Key Points
The likelihood of infections/severe infections and associated antimicrobial use decreased after IgRT initiation.
Among patients with accessible SPEP test results, IgRT use was associated with significant reductions in hypogammaglobulinemia.
Visual Abstract
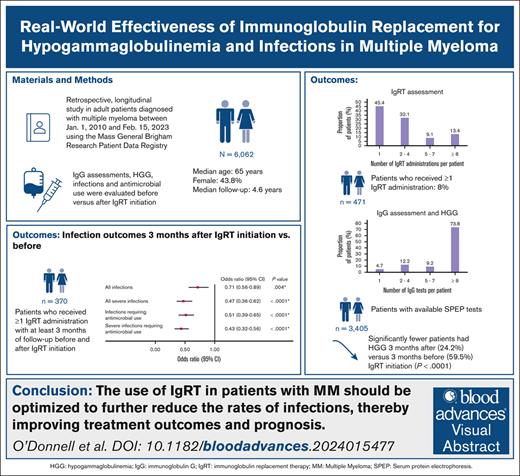
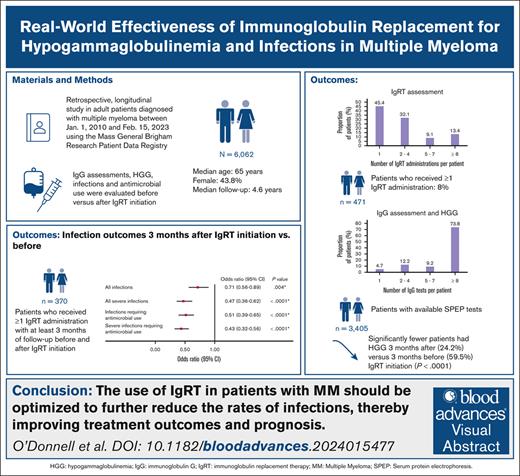
This study assessed the real-world effectiveness of immunoglobulin replacement therapy (IgRT) for treatment of hypogammaglobulinemia and infections in patients with multiple myeloma (MM). A retrospective study was conducted on adult patients diagnosed with MM on or after 1 January 2010 using the Mass General Brigham Research Patient Data Registry. Infections were compared before and after IgRT initiation. Generalized estimating equation logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). In patients with accessible serum protein electrophoresis (SPEP) test results, a Natural Language Processing program supported the extraction of immunoglobulin G (IgG) data. The IgG assessments and incidence of hypogammaglobulinemia (defined as IgG level <500 mg/dL) were compared before and after IgRT initiation. The results were reported using descriptive statistics. A total of 6062 patients with MM were identified (56.2% male; median age, 65.0 years). Of the 6062 patients, 471 (7.8%) received ≥1 IgRT administrations. At 3 months, significantly lower odds of infections (OR, 0.71; 95% CI, 0.56-0.89; P = .0004) were observed after IgRT initiation than before IgRT. Among patients with accessible SPEP results (n = 3405), 3231 (94.9%) underwent ≥1 IgG test with a median of 18.0 (interquartile range, 7.0-40.0) IgG tests per patient. Hypogammaglobulinemia was experienced by 2075 of the 3231 patients (64.2%) who had ≥1 IgG test. Significantly fewer patients had hypogammaglobulinemia after IgRT initiation. In conclusion, IgRT use was associated with significant reductions in hypogammaglobulinemia and infections. Although IgRT is currently used for MM treatment, there is potential to optimize its dosing and treatment duration to reduce the morbidity and mortality associated with infections.
