Key Points
BMP-2 can generate an eBM microenvironment capable of housing and maintaining a functional BM niche.
In situ adipose tissue–derived CD51highCxcl12-GFP– cells are the major cell source giving rise to CAR cells in the BMP-eBM.
Visual Abstract
Ectopic bone marrow (eBM) holds tremendous potential as an artificial organ, serving not only in stem cell transplantation therapies but also as a controlled experimental system for analyzing cellular dynamics and interactions between cells and the matrix during the formation, maintenance, and aging of BM. Although bone morphogenetic protein-2 (BMP-2) has been reported to induce eBM formation, it remains unproven whether BMP-2–induced eBM (BMP-eBM) can provide a functional BM niche that is comparable with native BM in long bones (LB-BM). In this study, through the use of single-cell RNA sequencing and transplantation models, we demonstrate that BMP-eBM displays a microstructure, cellular composition, and functional hematopoiesis similar to LB-BM. BMP-eBM establishes an optimized microenvironment capable of supporting hematopoietic stem cells and CXC chemokine ligand 12 (CXCL12)–abundant reticular (CAR) cells, which are critical components of the BM niche. BMP-eBM was able to significantly restore survival in irradiated mice. Through parabiosis and cell transplantation experiments, we identified that in situ adipose tissue–derived CD51highCxcl12-GFP– cells are the principal source of CAR cells within BMP-eBM. Furthermore, BMP-eBM can be isolated and after preconditioning, retransplanted as an independent, functional hematopoietic organ. In conclusion, our study confirms that BMP-eBM functions effectively as a hematopoietic organ, capable of supporting and maintaining a functional BM niche. These findings underscore BMP-2 as a crucial molecule for eBM generation and suggest its potential for addressing BM-related diseases and for use as a platform for in vitro and ex vivo biomedical applications.
Introduction
Bone marrow (BM) is a richly vascularized spongy tissue where mesenchymal and hematopoietic stem cells (MSCs and HSCs) reside and coordinate blood and immune cell production throughout life.1,2 During early developmental stages, BM formation in the diaphysis (primary ossification center) and epiphysis (secondary ossification center [SOC]) of long bones (LBs) begins around embryonic day 14 and postnatal day 6, respectively. This process is characterized by the invasion of mesenchymal cells, including periochondrial, neural crest progenitors, and endothelial cells, into the cartilage anlagen.3-6 In growing bones, a subset of SRY-box9–positive (Sox9+), collagen type 2–positive (Col2+), and aggrecan–positive (Acan+) chondrocytes self-renews and provides Osterix–positive (Osx+) precursors in the metaphysis. These Osx+ precursors differentiate into collagen type 1–positive (Col1+) osteoblasts on the bone surface and CXC chemokine ligand 12–positive (Cxcl12+) stromal cells in the BM, but they are restricted to the osteogenic lineage in adults.5,7 In contrast, distal-less homeobox 5–positive (Dlx5+) fetal perichondrial cells differentiate directly into BM stromal cells through a Sox9-independent pathway, continuing to supply osteoblasts and stromal cells after birth.8 In the early postnatal BM, leptin receptor–positive (LepR+) stromal and endothelial cells express high levels of stem cell factor, which is essential for HSCs and progenitor cells, with their properties changing within weeks after birth.9 In adult BM, HSCs are maintained in the so-called “BM niche” in the perivascular region, often near the trabecular bone.2 Within the BM niche, the self-renewal and differentiation of HSCs are regulated by various cells, including endothelial cells,10-12 perivascular cells,12-15 and Schwann cells.16 Among these, Cxcl12-abundant reticular (CAR) cells, also known as one type of MSCs and adipo-osteogenic progenitors, are considered key to maintaining the BM niche.17-19 CAR cells also express high levels of stem cell factor18 and strongly overlap with LepR+ cells.20 Their ablation has been shown to significantly reduce the number of HSCs within the BM.18 However, certain mechanistic details, such as the precise dynamics of BM formation during cartilage invasion and the regulatory pathways driving CAR cell differentiation, remain incompletely understood. This is largely because the BM niche exhibits high complexity in structure and function and is often inaccessible for controlled experimentation.
In this context, ectopic BM (eBM) microenvironments serve as valuable tools for understanding the intricate process of BM formation, as they allow for diverse functional analyses of their cellular constituents within short time frames. In addition, eBM holds tremendous potential for applications in transplantation therapeutics for managing lethal blood diseases, such as leukemia, and in vitro biomedical platforms like BM-on-chips,21 for drug screening and analyzing cellular dynamics. Numerous methods have been developed to fabricate eBM; however, most fail to replicate the detailed microstructure and macrostructure, cellularity, and, more importantly, the functionality of native BM. For example, conventional 2-dimensional BM models and biochips lack the specialized stroma, vascularization, and active hematopoiesis.22-25 Although 3-dimensional BM organoids have been developed to overcome some of these limitations, they still fall short in reconstituting inorganic tissue and/or sinusoidal endothelial cellular subtypes.26 Accurate replication of BM architecture has only been achieved with in vivo ectopic transplantation of BM stromal cells,27 mesenchymal progenitor cells28 or nonmineralized cartilage pellets.29 Nevertheless, these techniques require sophisticated sample processing and extensive periods for in vitro cell culture and differentiation, as well as BM development after in vivo transplantation.
A simple and efficient method for fabricating an eBM niche using only a single molecule, bone morphogenetic protein-2 (BMP-2), without any cell source, has been achieved.30 BMP-2 is a member of the transforming growth factor-β superfamily and strongly induces both orthotopic and ectopic bone formation31,32 through stimulation of osteoblast differentiation.31,33,34 BMP-2 also plays a critical role in maintaining bone and BM homeostasis by directly enhancing osteoclast activities,35,36 inducing angiogenesis,37 and promoting HSC proliferation of HSCs.30,38-41 Therefore, BMP-2–induced eBM (BMP-eBM) has great potential for clinical applications as both an adjunct and an independent hematopoietic site. However, no study has yet proven whether BMP-eBM presents similar cellularity and hematopoiesis-related functionality, or the capability as a bioscaffold, for engraftment and maintaining a functional BM niche compared with native BM in LB (LB-BM).
In this study, through single-cell profiling and in vivo functional analyses based on bone marrow transplantation models, we demonstrated that BMP-eBM undergoes similar developmental stages, specifically endochondral ossification, and is a functional hematopoietic organ that closely mimics native LB-BM. Moreover, we clarified the critical roles of adipose tissue–derived mesenchymal cells in the formation of the BM niche within BMP-eBM by differentiating into CAR cells.
Methods
Mice and BMP-2/β-TCP subcutaneous implantation
C57BL6J mice were sourced from CLEA Japan, Inc (Tokyo, Japan). For aging experiments, C57BL6J mice were purchased from The Jackson Laboratory Japan (Kanagawa, Japan). CAG-EGFP mice were purchased from Japan SLC, Inc (Shizuoka, Japan). C57BL/6-CD45.1, B6.129-Leprtm2(cre)Rck/J (LepR-cre), and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (tdTomato) mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Cxcl12-GFP mice42 were generated at one of the authors’ laboratories. Col1a1(2.3)-GFP mice43 and Trap-tdTomato mice44 were kindly gifted by Koichi Matsuo (Keio University, Tokyo, Japan) and Masaru Ishii (Osaka University, Osaka, Japan), respectively.
To prepare the BMP-2/β-tricalcium phosphate (β-TCP) complex, 40 mg of β-TCP (Superpore KG-2, particle size 0.6-1.0 mm, porosity 75%; HOYA, Tokyo, Japan) was soaked in 40 μL of 0.5 mM HCl containing 8 μg of soluble human recombinant BMP-2 (Osteopharma Inc, Osaka, Japan). These materials were gently mixed with a stainless-steel micro spatula and incubated for 5 minutes at room temperature. After incubation, almost all the solution was absorbed by the pores of the β-TCP. The BMP-2/β-TCP complexes were then implanted subcutaneously into anesthetized mice. For subcutaneous delivery, a small incision was made on the dorsal side of each mouse under general anesthesia induced with isoflurane. The BMP-2/β-TCP complex was placed into the subcutaneous pocket between the external oblique muscle and the skin, and the incision was closed with absorbable sutures.
