Visual Abstract
TO THE EDITOR:
B-cell maturation antigen–directed chimeric antigen receptor (CAR) T-cell therapy has been transformational in relapsed/refractory multiple myeloma. Ciltacabtagene autoleucel (cilta-cel) led to a median progression-free survival of 34.9 months.1,2 However, cilta-cel can rarely lead to persistent movement and neurocognitive toxicities (MNTs) including parkinsonism.1-3 It is a subacute hypokinetic movement disorder emerging ∼1 to 2 months after CAR T-cell infusion and characterized by tremors, bradykinesia, limb rigidity, micrographia, shuffling gait, memory loss, diminished attention, reduced facial expression, and/or flat affect.2-4
We analyzed 5 cases of cilta-cel–associated parkinsonism events out of the first 60 consecutive patients who received cilta-cel at the University of Chicago. Patient characteristics, CAR T-cell course, manifestations of MNTs, and treatment were reviewed to analyze contributing factors and strategies that may have led to recovery.
Patient characteristics can be found in supplemental Table 1. All patients received bridging therapy, resulting in 2 objective responses. The median vein-to-vein time was 56 days. All received fludarabine and cyclophosphamide lymphodepletion, followed by cilta-cel (median dose, 0.6 × 106 CAR+ viable T cells per kg).
All patients experienced grade 1 to 2 cytokine release syndrome and were treated with tocilizumab (100%) and dexamethasone (100%); anakinra was administered to 2 (40%) patients for IEC-HS (immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome). The median peak absolute lymphocyte count after CAR T-cell therapy for patients experiencing MNTs was 2.93 × 109/L (range, 1.75× 109/L to 3.74 × 109/L) compared to 1.45 × 109/L (range, 0.33 × 109/L to 37.7 × 109/L) in those who did not (P = .2). At median 32 months follow-up, 3 patients achieved at least a complete response, and 2 patients reached a very good partial response; there were 2 progression events, both of which were responsible for the 2 patient deaths in this cohort.
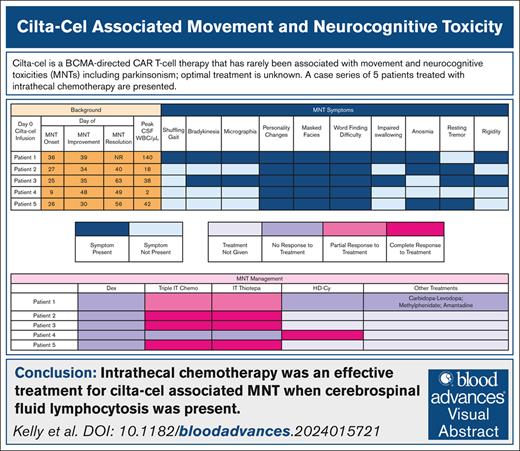
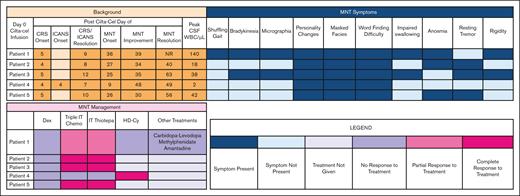
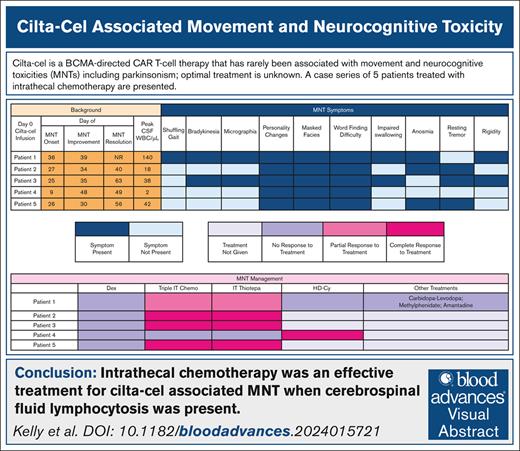
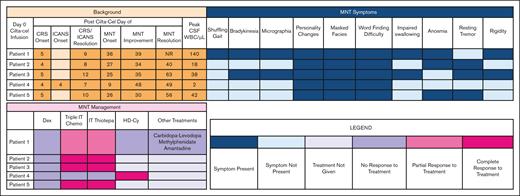
The median time to onset of parkinsonism was 26 (range, 9-36) days (Table 1). The most common symptoms were personality changes characterized by flat affect, anhedonia, and avolition (100%), difficulty with word finding (100%), and masked facies (100%) (Figure 1). Abnormal magnetic resonance imaging findings were present only in patient 1, showing an asymmetric T2 signal hyperintensity within the bilateral caudate heads and putamen, and then subsequent cerebral white matter Fluid Attenuated Inversion Recovery signal abnormality in the frontal lobes and posterior limbs of the internal capsules (supplemental Figure 1). A dopamine transporter (DaT) scan in patient 1 showed reduced uptake in the putamen, a pattern consistent with parkinsonism (supplemental Figure 2). The median cerebrospinal fluid (CSF) white blood count was 42/μL (range, 2-140). The median CSF lymphocyte percentage was 90% (range, 89%-95%); flow cytometry confirmed that these were almost exclusively made up of T cells.
