200 (51.9) | 28 (50) | |
| >1 | 185 (48.1) | 28 (50) | |
| Involved organs, n (%) | |||
| Kidney involvement | 230 (59.7) | 30 (53.6) | .38 |
| Heart involvement | 184 (47.8) | 29 (52.8) | .57 |
| Gastrointestinal involvement | 61 (15.8) | 8 (14.6) | .8 |
| Peripheral nerve involvement | 47 (12.2) | 7 (12.5) | .95 |
| Autonomic nerve involvement | 42 (10.9) | 7 (12.5) | .72 |
| Liver involvement | 38 (9.9) | 2 (3.6) | .09 |
| Baseline dFLC (mg/L), median (IQR) | 165 (61-553) | 189 (85-604) | .72 |
| Baseline BMPCs (%), median (IQR) | 8 (5-15) | 14 (6-30) | .008 |
| Cardiac stage at baseline, 1/2/3A/3B, % | 44/44/10/2 | 40/38/16/6 | .28 |
| Cardiac stage at ASCT, 1/2/3A/3B, % | 43/45/9/3 | 37/45/14/4 | .64 |
| ECOG PS at ASCT, 0/1/2-3, % | 46/51/3 | 19/81/0 | .01 |
BMPCs, bone marrow plasma cells; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; IgG, immunoglobulin G.
Statistically significant results (P < .05) are shown in bold.
Pre-ASCT induction therapy, irrespective of disease status (newly diagnosed or relapsed/refractory), was significantly more common in cohort 2 than in cohort 1 (89.3% vs 56.4%; P < .001), with the proportion of patients receiving induction in cohort 1 increasing over time (Figure 1). The list of regimens used as induction therapy are listed in supplemental Table 1. Among the newly diagnosed patients who received induction, CyBorD was the predominant induction regimen in cohort 1 (64.4% of patients), whereas Dara-CyBorD was the most common in cohort 2 (36.8%).
In the whole cohort, more patients in cohort 2 received >1 line of therapy before proceeding to ASCT compared to cohort 1 (49.1% vs 22.8%, P < .001). Although prior bortezomib (or other proteasome inhibitor) use remained consistent between cohorts (90.6% in cohort 2% and 88.2% in cohort 1, P = .61), Dara use significantly increased from cohort 1 to cohort 2 (5.3% vs 77.4%, P < .001), whereas immunomodulatory drug use remained unchanged (24.5% vs 20.6%, P = .53). Lymphoma-based regimens (such as bendamustine-rituximab used for lymphoplasmacytic marrow morphology) were more common in cohort 2 (15.1% vs 5.3%, P = .02). Across both cohorts, 20 patients received lymphoma-based regimens, of whom 16 patients (80%) had immunoglobulin M–associated AL amyloidosis.
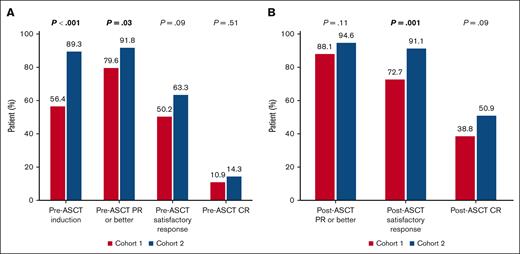
Pre-ASCT hematological response was assessable in 258 of the 267 patients who received induction prior to ASCT (96.6%). More patients in cohort 2 proceeded to ASCT after achieving a satisfactory hematological response compared to cohort 1 (63.3% vs 50.2%), although this difference was not statistically significant (P = .09; Figure 2A).
Response to treatment. (A) Pre-ASCT response. (B) Post-ASCT response. CR, complete response; PR, partial response.
Response to treatment. (A) Pre-ASCT response. (B) Post-ASCT response. CR, complete response; PR, partial response.