Female mice aged 8 to 18 weeks were used, except in development and aging experiments. The animal experiment protocols for this study (OKU-2020818, OKU-2021368, OKU-2022242, OKU-2022941, OKU-2022942, OKU-2022962, and OKU-2022963) were approved by the Okayama University Animal Research Committee. All animals were handled according to the guidelines of the Okayama University Animal Research Committee.
HSC and BMP-eBM functional assays
In the HSC transplantation assay, a total of 1 × 107 BMP-eBM cells were harvested from CAG-EGFP mice and transplanted into lethally irradiated (12 Gy) C57BL6J recipients. Control mice did not undergo transplantation. After 24 weeks, the recipients were euthanized, and samples were collected for further analysis.
For the HSC engraftment assay, BMP-2/β-TCP complexes were implanted subcutaneously into C57BL6J recipients. After 4 weeks, these recipients were lethally irradiated (12 Gy) and transplanted with 1 × 107 LB-BM cells derived from C57BL6J-Ly5.1 mice. Eighteen weeks later, the recipients were euthanized, and samples were collected for further analysis.
In the survival assay, C57BL6J recipients were irradiated with the LD60/30 dose (7.5 Gy), at which 60% of the mice die within 30 days,45 and subsequently transplanted subcutaneously with either BMP-2 (16 μg)/β-TCP (80 mg), BMP-eBM, or BMP-eBM/BMP-2 (2 μg). In this assay, BMP-eBM was generated using BMP-2 (16 μg) and β-TCP (80 mg).
In the retransplantation assay, BMP-eBM was collected from donor mice and transplanted subcutaneously into recipient mice with 0.5 mM HCl (vehicle) or BMP-2 (1 μg). Two weeks later, the recipients were euthanized, and samples were collected for further analysis. In this assay, BMP-eBM was produced from BMP-2 (8 μg) and β-TCP (40 mg).
Parabiosis surgery
C57BL6J and Cxcl12-GFP mice were habituated in the same cage to ensure harmonious cohabitation. Following anesthesia, a longitudinal skin incision was performed from the elbow to the knee joint on each mouse. The olecranon was then sutured, and the muscle and subcutaneous tissues of the 2 mice were joined with continuous sutures. A skin suture was performed in the area stretching from the elbow to the knee joint. The mice were monitored daily for signs of pain and distress, such as shaking, lethargy, tail chewing, arched back, and lack of grooming. After 3 weeks of combination, the C57BL6J mice had BMP-2/β-TCP implanted subcutaneously. Four weeks after implantation, the mice were euthanized, and samples were collected for further analysis.
Cell transplantation experiments
LB-BM stromal cells and inguinal white adipose tissue (iWAT) cells were harvested from Cxcl12-GFP mice, as detailed in the supplemental Methods. Hematopoietic cells were depleted using biotin-conjugated anti-CD45 (103104, BioLegend, clone: 30-F11), biotin-conjugated anti-Ter119 (116204, BioLegend, clone: TER119), and Streptavidin Microbeads (130-048-102, Miltenyi Biotec) with the autoMACS Pro Separator (Miltenyi Biotec). The cells were then stained with fluorochrome-conjugated antibodies: APC-Cy7 anti-CD45 (103115, BioLegend, clone: 30-F11), APC anti-CD31 (102509, BioLegend, clone: MEC13.3), PE-Cy7 anti-Ter119 (116221, BioLegend, clone: TER119), and phycoerythrin (PE) anti-CD51 (104105, BioLegend, clone: RMV-7). Flow cytometry analyses were performed using the FACSDiva 8.0.1 software (BD Biosciences). Dead cells and debris were excluded based on forward scatter, side scatter, and DAPI (4',6-diamidino-2-phenylindole) staining. Cell sorting was conducted on a FACSAria III Cell Sorter (BD Biosciences).
Cell suspensions (LB-BM CD51+Cxcl12-GFP–, 7122-11 506 cells; LB-BM CAR cells, 34 193-70 972 cells; iWAT CD51+Cxcl12-GFP–, 10 000 cells; iWAT CD51+Cxcl12-GFPlow, 147 013-276 048 cells; iWAT CD51lowCxcl12-GFP–, 3509-50 000 cells; iWAT CD51highCxcl12-GFP–, 19 422-50 000 cells) were incubated with β-TCP (10 mg) for 90 minutes at 37°C, mixed with BMP-2 (2 μg), incubated for 5 minutes at room temperature and then transplanted subcutaneously into anesthetized mice. Two weeks after transplantation, the mice were euthanized, and samples were collected for further analysis.
Detailed methods and statistical analyses are provided in the supplemental Methods.
Results
BMP-eBM and LB-BM show similar cellularity
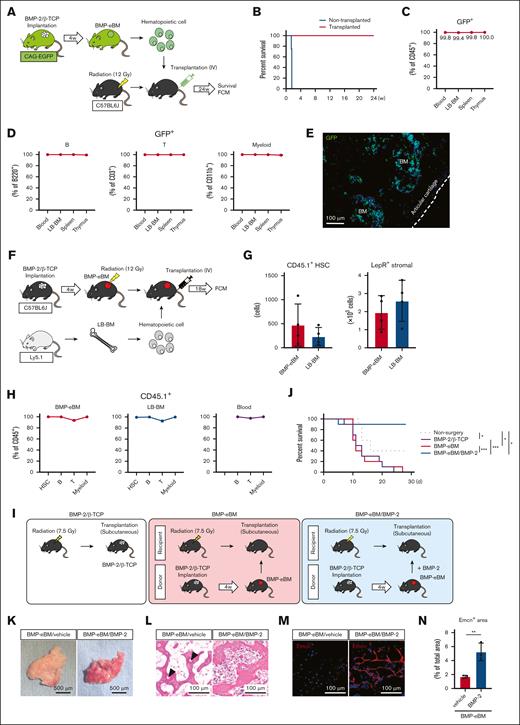
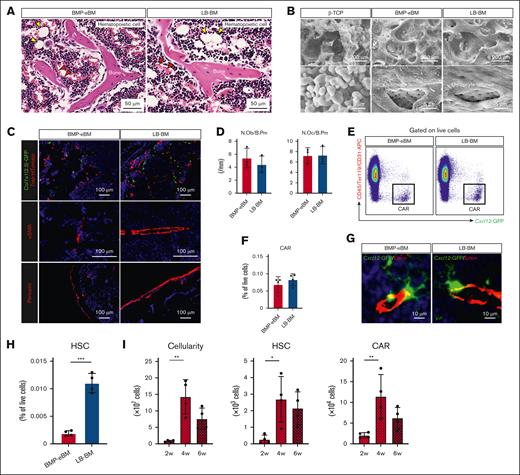
Subcutaneous implantation of BMP-2 (8 μg) with β-TCP (40 mg) successfully induced the formation of a mature eBM within 4 weeks. The chosen concentration of BMP-2 was based on prior studies46 and we verified that increasing doses of BMP-2 led to larger BMP-eBM sizes without significant morphological and histological differences within a dose range of 2 to 16 μg (supplemental Figure 1A-D). Notably, β-TCP alone could not generate an eBM (data not shown). The BMP-eBM developed a BM cavity filled with hematopoietic cells and mature trabecular bones containing embedded osteocytes (Figure 1A). Scanning electron microscope images clearly illustrated the similarity in microstructure between BMP-eBM and LB-BM, including osteocyte lacunae, which indicated the resorption and remodeling of β-TCP granules (Figure 1B). Various cell types, including osteoblasts (Col1a1(2.3)-GFP+), osteoclasts (Trap-tdTomato+), adipocytes, α-smooth muscle actin–positive endothelial cells, periostin-positive periosteum cells, and red blood cells were also observed in the BMP-eBM, confirming the formation of mature BM tissue during this period (Figure 1A,C-D).