MNT symptoms, management, and resolution. CRS, cytokine release syndrome; Dex, dexamethasone; ICANS, immune effector cell–associated neurotoxicity syndrome; NR, no resolution; WBC, white blood cell.
MNT symptoms, management, and resolution. CRS, cytokine release syndrome; Dex, dexamethasone; ICANS, immune effector cell–associated neurotoxicity syndrome; NR, no resolution; WBC, white blood cell.
For parkinsonism-directed therapies, all patients received systemic corticosteroids (dexamethasone 20-40 mg daily) without effect (Figure 1). Intrathecal (IT) chemotherapy was administered to all patients. Intrathecal methotrexate, cytarabine, and hydrocortisone (“triple IT”) chemotherapy with cytarabine 50 mg, methotrexate 12 mg, and hydrocortisone 50 mg was administered first.4,5 For patients with only partial improvement in MNT or persistent CSF lymphocytosis, IT thiotepa 10 mg with hydrocortisone 50 mg was given due to its superior antimyeloma properties in the event of occult disease driving persistent CSF lymphocytosis.6 Three (60%) patients had a complete resolution of MNT symptoms with IT chemotherapy. patient 1 had a partial improvement and patient 4 had no response. All 4 patients with CSF lymphocytosis had at least partial improvement in MNT symptoms with IT chemotherapy; the 1 patient without any improvement did not have a CSF lymphocytosis.
For the 2 patients without complete resolution to IT chemotherapy, IV high-dose cyclophosphamide (HD-Cy) 1 g/m2 was administered.5,7,8 Patient 4 had a rapid improvement. The remaining patient with persistent symptoms also received carbidopa-levidopa, methylphenidate, and amantadine to no benefit.9,10 The median time-to-first improvement in MNT symptoms was 7 (range, 3-39) days; the median time-to-full MNT resolution was 34 (range, 13-40) days.
We report an 8% institutional incidence of cilta-cel–associated parkinsonism and the presence of CSF CAR T-cell pleocytosis as a marker for response to IT chemotherapy. In addition, anosmia may be a potential feature of parkinsonism. In CARTITUDE-1, 6% experienced parkinsonism and only 1 patient had a full recovery.1,2 In CARTITUDE-4, 1% experienced parkinsonism.11 The median time to onset was 60 days with a median duration of 265 days. In patients treated with commercial cilta-cel, parkinsonism occurred in 2%; only 1 patient improved. Treatments attempted include levodopa/carbidopa (n = 1), amantadine (n = 1), corticosteroids (n = 1).12 In a registry analysis of 16 cases of parkinsonism, the median peak absolute lymphocyte count was 9.5 × 109/L and 73% of T cells in the CSF were CAR T cells. Corticosteroids led to at least a partial response in 7 of 10 (70%), IT methotrexate–based therapy in 0 of 2 (0%), HD-Cy in 3 of 5 (40%), and IV immunoglobulin in 2 of 4 (50%) patients.13 Although 3 of 5 patients in our cohort received IV immunoglobulin for secondary hypogammaglobulinemia, it did not improve symptoms.