Stem cell mobilization methods varied between cohorts, despite no significant change in availability of mobilization agents. In cohort 2, 66.1% of patients received on-demand plerixafor compared to 39.7% in cohort 1 (P < .001). A lower infused CD34+ cell dose was observed in cohort 2 compared to cohort 1 (3.8 × 106 vs 4.3 × 106 CD34+ cells per kg; P = .008), reflecting the impact of induction therapy (particularly daratumumab) on stem cell yields with higher requirements for plerixafor use and lower stem cell yields. Patients without prior induction received a median infused CD34+ cell dose of 4.5 × 106 cells per kg, compared to 4.1 × 106 cells per kg in those who received any induction before ASCT. When stratified by induction component (with potential overlap), the median infused CD34+ cell doses were 3.7 × 106, 4.1 × 106, 4.2 × 106, and 4.4 × 106 cells per kg for patients receiving prior Dara, bortezomib, immunomodulatory drug, and lymphoma-based regimens, respectively. Full-dose conditioning (melphalan ≥180 mg/m2 or BCNU-etoposide-cytarabine-melphalan [BEAM] conditioning) was more common in cohort 2 than in cohort 1 (91.1% vs 81%, P = .048). Day-100 transplant-related mortality was similar between the cohorts (2.6% vs 1.8%, P = .7).
Day-100 hematological response was available in 416 of 441 (94.3%) patients. Although the rate of satisfactory post-ASCT response was higher in cohort 2 than in cohort 1 (91.1% vs 72.7%, P = .001), there was only a statistical trend in post-ASCT CR between the 2 cohorts in favor of cohort 2 (50.9% vs 38.8%, P = .09; Figure 2B). When the analysis was stratified by disease status at ASCT (newly diagnosed vs relapsed refractory), post-ASCT CR was higher in cohort 2 than in cohort 1 only among the relapsed/refractory patients (71.4% vs 37.5%, P = .03), but not among the newly diagnosed patients (43.9% vs 39%, respectively; P = .54). The changes in hematological response observed from pre-ASCT to post-ASCT are detailed in supplemental Table 2. Overall, there was no difference between the 2 cohorts in the proportion of patients who experienced response improvement between the 2 time points (P = .5).
Discussion
This study highlights an important shift in the application of ASCT for AL amyloidosis within a major referral center over the past 15 years. We observed a gradual but consistent increase in the utilization of induction therapy, a practice that has now become nearly universal. However, the introduction of CyBorD induction therapy alone did not result in a noticeable change in the number of ASCTs performed. It was only with the implementation of Dara-CyBorD that a significant decline in ASCT was observed. This finding underscores the superior ability of Dara-CyBorD to induce deep and satisfactory hematological responses, including a threefold higher probability of achieving a complete hematological response.6 This enhanced efficacy significantly reduces the perceived need to proceed with ASCT, a procedure that carries inherent risks of morbidity and treatment-related mortality. Considering our earlier practice patterns before 2010, our center performed an average of 41 ASCTs for AL amyloidosis annually between 2003 and 2009. Throughout this 8-year period, the majority of patients (an average of 68.5%) proceeded to ASCT without prior induction therapy, further supporting our conclusion when examining a broader timeframe.
Unlike the approach taken in multiple myeloma, where the decision to proceed with ASCT is frequently based on the strategy of consolidating the response obtained from induction therapy to prolong remission duration,14 the decision to proceed with ASCT in AL amyloidosis is predominantly predicated upon the level of the hematological response. Consequently, it is not unexpected that, despite a marginally improved response rate observed in cohort 2 compared to cohort 1, the observed differences were subtle. In this context, we have identified specific patient groups who are more likely to undergo ASCT in the current era. These include patients who fail to achieve a deep hematological response (very good partial response or CR, depending on the treating physician’s clinical discretion), patients with relapsed or refractory disease, and patients with lymphoplasmacytic lymphoma (usually with serum immunoglobulin M monoclonal protein). This latter group tends to have a lower hematological response rate15 and may not derive the same benefit from treatment advancements that are primarily targeted at pure plasma cell–based clones (more typical in AL amyloidosis). In addition, AL patients with a higher bone marrow plasma cell burden may be in greater need of ASCT, possibly related to a higher recurrence rate in these patients or suboptimal response to current available therapies.16 This requires further attention in future studies. Another aspect worth investigating in the future is the application of fluorescence in-situ hybridization in ASCT utilization. Patients with gain(1q) may be less responsive to daratumumab-based frontline therapy,17 whereas patients with t(11;14) have excellent response to venetoclax,18 which may decrease ASCT utilization in this large cohort of patients.