BMP-eBM and LB-BM show similar cellularity. Bone morphogenetic protein (BMP)-2 (8 μg)/beta-tricalcium phosphate (β-TCP; 40 mg) was implanted into C57BL6, Col1a1(2.3)-GFP; Trap-tdTomato, or Cxcl12-GFP mice. BMP-2–induced ectopic bone marrow (BMP-eBM) was harvested 4 weeks after implantation and compared with native bone marrow in long bones (LB-BM). (A) Representative hematoxylin and eosin (HE) staining images of BMP-eBM and LB-BM. Yellow arrowhead, adipocyte; red arrowhead, red blood cell. (B) Scanning electron microscope images of β-TCP before implantation, BMP-eBM (4 weeks), and LB-BM. Lower panels show high-magnification images of the upper panels. (C) Fluorescence images: upper panel (green, Col1a1(2.3)-GFP; red, Trap-tdTomato); middle panel (red, α-smooth muscle actin [αSMA]); and lower panel (red, periostin in BMP-eBM [4 weeks] and LB-BM). The blue color represents DAPI staining. (D) Quantification analysis of the number of osteoblasts per bone perimeter (N.Ob/B. Pm) and the number of osteoclasts per bone perimeter (N.Oc/B. Pm) in upper image of panel C (n-3). (E-F) Frequency of CXC-chemokine-ligand-12 (CXCL12)-abundant reticular (CAR) cell analyzed by flow cytometric analysis (FCM; n = 4). CAR cell: CD45–Ter119–CD31–Cxcl12-GFPhigh. (G) Fluorescence images (green, Cxcl12-GFP and red, Endomucin (Emcn) in BMP-eBM [4 weeks] and LB-BM). The blue color indicates DAPI staining. (H) Frequency of hematopoietic stem cells (HSCs) analyzed by FCM (n = 4). HSC: Lin–Sca-1+c-Kit+CD150+CD48–. (I) Cellularity, number of HSCs, and number of CAR cells in BMP-eBM after 2, 4, and 6 weeks of implantation (n = 4). (J) Frequency of common lymphoid progenitor (CLP), common myeloid progenitor (CMP), pre–pro-B, pro-B, pre-B, total B cell, T cell, natural killer (NK) cell, myeloid cell, or erythroid cell (n = 4). CLP, Lin–Sca-1lowc-KitlowCD127+CD135+; CMP, Lin–Sca-1–c-Kit+CD34+CD16/32–; pre–pro-B, B220+sIgM–CD43+CD24–; pro-B, B220+sIgM–CD43+CD24+; pre-B, B220+sIgM–CD43–CD24+; total B cell, B220+; T cell, CD3+CD45+; NK cell, NK1.1+CD3–; myeloid cell, CD11b+Ly6G/C+; erythroid cell, Ter119+CD45–. (K) Representative HE staining images of megakaryocytes in BMP-eBM and LB-BM. (L) Cellularity and number of HSCs in BMP-2–implanted or –naive mouse LB-BM (n = 4). (M-P) single-cell RNA sequencing (scRNA-seq) analysis of hematopoietic and CAR cells. (M) Schematic overview. Hematopoietic cells were collected from C57BL6J mice and CAR cells were collected from Cxcl12-GFP mice by collagenase digestion. (N) Uniform manifold approximation and projection (UMAP)-based visualization (14 clusters). Cells were colored by groups (left panel) or clusters (right panel). Biological replicate 1, n = 14 934 cells (BMP-eBM, 5894 cells; LB-BM, 9040 cells). HSPC, hematopoietic stem and progenitor cell; DC, dendritic cell. (O) Frequencies of each hematopoietic cell cluster in panel N. (P) Number of differentially expressed genes (DEGs) (|log2FC| > 1; P < .05) between BMP-eBM and LB-BM for each cell cluster. FC, fold change.
BMP-eBM and LB-BM show similar cellularity. Bone morphogenetic protein (BMP)-2 (8 μg)/beta-tricalcium phosphate (β-TCP; 40 mg) was implanted into C57BL6, Col1a1(2.3)-GFP; Trap-tdTomato, or Cxcl12-GFP mice. BMP-2–induced ectopic bone marrow (BMP-eBM) was harvested 4 weeks after implantation and compared with native bone marrow in long bones (LB-BM). (A) Representative hematoxylin and eosin (HE) staining images of BMP-eBM and LB-BM. Yellow arrowhead, adipocyte; red arrowhead, red blood cell. (B) Scanning electron microscope images of β-TCP before implantation, BMP-eBM (4 weeks), and LB-BM. Lower panels show high-magnification images of the upper panels. (C) Fluorescence images: upper panel (green, Col1a1(2.3)-GFP; red, Trap-tdTomato); middle panel (red, α-smooth muscle actin [αSMA]); and lower panel (red, periostin in BMP-eBM [4 weeks] and LB-BM). The blue color represents DAPI staining. (D) Quantification analysis of the number of osteoblasts per bone perimeter (N.Ob/B. Pm) and the number of osteoclasts per bone perimeter (N.Oc/B. Pm) in upper image of panel C (n-3). (E-F) Frequency of CXC-chemokine-ligand-12 (CXCL12)-abundant reticular (CAR) cell analyzed by flow cytometric analysis (FCM; n = 4). CAR cell: CD45–Ter119–CD31–Cxcl12-GFPhigh. (G) Fluorescence images (green, Cxcl12-GFP and red, Endomucin (Emcn) in BMP-eBM [4 weeks] and LB-BM). The blue color indicates DAPI staining. (H) Frequency of hematopoietic stem cells (HSCs) analyzed by FCM (n = 4). HSC: Lin–Sca-1+c-Kit+CD150+CD48–. (I) Cellularity, number of HSCs, and number of CAR cells in BMP-eBM after 2, 4, and 6 weeks of implantation (n = 4). (J) Frequency of common lymphoid progenitor (CLP), common myeloid progenitor (CMP), pre–pro-B, pro-B, pre-B, total B cell, T cell, natural killer (NK) cell, myeloid cell, or erythroid cell (n = 4). CLP, Lin–Sca-1lowc-KitlowCD127+CD135+; CMP, Lin–Sca-1–c-Kit+CD34+CD16/32–; pre–pro-B, B220+sIgM–CD43+CD24–; pro-B, B220+sIgM–CD43+CD24+; pre-B, B220+sIgM–CD43–CD24+; total B cell, B220+; T cell, CD3+CD45+; NK cell, NK1.1+CD3–; myeloid cell, CD11b+Ly6G/C+; erythroid cell, Ter119+CD45–. (K) Representative HE staining images of megakaryocytes in BMP-eBM and LB-BM. (L) Cellularity and number of HSCs in BMP-2–implanted or –naive mouse LB-BM (n = 4). (M-P) single-cell RNA sequencing (scRNA-seq) analysis of hematopoietic and CAR cells. (M) Schematic overview. Hematopoietic cells were collected from C57BL6J mice and CAR cells were collected from Cxcl12-GFP mice by collagenase digestion. (N) Uniform manifold approximation and projection (UMAP)-based visualization (14 clusters). Cells were colored by groups (left panel) or clusters (right panel). Biological replicate 1, n = 14 934 cells (BMP-eBM, 5894 cells; LB-BM, 9040 cells). HSPC, hematopoietic stem and progenitor cell; DC, dendritic cell. (O) Frequencies of each hematopoietic cell cluster in panel N. (P) Number of differentially expressed genes (DEGs) (|log2FC| > 1; P < .05) between BMP-eBM and LB-BM for each cell cluster. FC, fold change.
To investigate the existence and spatial localization of the eBM niche, BMP-2/β-TCP was implanted into Cxcl12-GFP mice. Similar to observations in the LB-BM niche, CD45–Ter119–CD31–Cxcl12-GFPhigh CAR cells were found around endomucin-positive (Emcn+) cells in the sinusoids (Figure 1E-G).
Flow cytometric analysis further confirmed the existence of Lin–Sca-1+c-Kit+CD150+CD48– HSCs within the BMP-eBM, although in a lower percentage compared with that in LB-BM (Figure 1H). Notably, the number of HSCs and CAR cells peaked at 4 weeks after implantation, suggesting optimal tissue maturation at this stage (Figure 1I). In addition, the increase in total cellularity (≈17 times) from the second to the fourth week after implantation was significantly higher than the increase in the number of HSCs (≈10 times) and CAR cells (≈6 times; Figure 1I). Immature hematopoietic cells, including common lymphoid progenitors, common myeloid progenitors, and maturing B cells (pre–pro-B, pro-B, and pre-B), as well as mature hematopoietic cells such as T cells, natural killer cells, myeloid cells, and erythroid cells, were also detected within BMP-eBM in similar or greater amounts compared to LB-BM, with the exception of common myeloid progenitors and pre–pro-B (Figure 1J). Megakaryocytes, responsible for platelet production, were identified within BMP-eBM, comparable with LB-BM by histological analysis (Figure 1K). Interestingly, the number of HSCs in native LB-BM did not decrease following BMP-eBM formation (Figure 1L), indicating an increase in the total number of HSCs in each individual mouse. There was no difference in the blood, spleen, and thymus cellularity between implanted and naive mice (supplemental Figure 2A).