Introduction
Multiple myeloma (MM) is a malignancy characterized by elevated serum levels of monoclonal immunoglobulin secreted by abnormally proliferating plasma cells.1 The annual incidence of MM in the United States is 7.2 cases per 100 000 persons and the mortality rate is 3.3 per 100 000 persons.2,3 Although mortality from MM is decreasing in the United States, the incidence rates are increasing,4 and this trend is expected to continue over the next decade.5
A major cause of morbidity and mortality in patients with MM is infection6,7 owing to the immunosuppressive nature of the disease itself and emerging treatments, such as monoclonal antibodies, chimeric antigen receptor (CAR) T-cell therapy, antibody-drug conjugates, and bispecific antibodies (BsAbs). These agents can cause secondary immune deficiency (SID), including hypogammaglobulinemia,8 thereby increasing the risk of infections.9
Increasing immunoglobulin G (IgG) levels with immunoglobulin replacement therapy (IgRT) can enhance patients’ ability to fight infection.8 IgRT has been shown to be effective in reducing the infection rates in patients with hematologic malignancies10-13 and is recommended by guidelines, such as the National Comprehensive Cancer Network14 and the Society for Immunotherapy of Cancer,15 and the consensus statement from the International Myeloma Working Group.16 In the United States, IV immunoglobulin is recommended as supportive care for patients with MM who experience recurrent serious infection and/or hypogammaglobulinemia (IgG ≤ 400 mg/dL).8
Despite the guideline recommendations, there is a substantial lack of awareness regarding optimizing IgRT use and the management of hypogammaglobulinemia in the context of SID in MM. Studies with more recent real-world data and larger sample sizes are necessary to evaluate the effectiveness of IgRT in reducing infection rates and to better understand the unmet clinical needs. In addition, a gap remains in demonstrating the real-world effectiveness of IgRT in treating hypogammaglobulinemia among patients diagnosed with MM. The objective of this study was to evaluate the effectiveness of IgRT in treating hypogammaglobulinemia and reducing infection rates and to assess patterns of IgG testing in patients with MM.
Methods
Data source
This study used clinical data (inpatient and outpatient) from the Mass General Brigham (MGB) Research Patient Data Registry. The database contains data from 8 affiliated hospitals (Massachusetts General Hospital, Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, Massachusetts Eye and Ear Hospital, McLean Hospital, Newton-Wellesley Hospital, North Shore Medical Center, and Spaulding Rehabilitation Hospital) and the Dana-Farber Cancer Institute in Massachusetts. The data that are presented in this study are from a preplanned study of data from the database. The MGB Institutional Review Board reviewed the study protocol (number 2022P000465) and waived the requirement for documentation of informed consent.
Study design and study population
This was a retrospective, longitudinal, observational study of patients with a diagnosis of MM (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 203.0x; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] C90.0x) between 1 January 2010 and 15 February 2023 (ie, the study period). The follow-up period spanned from the diagnosis of MM to the end of clinical information, the end of data availability (ie, 15 February 2023), or death, whichever came first.
We included adult patients (≥18 years old) at the time of their MM diagnosis who had ≥12 months of clinical data after the diagnosis unless the patients passed away. In addition, to ensure that patients received continuous care within the MGB network, patients were required to have ≥3 annual visits (outpatient, inpatient, or emergency department visits) during the study period. Patients were excluded if they were diagnosed with another primary malignancy that required systemic therapy or a primary immunodeficiency disease or HIV/AIDS or if they had a history of solid organ transplantation.
The IgRT subcohort included patients with ≥1 administration of IgRT during the follow-up period. The date of first receipt of IgRT was referred to as the date of IgRT initiation. Patients were also required to have ≥3, 6, or 12 months of follow-up before and after IgRT initiation to measure the outcomes at these respective timeframes.