Initially, ASCT in AL amyloidosis was a viable option for only a limited subset of patients, typically 20% to 30% (depending on referral patterns), primarily due to advanced age or significant organ dysfunction rendering the procedure unsafe. Now, for the first time, we observe a decline in ASCT utilization not due to patient frailty or ineligibility, but rather as a consequence of improved treatment options that are reducing the necessity for ASCT. However, it is important to note that long-term follow-up data for these newer regimens, such as Dara-CyBorD, are not yet mature, whereas ASCT has over 2 decades of follow-up in this disease. Patients achieving hematologic CR following ASCT often experience excellent outcomes, with a subset potentially achieving an operational cure and remaining treatment-free for over a decade.1
This study is inherently limited by its retrospective design and its single-center data source, which may restrict the generalizability of our findings. Additionally, the shorter duration of monitoring of cohort 2, spanning 5 years, compared to the 10-year span of cohort 1, may introduce potential biases when comparing trends and outcomes between the 2 periods.
In conclusion, we report a major shift in the utilization of ASCT for AL amyloidosis over the past 5 years. ASCT is currently utilized in several key groups of patients, including those with suboptimal hematological response to previous therapies, patients with relapsed or refractory disease, those with a lymphoplasmacytic clone, and possibly those with a higher bone marrow plasma cell burden. We also reconfirm the impact of daratumumab on reducing stem cell yields during mobilization as has been previously reported for multiple myeloma.19,20
Acknowledgment
This study was supported by the Mayo Clinic Transplant Center Scholarly Award.
Authorship
Contribution: E.M. and M.A.G. conceptualized the study, performed data curation and formal and statistical analysis, and wrote the original draft; and all authors contributed to draft editing and critical review of the manuscript and provided approval of the final draft.
Conflict-of-interest disclosure: E.M. receives consultancy fees (paid to the institution) from Protego. A.D. receives research funding from Janssen; consultancy and research funding from Alexion, Takeda, and Bristol Myers Squibb (BMS); and research funding from HaemaloiX, Alnylam, and Pfizer. P.K. receives research funding from Loxo Pharmaceuticals, IchnosKaryopharm, BMS, Regeneron, and Amgen; has membership on the board of directors or advisory committees of Kite, Oncopeptides, Pharmacyclics, Janssen, Mustang Bio, Angitia Bio, and ×4 Pharmaceuticals; consultancy for CVS Caremark and Keosys; has membership on an entity’s board of directors or advisory committees and research funding from GlaxoSmithKline, AbbVie, Sanofi, and BeiGene. D.D. reports receiving consultancy from Genentech; consultancy and honoraria from Sorrento, Janssen, Novartis, Regeneron, BMS, Sanofi, Merck & Co, and Alexion; research funding from K36 Therapeutics: and consultancy, honoraria, research funding from Apellis. T.V.K. reports receiving research funding from Novartis and Pfizer. N.L. is the current holder of stock options in privately held companies, Checkpoint Therapeutics and AbbVie. S.K.K. reports membership on an entity’s board of directors or advisory committees and research funding from Celgene, Takeda, KITE MedImmune/AstraZeneca, Adaptive, AbbVie, and Janssen; research funding from Roche, Merck, Novartis, and Sanofi; and independent review committee participation in Oncopeptides and others. M.A.G. served as a consultant for Millennium Pharmaceuticals and received honoraria from Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Eli Muchtar, Division of Hematology, Mayo Clinic, 200 First St, SW, Rochester, MN 55905; email: muchtar.eli@mayo.edu.
References
Author notes
The data set used in this study is not publicly available due to the sensitive nature of electronic health records and the need to protect patient anonymity. An anonymized data set may be made available upon reasonable request from the corresponding author, Eli Muchtar (muchtar.eli@mayo.edu).
The full-text version of this article contains a data supplement.