Furthermore, single-cell RNA-sequencing analysis of hematopoietic cells and CAR cells showed that BMP-eBM contained identical cellular populations compared with LB-BM (Figure 1M-O; supplemental Figure 3A). The number of differentially expressed genes (|log2 fold change [FC]| > 1; P < .05) was fewer than 10 in all cell populations except for CAR cells (Figure 1P; supplemental Figure 3B). Functional enrichment analysis of differentially expressed genes revealed that CAR cells in BMP-eBM exhibited higher expression of lipid-related genes and lower expression of bone mineralization–related genes than those in LB-BM (supplemental Figure 3C-D). However, no differences were observed in the expression of BM niche factors such as Cxcl12 and Kitl between CAR cells in BMP-eBM and LB-BM. The expression of genes critical for CAR cell differentiation and BM formation, such as Foxc1 and Ebf3, showed a decreasing trend (supplemental Figure 3E). Some CAR cells in BMP-eBM were also positive for LepR, a surface marker for BM niche cells, which has been reported to overlap with CAR cells (supplemental Figure 3E-F). These results indicate that BMP-eBM and LB-BM display similar cellularity and spatial distribution of CAR cells near the sinusoids.
Functional characterization of BMP-eBM
To evaluate the function of HSCs and their long-term multilineage reconstitution, hematopoietic cells, including HSCs isolated from BMP-eBM generated in CAG-EGFP mice, were transplanted IV into lethally irradiated (12 Gy) C57BL6J counterparts. These were compared with lethally irradiated nontransplanted controls (Figure 2A). As demonstrated in Figure 2B-C, hematopoietic cell transplantation rescued the lethality of BM irradiation and reconstituted over 99% of the CD45+ hematopoietic cell population in the peripheral blood, LB-BM, spleen, and thymus. Additionally, donor GFP+ cells from BMP-eBM contributed to multilineage reconstitution, including B, T, and myeloid cells (Figure 2D). The GFP+ hematopoietic cells were clearly detected in the LB-BM space (Figure 2E). These results confirm that the HSCs within BMP-eBM can promote multilineage reconstitution.
Functional characterization of BMP-eBM. (A-E) HSC transplantation assay. (A) Schematic overview. BMP-eBM hematopoietic cells (1 × 107) were collected from CAG-EGFP mice and transplanted IV into lethally irradiated (12 Gy) C57BL6J mice. Control mouse represents no transplantation. (B) Survival curve of irradiated mice after 24 weeks (n = 4). (C) Frequency of donor contribution (GFP+) within CD45+ cells (n = 3). Recipient mice were euthanized 24 weeks after transplantation, and the peripheral blood, LB, Spleen, and thymus were harvested for FCM and histological analysis. (D) Frequency of GFP+ B cell (B220+), T cell (CD3+), and myeloid cells (CD11b+) in the peripheral blood, LB-BM, spleen and thymus after 24 weeks of transplantation (n = 3). (E) A frozen section of the femur showing engrafted GFP+ hematopoietic cells (green). The blue color represents DAPI staining. (F-H) HSC engraftment assay. (F) Schematic overview. BMP-2/β-TCP complexes were implanted subcutaneously into C57BL6J recipients. After 4 weeks of implantation, recipients were lethally irradiated (12 Gy) and transplanted with LB-BM cells (1 × 107) from C57BL6J-Ly5.1 mice. Eighteen weeks later, recipients were euthanized, and tissue samples were harvested for FCM analysis. (G) The number of CD45.1+ HSCs and Leptin receptor (LepR)+ stromal cells (CD45–Ter119–CD31–LepR+; n = 4). (H) Frequency of donor contribution (CD45.1+) in the HSC, B cell, T cell, and myeloid cell populations (n = 4). (I-J) Survival assay. (I) Schematic overview. BMP-eBM was collected from C57BL6J donor and transplanted subcutaneously into irradiated (LD60/30 dose, 7.5 Gy) C57BL6J recipient, with or without supplemental BMP-2 (2 μg). Control mouse represents BMP-2 (16 μg)/β-TCP (80 mg) transplantation. In this assay, BMP-eBM was produced from BMP-2 (16 μg)/β-TCP (80 mg). (J) Survival curve irradiated mice after 28 days (n = 10). (K-N) Retransplantation assay. BMP-eBM was produced from BMP-2 (8 μg)/β-TCP (40 mg) and immersed in 0.5 mM HCl (vehicle) or BMP-2 (1 μg) before retransplantation in other mice. (K) Photographs, (L) HE staining, and (M) immunostaining images of retransplanted BMP-eBMs 2 weeks after retransplantation (n = 3). In panel L, arrowheads indicate empty lacunae. For panel M: red, Emcn; blue, DAPI. (N) Quantification of Emcn+ area per total area of the photo shown in panel M (n = 3).
Functional characterization of BMP-eBM. (A-E) HSC transplantation assay. (A) Schematic overview. BMP-eBM hematopoietic cells (1 × 107) were collected from CAG-EGFP mice and transplanted IV into lethally irradiated (12 Gy) C57BL6J mice. Control mouse represents no transplantation. (B) Survival curve of irradiated mice after 24 weeks (n = 4). (C) Frequency of donor contribution (GFP+) within CD45+ cells (n = 3). Recipient mice were euthanized 24 weeks after transplantation, and the peripheral blood, LB, Spleen, and thymus were harvested for FCM and histological analysis. (D) Frequency of GFP+ B cell (B220+), T cell (CD3+), and myeloid cells (CD11b+) in the peripheral blood, LB-BM, spleen and thymus after 24 weeks of transplantation (n = 3). (E) A frozen section of the femur showing engrafted GFP+ hematopoietic cells (green). The blue color represents DAPI staining. (F-H) HSC engraftment assay. (F) Schematic overview. BMP-2/β-TCP complexes were implanted subcutaneously into C57BL6J recipients. After 4 weeks of implantation, recipients were lethally irradiated (12 Gy) and transplanted with LB-BM cells (1 × 107) from C57BL6J-Ly5.1 mice. Eighteen weeks later, recipients were euthanized, and tissue samples were harvested for FCM analysis. (G) The number of CD45.1+ HSCs and Leptin receptor (LepR)+ stromal cells (CD45–Ter119–CD31–LepR+; n = 4). (H) Frequency of donor contribution (CD45.1+) in the HSC, B cell, T cell, and myeloid cell populations (n = 4). (I-J) Survival assay. (I) Schematic overview. BMP-eBM was collected from C57BL6J donor and transplanted subcutaneously into irradiated (LD60/30 dose, 7.5 Gy) C57BL6J recipient, with or without supplemental BMP-2 (2 μg). Control mouse represents BMP-2 (16 μg)/β-TCP (80 mg) transplantation. In this assay, BMP-eBM was produced from BMP-2 (16 μg)/β-TCP (80 mg). (J) Survival curve irradiated mice after 28 days (n = 10). (K-N) Retransplantation assay. BMP-eBM was produced from BMP-2 (8 μg)/β-TCP (40 mg) and immersed in 0.5 mM HCl (vehicle) or BMP-2 (1 μg) before retransplantation in other mice. (K) Photographs, (L) HE staining, and (M) immunostaining images of retransplanted BMP-eBMs 2 weeks after retransplantation (n = 3). In panel L, arrowheads indicate empty lacunae. For panel M: red, Emcn; blue, DAPI. (N) Quantification of Emcn+ area per total area of the photo shown in panel M (n = 3).