Outcomes
Assessment of infections and antimicrobial use before and after IgRT initiation
In the IgRT subcohort, the likelihood of any infections, any severe infections, and the associated antimicrobial use were compared at 3, 6, and 12 months before and after IgRT initiation. Infections (including pneumonia, pyrexia of unknown origin, ear-nose-throat infections, bronchitis, skin/soft tissue/joint/bone infections, sepsis, bacteremia, genitourinary infections, gastrointestinal infections, brain/spinal infections, COVID-19, blood infections other than sepsis, cardiac infections, and other infections [not otherwise specified]) were identified using the associated ICD-9-CM and ICD-10-CM diagnosis codes. Severe infection was defined as an infection that led to hospitalization or treatment with any IV antibiotic, antiviral, or antifungal medication. Antimicrobial use (ie, antibiotics, antivirals, antifungals) associated with infections and severe infections was defined as a prescription for an antimicrobial agent within 30 days of the diagnosis of an infection or a severe infection.17
Identification of IgG testing and hypogammaglobulinemia
Among patients with accessible serum protein electrophoresis (SPEP) results (as documented in pathology reports, commentary sections of laboratory reports, or discharge summaries), the IgG, IgA, IgM, and monoclonal spike (M-spike) levels were extracted using Natural Language Processing. The Natural Language Processing algorithm was first validated for accuracy through review of 100 SPEP test results before it was used to identify laboratory test results of interest (ie, IgG, IgA, IgM, and M-spike). Hypogammaglobulinemia was identified if patients had a quantitative IgG level of <500 mg/dL.18,19 The threshold of <500 mg/dL to define hypogammaglobulinemia was determined based on a combination of literature review and clinical insights.18,19 The quantitative IgG was calculated by subtracting the M-spike level from the reported IgG level, provided that the IgG level exceeded the IgA and IgM levels by >200 mg/dL; IgG was presumed to be the clonal protein. IgG levels and the proportion of patients with hypogammaglobulinemia were compared at 3, 6, and 12 months before and after IgRT initiation.
Statistical analyses
Patient demographics (eg, age, sex, race etc), clinical characteristics (eg, neutropenia, diabetes, sinusitis, bronchitis), and treatment characteristics (eg, BsAbs, CAR T-cell therapy) were summarized using descriptive statistics, including the mean (±standard deviation [SD]) and median and interquartile range (IQR) for continuous variables and the frequency (proportion) for categorical variables. In the IgRT subcohort, generalized estimating equation logistic regression models were used to estimate the odds ratios (ORs), 95% confidence intervals (CIs), and P values for infections, severe infections, and antimicrobial use associated with infections and severe infections. Among patients with accessible SPEP test results, the change in IgG level at 3, 6, and 12 months before and after IgRT initiation was assessed using the Wilcoxon signed-rank test, and the proportion of patients with hypogammaglobulinemia at 3, 6, and 12 months before and after IgRT initiation was evaluated using McNemar test. Statistical significance was indicated by P values < .05.
Subgroup analyses
The likelihood of infection/severe infection and the associated antimicrobial use were evaluated in subgroups of patients who received Gammagard Liquid (GGL) and CAR T-cell therapy.
Results
Patient characteristics
A total of 6062 patients with MM were included in this study (supplemental Figure 1). The mean ± SD follow-up time was 5.0 ± 3.2 years. The mean ± SD age at MM diagnosis was 65.0 ± 11.6 years (median, 65.0 years [IQR, 58.0-73.0]). More than half of the patients (53.5%) were aged >65 years and male (56.2%), and most were White (85.2%). The most common clinical conditions during the follow-up period were neutropenia (23.6%), diabetes (15.4%), sinusitis (5.9%), and bronchitis (4.4%). Treatments received were IgRT (n = 471), proteasome inhibitors (49.7%; ie, bortezomib, carfilzomib, and ixazomib), immunotherapy (42.9%; ie, daratumumab, elotuzumab, and isatuximab), immunomodulatory agents (40.6%; ie, lenalidomide, pomalidomide, and thalidomide), alkylating agents (36.5%; eg, melphalan, bendamustine, doxorubicin, and cyclophosphamide), hematopoietic stem cell therapy (34.8%), CAR T-cell therapy (5.6%), and BsAbs (1.1%; Table 1). The demographic and clinical characteristics of patients included in the IgRT subcohort (n = 471) are summarized in supplemental Table 1.