The mechanism of cilta-cel–associated parkinsonism is not well-elucidated. In CARTITUDE-1, patients with MNTs had high tumor burden, grade ≥2 cytokine release syndrome or any grade immune effector cell–associated neurotoxicity syndrome, and robust CAR T-cell expansion.1-3 A postmortem analysis following a parkinsonism event found CAR T-cell persistence in the blood and CSF, basal ganglia lymphocytic infiltration, and B-cell maturation antigen expression on neurons and astrocytes to suggest that this could be an on-target, off-tumor effect.14 In this case series, neither high tumor burden nor high CAR T-cell dose were common features. However, we did note a possible disproportionate expansion of CAR T cells among those with MNTs compared to those without MNTs, suggesting that early CAR T-cell kinetics may play a role in the emergence of parkinsonism.12,13 The role of hyperinflammation (IEC-HS) as a risk factor for parkinsonism is unclear.
A DaT scan may be useful for evaluating the diagnosis of CAR T-cell–associated parkinsonism, although some reports suggest otherwise.8,14,15 By identifying hallmark dopamine deficiency in the basal ganglia, DaT scans could help distinguish parkinsonism from other movement disorders. DaT scans warrant investigation in CAR T-cell–associated parkinsonism.
A CSF pleocytosis comprising T cells is consistent with cilta-cel CSF penetration and supports existing evidence that CAR T cells can traffic into the CSF. It is unknown whether a CSF pleocytosis typically occurs following CAR T-cell therapy, even in patients without neurologic symptoms. For instance, patients with steroid-refractory neurotoxicity following CD19-directed CAR T-cell therapy had CSF T-cell lymphocytosis and triple IT chemotherapy was also effective.4,16-18 CSF lymphocytosis in the setting of cilta-cel–related parkinsonism aligned with patients who responded to IT chemotherapy. We theorize that IT thiotepa may have been more effective than triple IT chemotherapy due to eradication of occult myeloma cells in the CSF and/or superior elimination of T cells in the CSF.
Importantly, IT chemotherapy administration was safe. As for other treatments, carbidopa/levodopa is not reliably effective.10 HD-Cy has been used successfully but may carry the unintended risk of prematurely eradicating CAR T cells systemically and potentially increasing the risk for early relapse; this is less of a concern with IT chemotherapy.7 HD-Cy was effective in 1 of 2 IT chemotherapy-refractory cases, but led to severe cytopenias and early relapse. This approach can be a rescue intervention if IT therapy is ineffective.
In conclusion, cilta-cel–associated parkinsonism remains an important and potentially reversible toxicity. DaT scan as a tool for early diagnosis of parkinsonism requires further investigation. IT chemotherapy may be an effective treatment for parkinsonism with CSF lymphocytosis, whereas HD-Cy may be useful for refractory cases. Further elucidation of the pathogenesis and treatment of parkinsonism is urgently needed.
This study was approved by the institutional review board at the University of Chicago.
Contribution: K.K. and B.A.D. developed the study concept, performed data acquisition and data analysis, and drafted and revised the manuscript; J.H.C., M.R.B., S.K., and A.J. performed data analysis and drafted and revised the manuscript; and all authors approved the content of the manuscript.
Conflict-of-interest disclosure: J.H.C. declares that her spouse is employed by AbbVie, Inc. M.R.B. reports consultancy for Arcellx, Kite/Gilead, and Bristol Myers Squibb (BMS); serving on speakers’ bureaus for BMS and Kite/Gilead; and research funding from Arcellx and Kite/Gilead. A.J. reports involvement of advisory boards and consultations with honoraria for AbbVie, Amgen, BMS, GlaxoSmithKline (GSK), Janssen, and Sanofi-Aventis. B.A.D. reports consultancy for Johnson & Johnson, Sanofi, Canopy, and COTA; is an independent trial reviewer for BMS; and reports research funding from Amgen and GSK. The remaining authors declare no competing financial interests.
Correspondence: Benjamin Derman, Section of Hematology/Oncology, University of Chicago, 5841 S Maryland Ave, M/C 2115, Chicago, IL 60637; email: bderman@bsd.uchicago.edu.
References
Author notes
Original data are available on request from the author, Kaitlin Kelly (kaitlin.kelly2@uchicagomedicine.org).
The full-text version of this article contains a data supplement.