To determine if BMP-eBM provides a suitable environment for HSC engraftment, C57BL6J mice harboring a functional BMP-eBM (after 4 weeks of BMP-2/β-TCP implantation) were lethally irradiated (12 Gy) and transplanted IV with LB-BM cells from C57BL6J-Ly5.1 counterparts (Figure 2F). After 18 weeks, CD45.1+ donor–derived HSCs and LepR+ stromal cells were detected in both BMP-eBM and LB-BM (Figure 2G). Most (>92%) hematopoietic cell populations (HSCs, B cells, T cells, and myeloid cells) in BMP-eBM, LB-BM, and peripheral blood were replaced by these CD45.1+ donor–derived cells (Figure 2H).
To investigate whether BMP-eBM transplantation can be used as a therapeutic tool for managing hematopoietic dysfunctions, functional BMP-eBM organ were transplanted into irradiated (LD60/30 dose, 7.5 Gy) C57BL6J mice. However, all mice unexpectedly died within 30 days of transplantation due to a lack of blood supply, resulting in necrosis within the transplanted BMP-eBM. Interestingly, pretreatment with BMP-2 (2 μg) prior to the retransplantation of functional BMP-eBM organs promoted de novo vascularization and its in situ integration with the donor tissue, ultimately rescuing the irradiated mice (Figure 2I-J). As a control, mice transplanted with BMP-2/β-TCP did not survive due to the absence of eBM formation within the BMP-2/β-TCP graft. We further confirmed that supplementation of BMP-2 supported BMP-eBM engraftment in a nonirradiated mouse model. Note the marked difference in cellularity and blood vessel formation, as indicated by Emcn immunostaining, following BMP-2 supplementation (Figure 2K-N).
BMP-eBM niche formation involves a timely orchestration of cellular differentiation
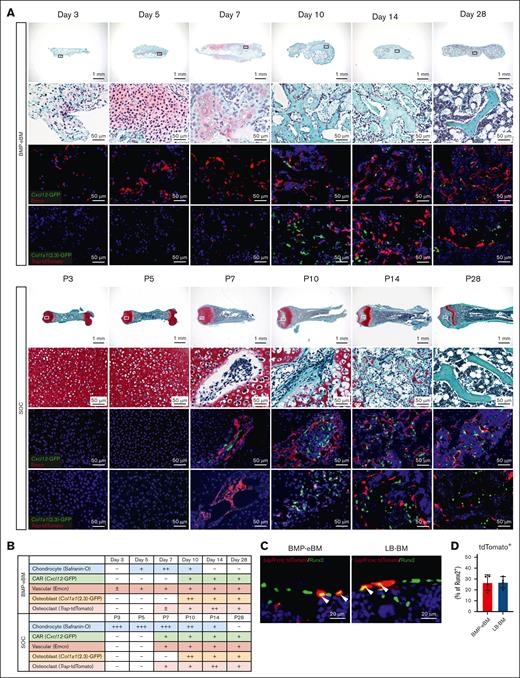
At earlier stages following BMP-2/β-TCP implantation, mesenchymal cells, including Emcn+ endothelial cells, infiltrated and accumulated around the implantation site within 3 to 5 days, confirming the known effect of BMP-2 in promoting vascularization (Figure 3A-B; supplemental Figure 4A).37 Since BMP signaling is also essential for endochondral ossification,47-51 we compared the BM formation process in BMP-eBM and LB-BM (SOC). Indeed, chondrocytes were observed in restricted areas between 5 to 10 days after BMP-2/β-TCP implantation, as demonstrated by safranin-O staining (Figure 3A-B) and an increase in the expression of major chondrocyte markers, Acan and Col2a1 (supplemental Figure 5A). These results suggest that BMP-2 induced chondrocyte differentiation possibly by acting on local MSCs.
BMP-eBM niche formation involves a timely orchestration of cellular differentiation. (A-B) Analysis of BMP-eBM development compared to femoral secondary ossification center (SOC). BMP-eBMs were harvested 3, 5, 7, 10, 14, or 28 days after implantation. Femurs were harvested from C57BL6J mice at postnatal day 3 (P3), P5, P7, P10, P14, or P28. (A) Safranin-O staining images (upper panels). High-middle panels are high-magnification images of the squares in the upper images. Middle-lower panels: green, Cxcl12-GFP; red, Emcn. Lower panels: green, Col1a1(2.3)-GFP; red, Trap-tdTomato. The blue color represents DAPI staining. (B) Semiquantitative analysis of the cellular populations during BMP-eBM and LB-BM development (illustrative). (C) Fluorescence images of BMP-eBM or LB-BM 4 weeks after implantation in LepR-cre; tdTomato mice. Red, LepR-cre; tdTomato; green, Runt-related Transcription Factor 2 (Runx2); blue, DAPI. (D) Frequency of tdTomato+ per Runx2+ cells (n = 4).
BMP-eBM niche formation involves a timely orchestration of cellular differentiation. (A-B) Analysis of BMP-eBM development compared to femoral secondary ossification center (SOC). BMP-eBMs were harvested 3, 5, 7, 10, 14, or 28 days after implantation. Femurs were harvested from C57BL6J mice at postnatal day 3 (P3), P5, P7, P10, P14, or P28. (A) Safranin-O staining images (upper panels). High-middle panels are high-magnification images of the squares in the upper images. Middle-lower panels: green, Cxcl12-GFP; red, Emcn. Lower panels: green, Col1a1(2.3)-GFP; red, Trap-tdTomato. The blue color represents DAPI staining. (B) Semiquantitative analysis of the cellular populations during BMP-eBM and LB-BM development (illustrative). (C) Fluorescence images of BMP-eBM or LB-BM 4 weeks after implantation in LepR-cre; tdTomato mice. Red, LepR-cre; tdTomato; green, Runt-related Transcription Factor 2 (Runx2); blue, DAPI. (D) Frequency of tdTomato+ per Runx2+ cells (n = 4).
BMP-2 is also known to directly induce osteoblast differentiation of MSCs, independent of the SOX-9-associated chondrocyte differentiation pathway and endochondral ossification.52 In agreement, bone-lining osteoblasts were observed from day 10 onward, indicating bone neoformation. This was confirmed by histological analyses of BMP-eBM generated in Col1a1(2.3)-GFP;Trap-tdTomato mice, in which Col1a1(2.3)-GFP+ osteoblasts were observed near Trap-tdTomato+ osteoclasts. Osteoclasts appeared earlier, by day 7, to resorb the cartilage and the bone-like inorganic matrix in both BMP-eBM and LB-BM. The presence of osteoclasts and osteoblasts indicates the remodeling of the inorganic matrix, including β-TCP, with concomitant formation of mature bone tissue enclosing osteocytes (observed around day 10). These dynamic cellular interchanges and matrix remodeling were also observed in the SOC of newborn mice (Figure 3A, lower panels). By day 28, most of this inorganic matrix was resorbed and replaced by the BM cavity containing hematopoietic cells (Figure 3A-B; supplemental Figure 4A). Expression levels of bone homeostasis-related markers (Runx2, Spp1, Sost, Nfatc1, and Ctsk) further confirmed the similar developmental pattern of BMP-eBM and LB-BM in SOC (supplemental Figure 5B).
By generating BMP-eBM in Cxcl12-GFP mice, we confirmed the presence of Cxcl12-GFP+ cells around day 10, although not adjacent to Emcn+ sinusoids. By day 28, Cxcl12-GFPhigh CAR cells were identified alongside Emcn+ sinusoids, displaying a reticular morphology (Figure 3A-B). Notably, CAR cells are known to differentiate into osteoblasts within the BM. To explore the contribution of CAR cells to osteogenesis, we generated BMP-eBM in LepR-cre; tdTomato mice. Immunohistochemical staining for a major preosteoblast marker, Runt-related transcription factor 2 (Runx2) showed that 26.68% of Runx2+ osteoblasts were LepR lineage cells (Figure 3C-D). These results indicate that CAR cells in BMP-eBM give rise to osteoblasts and contribute to bone formation.