IgRT assessment
Of the patients identified with MM (N = 6062), 471 (7.8%) received ≥1 IgRT administrations (most of these patients [87.9%] received GGL) of which 257 (54.6%) received ≥2 IgRT administrations (Table 2). The median number of IgRT administrations was 2.0 (IQR, 1.0-4.0). Among patients with only 1 IgRT administration (n = 214), the median time from the MM diagnosis to IgRT administration was 32.2 months (IQR, 10.2-58.4). Among patients with ≥2 IgRT administrations (n = 257), the median time from the first to the second IgRT administration was 3.1 months (IQR, 0.9-6.5).
Likelihood of infections and antimicrobial use associated with infections and severe infections before and after IgRT initiation
Of the patients in the IgRT subcohort (n = 471), 370, 175, and 137 patients were identified with at least 3, 6, and 12 months of follow-up before and after IgRT initiation, respectively (Table 3). In the logistic regression model, significantly lower odds of infections were observed at 3 and 6 months after IgRT initiation (3 months [OR, 0.71; 95% CI, 0.56-0.89; P = .0004]; 6 months [OR, 0.75; 95% CI, 0.58-0.96; P = .026]) and significantly lower odds of severe infection were observed at 3, 6, and 12 months after IgRT initiation (3 months [OR, 0.47; 95% CI, 0.36-0.62; P < .0001]; 6 months [OR, 0.46; 95% CI, 0.35-0.62; P < .0001]; 12 months [OR, 0.52; 95% CI, 0.39-0.70; P < .0001]). The odds of antimicrobial use associated with infections and severe infections (including antibiotics, antivirals, and antifungals) were significantly lower at all 3 time points after IgRT initiation (Table 3).
IgG assessment
Among the patients with accessible SPEP test results (n = 3405), 3231 (94.9%) had ≥1 IgG tests, and the median number of IgG tests per patient was 18 (IQR, 7-40; Table 4). The average number of IgG tests was 6.2 per patient per year. Among patients with ≥1 IgG tests (n = 3231), 2075 (67.0%) experienced hypogammaglobulinemia. Most patients (n = 2716 [84.1%]) underwent ≥1 IgG test after their MM diagnosis date and the mean ± SD time from the IgG test to the end of follow-up was 6.4 ± 3.9 years.
IgG levels and hypogammaglobulinemia before and after IgRT initiation
Among patients with ≥1 IgRT administrations and who underwent ≥1 IgG tests before and after IgRT initiation, the median IgG level at 3, 6, and 12-months after IgRT initiation was 609.0, 728.6, and 722.8 mg/dL, respectively, as opposed to IgG levels immediately before IgRT initiation of 410.0, 434.0, and 433.0 mg/dL, respectively (P < .0001; Table 5). The proportion of patients with hypogammaglobulinemia was also significantly lower after IgRT initiation (3 months: 24.2% vs 59.5%; 6 months: 26.3% vs 56.6%1; 12 months: 26.6% vs 56.2%; all P < .0001).
Subgroup analyses of patients who received GGL and CAR T-cell therapy
In the GGL subgroup, the odds of infection were significantly lower at 3 months (OR, 0.71; 95% CI, 0.55-0.90; P = .006) and 6 months (OR, 0.76; 95% CI, 0.58-1.00; P = .048) after IgRT initiation, whereas the odds of severe infection were significantly lower at all follow-up time points after IgRT initiation (3 months [OR, 0.46; 95% CI, 0.35-0.61; P < .0001]; 6 months [OR, 0.44; 95% CI, 0.32-0.60; P < .0001]; 12 months [OR, 0.50; 95% CI, 0.37-0.68; P < .0001]; supplemental Table 2). Similarly, the odds of antimicrobial use associated with infections and severe infections (including antibiotics, antivirals, and antifungals) were significantly lower at all time points after IgRT initiation.
In the CAR T-cell therapy subgroup, the odds of severe infections were significantly lower at 3 and 6 months after IgRT initiation (3 months [OR, 0.37; 95% CI, 0.22-0.64; P < .0001]; 6 months [OR, 0.42; 95% CI, 0.24-0.75; P = .003]; supplemental Table 3). The odds of antimicrobial use associated with infections and severe infections (including antibiotics, antivirals, and antifungals) were significantly lower at all time points after IgRT initiation.