CAR cells in BMP-eBM are derived from in situ adipose tissue mesenchymal cells
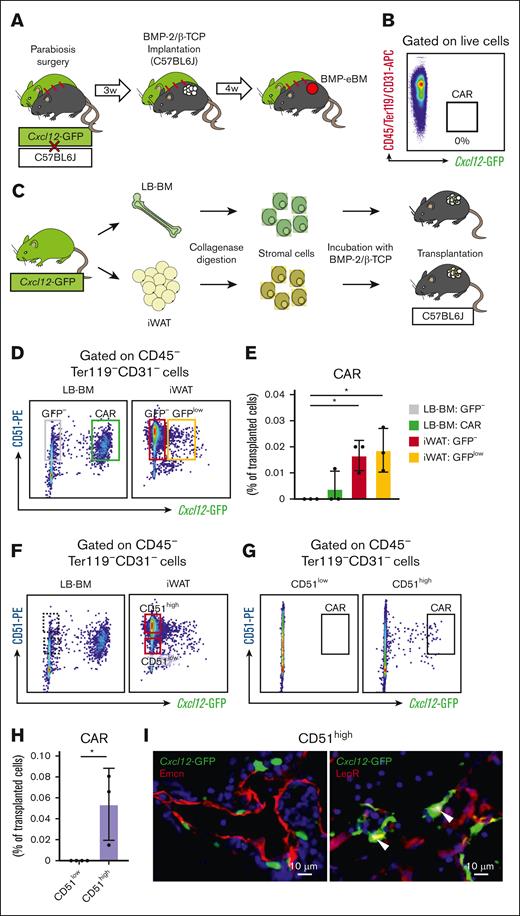
Given that BMP-eBM implantation experiments facilitate a controlled analysis of its cellular components, we aimed to clarify the origins of CAR cells in the BMP-eBM. Two hypotheses were considered: either innate CAR cells from LB-BM would mobilize to the implantation site via systemic blood circulation, or in situ non-CAR mesenchymal cells would migrate and differentiate into CAR cells. To test these hypotheses, we first conducted parabiosis experiments as previously reported.53 A Cxcl12-GFP mouse was surgically paired with a C57BL6J mouse, and after 3 weeks, BMP-2/β-TCP was implanted subcutaneously into the C57BL6J parabiont (Figure 4A). Four weeks post-implantation, the BMP-eBM was harvested, but no Cxcl12-GFP labeled CAR cells were detected (Figure 4B).
CAR cells in BMP-eBM are derived from in situ adipose tissue mesenchymal cells. (A-B) Parabiosis experiment. (A) Schematic overview. Cxcl12-GFP and C57BL6J mice were surgically unified. After 3 weeks, BMP-2/β-TCP was implanted subcutaneously into C57BL6J parabiont. Four weeks after implantation, BMP-eBM was harvested. (B) FCM analysis of CAR cells in BMP-eBM showing the absence of CAR cells (n = 4). (C-I) Cell transplantation experiment. (C) Schematic overview. Cells were isolated from LB-BM or inguinal white adipose tissue (iWAT) of Cxcl12-GFP mice and transplanted together with BMP-2/β-TCP into C57BL6J mice. Two weeks after transplantation, BMP-eBM was harvested. (D) Gating strategy for isolation of LB-BM–derived CD51+Cxcl12-GFP– and CAR cells, iWAT-derived CD51+Cxcl12-GFP– and CD51+Cxcl12-GFPlow cells. (E) FCM analysis of CAR cells in BMP-eBM after transplantation of cells isolated in panel D (n = 3). (F) Gating strategy for isolation of iWAT-derived CD51lowCxcl12-GFP– and CD51highCxcl12-GFP– cells. (G-H) FCM analysis of CAR cells in the BMP-eBM after transplantation of cells isolated as described in panel F (n = 3-4). (I) Fluorescence images of BMP-eBM transplanted with iWAT-derived CD51highCxcl12-GFP– cells. Left panel: green, Cxcl12-GFP; red, Emcn. Right panel: green, Cxcl12-GFP; red, LepR; blue, DAPI.
CAR cells in BMP-eBM are derived from in situ adipose tissue mesenchymal cells. (A-B) Parabiosis experiment. (A) Schematic overview. Cxcl12-GFP and C57BL6J mice were surgically unified. After 3 weeks, BMP-2/β-TCP was implanted subcutaneously into C57BL6J parabiont. Four weeks after implantation, BMP-eBM was harvested. (B) FCM analysis of CAR cells in BMP-eBM showing the absence of CAR cells (n = 4). (C-I) Cell transplantation experiment. (C) Schematic overview. Cells were isolated from LB-BM or inguinal white adipose tissue (iWAT) of Cxcl12-GFP mice and transplanted together with BMP-2/β-TCP into C57BL6J mice. Two weeks after transplantation, BMP-eBM was harvested. (D) Gating strategy for isolation of LB-BM–derived CD51+Cxcl12-GFP– and CAR cells, iWAT-derived CD51+Cxcl12-GFP– and CD51+Cxcl12-GFPlow cells. (E) FCM analysis of CAR cells in BMP-eBM after transplantation of cells isolated in panel D (n = 3). (F) Gating strategy for isolation of iWAT-derived CD51lowCxcl12-GFP– and CD51highCxcl12-GFP– cells. (G-H) FCM analysis of CAR cells in the BMP-eBM after transplantation of cells isolated as described in panel F (n = 3-4). (I) Fluorescence images of BMP-eBM transplanted with iWAT-derived CD51highCxcl12-GFP– cells. Left panel: green, Cxcl12-GFP; red, Emcn. Right panel: green, Cxcl12-GFP; red, LepR; blue, DAPI.
Next, to determine whether in situ non-CAR mesenchymal cells could migrate and differentiate into CAR cells, we conducted cell transplantation experiments using CD51 (integrin, Alpha V), a skeletal stem cell marker40 and expressed in all CAR cells. Mesenchymal cells from LB-BM (CD51+Cxcl12-GFP– or CAR cells) and iWAT (CD51+Cxcl12-GFP– or CD51+Cxcl12-GFPlow) were isolated from a Cxcl12-GFP mouse and transplanted into the BMP-2/β-TCP in a C57BL6J mouse (Figure 4C-D). Interestingly, 2 weeks later, Cxcl12-GFP labeled CAR cells were observed in recipients transplanted with iWAT-derived CD51+Cxcl12-GFP– and CD51+Cxcl12-GFPlow cells, but not in those receiving LB-BM-derived CD51+Cxcl12-GFP– and CAR cells (Figure 4E). The inability of LB-BM-derived CAR cells to engraft was likely because of the absence of a BM niche at the transplantation site. Given that CD51highCxcl12-GFP– cells are rarely found within LB-BM (Figure 4F), it suggests that CD51high cells could be the primary source differentiating into CAR cells. Indeed, Cxcl12-GFP labeled CAR cells were only detected in mice transplanted with iWAT-derived CD51highCxcl12-GFP– cells, and not in those receiving CD51lowCxcl12-GFP– cells (Figures 4G-H). Immunostaining analyses confirmed that these CAR cells were LepR+ and localized along Emcn+ sinusoids (Figure 4I). These findings suggest the potential for in situ mesenchymal cells to differentiate into CAR cells under BMP-2 stimulation.
BMP-eBM shows an aging phenotype similar to LB-BM
Aging is characterized by a decreased amount of trabecular bone due to increased bone resorption activity and enhanced MSC differentiation into the adipocyte lineage, with their accumulation within the BM.54 Consequently, the percentage of marrow space occupied by hematopoietic tissue decreases from 40% to 60% in young adults to 20% to 40% in older individuals.55
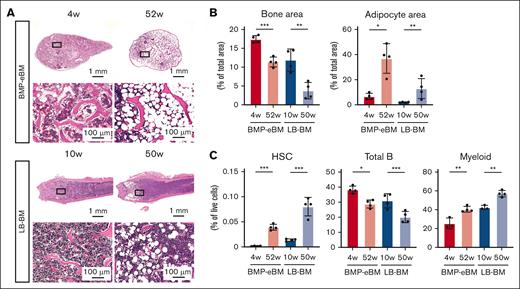
To evaluate the effect of aging on the phenotype of BMP-eBM, specimens were harvested at 4 or 52 weeks after implantation and compared with their LB-BM counterparts harvested at 10 or 50 weeks. Consistent with previous reports, the bone area decreased, while the adipocyte area increased in the aged specimens (Figure 5A-B). Flow cytometric analysis revealed that the number of HSCs and myeloid cells increased, while that of total B cells decreased in the aged BMP-eBM and LB-BM (Figure 5C). These results suggest that BMP-eBM undergoes a similar aging process to LB-BM.