Discussion
Patients with hematologic malignancies, including MM, have a higher risk of SID, SID-related infections, and mortality than immunocompetent individuals.9 In these patients, regular assessment of the IgG levels and IgRT are recommended to restore immune function and prevent recurrent infections.9,20 To the authors’ knowledge, this is the largest real-world study that evaluated the effectiveness of IgRT in reducing infection outcomes and the rates of hypogammaglobulinemia in patients with MM in the United States. The findings suggest that the use of IgRT was associated with significantly lower odds of both infections and severe infections and associated antimicrobial use. In addition, a significantly lower proportion of patients experienced hypogammaglobulinemia after IgRT use.
One of the key tools for monitoring MM is SPEP, which is used to detect and quantify abnormal proteins, particularly M-spike protein, that are often produced in excess by malignant plasma cells. The routine measurement of IgG levels, often through SPEP, also plays a critical role in managing MM, because it helps to diagnose and monitor hypogammaglobulinemia, a condition characterized by abnormally low levels of immunoglobulins. In this study, we found that the IgG levels in patients diagnosed with MM were routinely monitored with more than 95% of patients undergoing IgG testing. Moreover, in this study, we also observed that, among the patients who underwent IgG testing, 67.0% experienced hypogammaglobulinemia. These results are consistent with the hypogammaglobulinemia rates reported in trials of bispecifics among patients with MM (ie, 71%-85%).21-24 Despite the routine monitoring of IgG levels, there is a notable lack of consistency in the treatment of hypogammaglobulinemia across the United States. Consequently, treatment decisions are frequently tailored to the individual patient with consideration of their clinical history, infection risk, and overall health. Addressing these inconsistencies may require more standardized treatment protocols and a stronger emphasis on evidence-based practices to ensure that all patients receive optimal care for hypogammaglobulinemia.
IgRT is recommended by several guidelines as an important strategy for managing patients at risk of infections owing to SID, thereby effectively addressing underlying immune defects, such as hypogammaglobulinemia, and preventing infections. Numerous studies have presented compelling evidence for the efficacy of IgRT in reducing infection risk among patients with MM in the United States.11,12,25 Although IgRT is recommended for individuals with SID, IgRT relies on a finite supply of blood products. Consequently, it becomes crucial to administer IgRT to the appropriate patient at the optimal time. This complexity distinguishes the treatment of SID from that of primary immunodeficiency, because it necessitates ongoing monitoring to determine when a patient would derive the most benefit from IgRT. The timing may vary from months to years depending on the underlying cause of hypogammaglobulinemia. Despite the evidence of IgRT's effectiveness in preventing infections, the findings of this study indicated that only 7.8% of patients received ≥1 IgRT administrations with 45.4% of them receiving just 1 administration. In addition, the number of IgRT administrations per patient varied widely. These findings suggest that a more conservative approach to IgRT use may be used at the physicians’ discretion, typically at a dose of 400 mg/kg at 4-week intervals for up to a year, based on the current guidelines. Alternatively, the lower utilization of IgRT observed in this study might be because of the practice of ordering IgRT only after a patient has experienced an infection, indicating a reactive rather than proactive approach. Overall, these findings highlight a gap between the current recommendations and real-world practice related to the use of IgRT.