BMP-eBM shows an aging phenotype change similar to LB-BM. (A-C) Analysis of BMP-eBM aging compared with LB-BM. BMP-eBM was harvested 4 or 52 weeks after implantation. LB-BM was harvested from C57BL6J mice at 10 or 50 weeks of age. (A) HE staining images. Lower panels are high-magnification images of the squares shown in the upper images. (B) Quantification of bone area and adipocyte area per total area of the image shown in the lower panel of A (n = 4). (C) Frequency of HSCs, total B cells, and myeloid cells (n = 3-4).
BMP-eBM shows an aging phenotype change similar to LB-BM. (A-C) Analysis of BMP-eBM aging compared with LB-BM. BMP-eBM was harvested 4 or 52 weeks after implantation. LB-BM was harvested from C57BL6J mice at 10 or 50 weeks of age. (A) HE staining images. Lower panels are high-magnification images of the squares shown in the upper images. (B) Quantification of bone area and adipocyte area per total area of the image shown in the lower panel of A (n = 4). (C) Frequency of HSCs, total B cells, and myeloid cells (n = 3-4).
Discussion
BMP-2 is well established as a bone formation–inducing factor and is widely used in clinical conditions requiring bone augmentation.56,57 It is also known to generate eBM.40 In this study, we provide a detailed hematopoietic characterization of BMP-eBM and demonstrate its functional HSC support, showing it to be an optimized microenvironment capable of engrafting and sustaining HSCs, closely mimicking native LB-BM. Although several studies have demonstrated the generation of eBM with hematopoietic function through various approaches, these methods have typically involved complex protocols, often relying on extensive in vitro cell culture as a preparatory step, which can hinder reproducibility and practicability. In contrast, we demonstrate that functional eBM with hematopoietic capability can be generated using BMP-2 alone. Our approach employs a straightforward methodology that eliminates the time and labor-intensive processes associated with in vitro cell culture or other preparatory steps. By mixing BMP-2 with β-TCP and performing in vivo transplantation, BM-like organs were formed as early as 4 weeks after transplantation. BMP-eBM exhibited a mature architecture and function comparable with native BM. Furthermore, by eliminating the need for cell transplantation, we address concerns such as graft-versus-host disease and invasiveness associated with cell harvesting and reduce the risks of potential infections. These advantages position BMP-eBM as a particularly promising candidate for clinical applications. To our knowledge, this study is the first to demonstrate that CAR cells can be induced to differentiate without any tissue injuries and to provide insights into their origins. This represents a significant step toward understanding the mechanisms underlying BM formation and its role in hematopoietic support. Our findings offer novel insights into BM biology and lay the groundwork for future studies aimed at elucidating the precise mechanisms through which BM supports hematopoiesis.
Besides inducing chondrogenic differentiation of MSCs, BMP-2 is also known to promote angiogenesis and osteoblast differentiation. These pleiotropic effects are critically important for building the BM environment, that is, a highly vascularized bone matrix scaffold that houses the BM niche. β-TCP enhances bone formation due to its osteoinductive properties, which have been reported to depend more on its 3-dimensional architecture, including micropores within the macroporous structure, than on its chemical composition.58,59 Moreover, β-TCP can adsorb BMP-2 within its pores and subsequently optimize its in situ function, such as facilitating the recruitment and homing of surrounding mesenchymal cells in the early stages of BMP-eBM formation.
A previous study showed that LB-BM–derived MSCs spontaneously build a BM cavity through the formation of a vascularized cartilage intermediate in vivo, which was progressively replaced by bone and BM tissue.60 Similarly, primitive cartilage templates containing hypertrophic chondrocytes, formed by chondrogenic differentiation of MSCs in vitro, were replaced by BM tissue capable of maintaining hematopoiesis in vivo after implantation in the subcutis of mice.27,29,61 Furthermore, transplantation of LB cartilage diaphysis to subcutaneous sites resulted in eBM formation through endochondral ossification.62 In this study, BMP-eBM generation involved endochondral ossification. These results suggest that endochondral ossification is a key and necessary step preceding BM formation.60
In this study, BMP-eBM exhibited higher bone and adipocyte areas compared to LB-BM. Regarding gene expression patterns in CAR cells, previous reports have shown that the expressions of Ebf1/3 and Foxc1 are involved in BM cavity formation through the regulation of osteoblast and adipocyte differentiation, respectively.19,63 In our single-cell RNA-sequencing analysis, the expression of Ebf3 and Foxc1 showed a decreasing trend in BMP-eBM relative to LB-BM. This aligns with the observed higher bone and adipocyte areas in BMP-eBM compared to LB-BM.
Using parabiosis and cell transplantation models, we found that CAR cells in BMP-eBM were derived from CD51highCxcl12-GFP– cells in the tissue surrounding the BMP-2/β-TCP graft. CD51 has been reported to be expressed in MSCs in both mouse and human adipose tissue,64-68 but CD51highCxcl12-GFP– cells are rarely found within the flushed LB-BM in this study. These findings may help explain why BMP-2 cannot induce bone formation when delivered within a medullary cavity in LB-BM.46,69 However, our analysis of LB-BM focused on flushed marrow, without accounting for stromal cells potentially derived from compact bone, and the number of transplanted stromal cells varied across experimental conditions. These limitations restrict our ability to definitively conclude that CD51high cells from the BM do not engraft in the BMP-2/β-TCP nodules, as the experiment did not evaluate the entire bone environment. We are currently developing and validating a protocol for enzymatically digesting whole bones, including both compact bone and marrow, to enable a more comprehensive analysis of stromal populations and a direct comparison with iWAT-derived cells. Despite these limitations, the findings of this study suggest that heterogeneous CD51highCxcl12-GFP– cells, potentially including mouse skeletal stem cells,40 are a key source for differentiating into CAR cells under BMP-2 stimulation. This is a novel finding that BMP-2 can induce CAR cells, a key component of the BM niche, from non-BM sources.
The frequency of HSCs in BMP-eBM was significantly lower than in LB-BM. A possible explanation for this discrepancy could be the timing of the observation. In this study, we compared the frequency of HSCs in BMP-eBM, harvested 4 weeks after transplantation, with that in LB-BM from 8-week-old mice. However, as shown in the supplemental figure, the frequency of HSCs in both BMP-eBM and LB-BM increases with age. Therefore, the observed difference may be due to the comparison being limited to a single time point. In future studies, we plan to investigate BM maturation and aging by analyzing multiple time points.
BMP-eBM can be isolated and transplanted as an independent hematopoietic site, offering immense potential for diverse clinical applications requiring adjunctive BM support. Ectopically formed BM may serve as a reservoir for HSCs and promote hematopoiesis as needed. This approach also presents a novel strategy to enhance hematopoietic recovery following radiation therapy or chemotherapy. By utilizing the patient’s own stem cells and extracellular matrix, eBM can create an autologous hematopoietic-supportive environment, thereby minimizing the risk of allogeneic immune responses. Moreover, heterotopic BM offers a multimodal therapeutic advantage, as it simultaneously supports hematopoietic function and promotes bone regeneration. Consequently, eBM represents a novel regenerative medicine platform with hematopoietic-supportive capabilities, applicable in clinical trials and foundational for regenerative medicine. A key innovation of this study is the development of a method to harvest and re-transplant BMP-eBM while preserving its function. While the role of BMP-2 in neovascularization is well established, this study is the first to demonstrate its application in supporting live engraftment within an organ transplantation model. This breakthrough opens exciting possibilities for clinical applications, such as regenerating bone into specific shapes through molding prior to reimplantation or enhancing hematopoietic function with allografts from optimal hematopoietic donors. Furthermore, BMP-2 has already been widely and safely used in clinical applications for bone regeneration, which facilitates its translation into clinical practice for these novel applications. Humanized ossicles are also being actively investigated as an eBM model with hematopoietic function. Although humanized ossicles are valuable for basic research and as a preclinical model, their clinical applications face challenges related to cell supply and immune responses.
BMP-eBM can be utilized as hematopoietic organ models for more detailed investigations into the origins, multilineage differentiation analysis, and functions of HSCs and MSCs in both in vivo and ex vivo settings within short time frames. BMP-eBM also provides the advantage of compatibility with a variety of experimental tools, including genetically engineered mouse models. Generating BMP-eBM in specific mouse types or genetically engineered mice may further expand the possibilities for analyzing specific cell types under controlled experimental conditions, including studies on hematological malignancies, immune reactions such as graft-versus-host disease, and other pathological states. BMP-eBM presents unique opportunities for exploring fundamental mechanisms of BM development and aging. For instance, it would be valuable to investigate whether the age of the recipient affects eBM formation or whether HSC homing and frequency differ between young and old eBM. Such studies could provide critical insights into BM aging. Furthermore, BMP-eBM could serve as a platform for drug discovery or for studying cell-cell and cell-matrix interactions within a confined microenvironment.
In conclusion, although previous studies have shown the presence of HSCs in BMP-eBM, this study clarifies that BMP-eBM is a functional hematopoietic organ capable of housing and maintaining an eBM niche. An adipose tissue–derived CD51highCxcl12-GFP– mesenchymal cell population was shown to be a major cell source for the formation of the eBM niche by differentiating into CAR cells.
Acknowledgments
The authors thank Koichi Matsuo (Keio University, Tokyo, Japan) for providing the Col1a1(2.3)-GFP mice; Masaru Ishii and Junichi Kikuta (Osaka University, Osaka, Japan) for providing the Trap-tdTomato mice; Shiro Jochi and Hiroyuki Irie (Osteopharma Inc, Osaka, Japan) for providing the BMP-2; Tomoyuki Mukai (Kawasaki Medical School, Okayama, Japan) and Shuta Tomida (Okayama University, Okayama, Japan) for supporting with single-cell RNA-sequencing analysis; Yasuhito Yahara (University of Toyama, Toyama, Japan) for the instruction of the parabiosis surgery; Daisuke Yamada (Okayama University, Okayama, Japan) for providing illustrations; Chinatsu Hashimoto, Kami Sato, Shuji Nosho, Yukie Tanaka, Ryotaro Ide, Toshiyuki Tomisaka, and the member of Central Research Laboratory in Okayama University Medical School for technical assistance. The authors thank Editage for English language editing.
This study was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grants 19H03842 (M.O.), 20K21679 (T.K.), 20KK0377 (M.O.), 22K19625 (M.O.), and 24K21302 (M.O.).
Authorship
Contribution: M.O. conceptualized the study; I.T. and M.O. were involved in data curation; I.T., M.O., and A.T.D. performed the formal analysis; M.O. and T.K. were involved in funding acquisition; I.T., M.O., A.T.D., M.K., W.K., K.I., H.T.D., K.Z., and E.S.H. performed the investigation; I.T., M.O., M.K., and N.A. designed the methodology; I.T. and M.O. carried out project administration; M.O., E.S.H., N.A., T.N., T.O., and T.K. acquired resources; I.T., M.O. and Z.W. performed analysis on the software; T.K. provided supervision; I.T., M.O., A.T.D., W.K., K.I., H.T.D., and K.Z. performed validation; I.T., M.O., A.T.D., W.K., and E.S.H. performed visualization; I.T. and M.O. wrote the original draft; and E.S.H., N.A., T.N., T.O., N.O., and T.K were involved in writing, review, and editing the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mitsuaki Ono, Department of Oral Rehabilitation and Regenerative Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8525, Japan; email: mitsuaki@md.okayama-u.ac.jp.
References
Author notes
The single-cell RNA-sequencing data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and can be accessed through the GEO Series (accession number GSE283925).
Original data are available on request from the corresponding author, Mitsuaki Ono (mitsuaki@md.okayama-u.ac.jp).
The full-text version of this article contains a data supplement.

![BMP-eBM and LB-BM show similar cellularity. Bone morphogenetic protein (BMP)-2 (8 μg)/beta-tricalcium phosphate (β-TCP; 40 mg) was implanted into C57BL6, Col1a1(2.3)-GFP; Trap-tdTomato, or Cxcl12-GFP mice. BMP-2–induced ectopic bone marrow (BMP-eBM) was harvested 4 weeks after implantation and compared with native bone marrow in long bones (LB-BM). (A) Representative hematoxylin and eosin (HE) staining images of BMP-eBM and LB-BM. Yellow arrowhead, adipocyte; red arrowhead, red blood cell. (B) Scanning electron microscope images of β-TCP before implantation, BMP-eBM (4 weeks), and LB-BM. Lower panels show high-magnification images of the upper panels. (C) Fluorescence images: upper panel (green, Col1a1(2.3)-GFP; red, Trap-tdTomato); middle panel (red, α-smooth muscle actin [αSMA]); and lower panel (red, periostin in BMP-eBM [4 weeks] and LB-BM). The blue color represents DAPI staining. (D) Quantification analysis of the number of osteoblasts per bone perimeter (N.Ob/B. Pm) and the number of osteoclasts per bone perimeter (N.Oc/B. Pm) in upper image of panel C (n-3). (E-F) Frequency of CXC-chemokine-ligand-12 (CXCL12)-abundant reticular (CAR) cell analyzed by flow cytometric analysis (FCM; n = 4). CAR cell: CD45–Ter119–CD31–Cxcl12-GFPhigh. (G) Fluorescence images (green, Cxcl12-GFP and red, Endomucin (Emcn) in BMP-eBM [4 weeks] and LB-BM). The blue color indicates DAPI staining. (H) Frequency of hematopoietic stem cells (HSCs) analyzed by FCM (n = 4). HSC: Lin–Sca-1+c-Kit+CD150+CD48–. (I) Cellularity, number of HSCs, and number of CAR cells in BMP-eBM after 2, 4, and 6 weeks of implantation (n = 4). (J) Frequency of common lymphoid progenitor (CLP), common myeloid progenitor (CMP), pre–pro-B, pro-B, pre-B, total B cell, T cell, natural killer (NK) cell, myeloid cell, or erythroid cell (n = 4). CLP, Lin–Sca-1lowc-KitlowCD127+CD135+; CMP, Lin–Sca-1–c-Kit+CD34+CD16/32–; pre–pro-B, B220+sIgM–CD43+CD24–; pro-B, B220+sIgM–CD43+CD24+; pre-B, B220+sIgM–CD43–CD24+; total B cell, B220+; T cell, CD3+CD45+; NK cell, NK1.1+CD3–; myeloid cell, CD11b+Ly6G/C+; erythroid cell, Ter119+CD45–. (K) Representative HE staining images of megakaryocytes in BMP-eBM and LB-BM. (L) Cellularity and number of HSCs in BMP-2–implanted or –naive mouse LB-BM (n = 4). (M-P) single-cell RNA sequencing (scRNA-seq) analysis of hematopoietic and CAR cells. (M) Schematic overview. Hematopoietic cells were collected from C57BL6J mice and CAR cells were collected from Cxcl12-GFP mice by collagenase digestion. (N) Uniform manifold approximation and projection (UMAP)-based visualization (14 clusters). Cells were colored by groups (left panel) or clusters (right panel). Biological replicate 1, n = 14 934 cells (BMP-eBM, 5894 cells; LB-BM, 9040 cells). HSPC, hematopoietic stem and progenitor cell; DC, dendritic cell. (O) Frequencies of each hematopoietic cell cluster in panel N. (P) Number of differentially expressed genes (DEGs) (|log2FC| > 1; P < .05) between BMP-eBM and LB-BM for each cell cluster. FC, fold change.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/14/10.1182_bloodadvances.2024014062/2/m_blooda_adv-2024-014062-gr1b.jpeg?Expires=1769079915&Signature=y~zRhnuPPF9kZxDw4yYpL04d39SOU2fegadItNmyfLa~zeBe1w~94X2xL0cdxYw99WS3WM8mDyuX7lRrMmydBbIUGst90hMs4pQGLjy1PI3Gjcmj7-jY2YX-Pr8tnKTYL3OhcjbGOfIT42pc9tbHsh-2isuox-BQX5xSkIIWZn6-suyfO2Ttm5LHko~YGFx96RwkT7n1BRVHV5hT2layjY3D9Eetvpmu9owVVHt6JpfXgt7yyhLyxW11YTlWY~-xNWZeea4tXMM2stwDn5xjV9PF1LO5vDNovtgk~tOqvd1wf0AeM-CU~NJ4zUkG2RFRdvABYy8IrqZOG~0F0bFtUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)