Adherence to clinical guidelines for IgRT has been shown to improve infectious outcomes in MM.26 In this study, the use of IgRT was associated with a lower likelihood of infections and severe infections, which is in line with previous studies. In an administrative claims–based study of patients with MM, the initiation of IgRT (IgPro10/IgPro20) was associated with a 51% reduction in the total number of bacterial infections when compared with non-IgRT users.27 In a retrospective crossover study conducted by Lancman et al who used electronic medical records data from the Tisch Cancer Institute and Bone Marrow Transplant Department at Mount Sinai Hospital, there was no statistically significant difference in the infection rates per person per year for the period of receiving IV immunoglobulin and the period of no IV immunoglobulin use (incidence rate ratio, 0.92; 95% CI, 0.76-1.10; P = .376). However, in the nonprogressive disease subgroup, a statistically significant reduction in severe infection rates during IgRT use was observed when compared with the period of no IgRT use.25 In a different study conducted by Lancman et al,12 the authors conducted a retrospective study among patients with MM who were treated with bispecifics as monotherapy at the Mount Sinai Hospital in New York as part of 4 clinical trials (ClinicalTrials.gov identifiers: NCT03287908, NCT03761108, NCT04083534, and NCT03145181) between 1 January 2019 and 30 June 2022 (N = 37). The authors found that the use of IgRT significantly reduced the rate of grade 3 to 5 infections by 90% (incidence rate ratio, 0.10; 95% CI, 0.01-0.80; P = .0307). Finally, in a retrospective study conducted by Lahue et al that compared the infection rates among patients at risk of SID who underwent IgRT and those without IgRT,11 patients who received IgRT were much less likely to experience infections than those who did not receive IgRT. In addition, patients who received IgRT were significantly less likely to experience a severe infection (OR, 0.41; 95% CI, 0.24-0.70; P = .001). Taken together, evidence from these studies suggest that IgRT may be an effective treatment option for preventing infections in patients with compromised immune systems caused by SID. Overall, these data highlight that adhering to the guidelines for IgRT use in patients with MM could enhance their immune function and reduce the risk of infections, thereby improving patient outcomes. Future studies that adjust for time varying confounders, like seasonal trends, would be valuable in further exploring the association of IgRT use with infections.
Moreover, given that the data used in this study only captured the initial period following the US Food and Drug Administration approval of several CAR T-cell therapies and BsAbs, this study’s findings primarily reflect the clinical practices before the CAR T-cell therapies and bispecifics era. Consequently, the necessity for IgRT may be even more significant than initially recognized in this area given that CAR T-cell therapies and BsAbs have been linked to an increased risk of infections in several studies.11,12,25 As these new treatments are adopted more widely in clinical practice, the incidence of infections could increase, highlighting an urgent need for the implementation of IgRT to optimize patient outcomes. Additional study is warranted to examine the effectiveness of IgRT during the CAR T-cell therapies and bispecifics era.
Furthermore, our research sheds light on the fact that patients diagnosed with MM had a significantly lower likelihood of receiving antimicrobials associated with infections and severe infections after IgRT initiation. Before our study, no comprehensive investigations had explored the association between IgRT and the use of antimicrobials associated with infections and severe infections in a real-world setting in the US population. This finding holds importance in the context of efforts to mitigate antimicrobial resistance in patients who are experiencing infections in MM. The overuse of antibiotics can lead to the development of antibiotic-resistant pathogens. This can lead to more severe infections, thereby increasing the risk for complications and mortality.28
Although CAR T-cell therapy has shown remarkable success in achieving remission in many patients diagnosed with MM, it also presents unique challenges and considerations related to infections.29 CAR T-cell therapy, despite its effectiveness in targeting cancer cells, can inadvertently impact healthy B cells, which leads to a temporary reduction in antibody production and increased susceptibility of patients to infections. Despite the recommendations from national guidelines and the wide usage of IgRT in patients treated with CAR T-cell therapy,14,15 the corresponding data on the evaluation of the impact of IgRT on reducing the infection risk among patients with MM who were treated with CAR T-cell therapy is limited. The findings of our study suggest that IgRT use was associated with a significantly lower likelihood of infections and severe infections among patients with MM who were treated with CAR T-cell therapy. This underscores the critical importance of incorporating IgRT into the treatment regimen for patients who are undergoing CAR T-cell therapy, particularly for those diagnosed with MM, as recommended by established guidelines.
This was the largest study to date that evaluated the rates of IgG testing, infections, and hypogammaglobulinemia in a real-world population of patients diagnosed with MM in the United States. Nonetheless, there are limitations that should be noted, which are mostly related to the use of electronic medical records as the data source. First, diagnosis codes were used to identify the baseline clinical conditions and follow-up clinical outcomes (eg, infections) and there may have been misclassification because of miscoding. Second, the data source was limited to clinical sites within the MGB network, and the results may not be generalizable to the entire population of patients with MM in the United States. Third, IgRT use may have been underreported because of limited information on the care patients received outside of the MGB network. Similarly, the frequency of IgG testing may have been underreported in pathology reports if the tests were conducted outside of the network. Fourth, although treatments received by patients with MM were identified using both structured data and clinical notes (unstructured data), the exact dates of receipt of treatment were not available in the latter. Fifth, patients in the MGB Research Patient Data Registry were more likely to be White and therefore may not be fully representative of the US population. Finally, the generalizability of the study findings for hypogammaglobulinemia is limited by the cutoff value used as the definition (ie, IgG levels <500 mg/dL).
In conclusion, this study provides new and important insights regarding the use and effectiveness of IgRT in patients with MM in a real-world setting. To the best of our knowledge, this is the largest real-world cohort study, to date, of patients with MM who were treated with IgRT. IgRT use was associated with significant reductions in the rates of infection and severe infections and the use of antimicrobials. Consistent with clinical guidance, among the patients with accessible SPEP test results, most patients underwent IgG testing, and IgRT use was associated with significant reductions in hypogammaglobulinemia in these patients. Although IgRT is currently used in the management of MM, there is potential to optimize both the dosing and duration of treatment to further reduce the rate of infections and the associated risks of morbidity and mortality in patients, thereby improving their treatment outcomes and prognosis.
Acknowledgments
Medical writing assistance was provided by a professional medical writer, Janice Imai, an employee of Analysis Group, Inc, a consulting company that provided paid consulting services to Takeda Pharmaceuticals USA, Inc.
This study was funded by Takeda Pharmaceuticals USA, Inc.
The study sponsor was involved in several aspects of the study, including study design, data interpretation, manuscript writing, and the decision to submit the manuscript for publication.
Authorship
Contribution: R.D., L.H., M.Y., A.B., L.C., M.P., and M.S.D. contributed to study conception and design and data acquisition, assembly, analysis, and interpretation; E.O., N.K., T.G., M.S., and Z.Y. contributed to study conception and design and data interpretation; G.B., Y.G.H., C.H., and S.N.M. contributed to study conception; and all authors made substantial contributions to manuscript drafting and critical revision for important intellectual content, reviewed and approved the final version to be published, and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: R.D., L.H., M.Y., A.B., L.C., M.P., and M.S.D. report being employees of the Analysis Group, Inc, a consulting company that provided paid consulting services to Takeda Pharmaceuticals USA, Inc, the study sponsor. E.O. reports serving on advisory boards for and/or receiving honoraria from Janssen, Bristol Myers Squibb, Sanofi, Pfizer, Exact Sciences, and Grail; receiving consulting fees from Takeda; and participating in steering committees for Natera and Legend Pharmaceuticals. T.G., N.K., and M.S. report being employees of Takeda Pharmaceuticals USA, Inc and being Takeda shareholders. Z.Y. reports being an employee of the University of California San Diego. G.B., C.H., Y.G.H., and S.N.M. report being current employees of Mass General Brigham, a hospital and physician network that received funding from Takeda Pharmaceuticals USA, Inc for this research study.
Correspondence: Elizabeth O’Donnell, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: elizabeth_odonnell@dfci.harvard.edu.
References
Author notes
S.N.M. and M.S. are joint senior authors.
Presented, in part, as an oral presentation at the 66th annual meeting of the American Society of Hematology, San Diego, CA, 7 to 10 December 2024.
The data supporting the findings of this study are available from Mass General Brigham Research Patient Data Registry. Restrictions apply to the availability of these data, which were used under license for this study.
The full-text version of this article contains a data supplement.