Key Points
NTRK1-rearranged histiocytosis often presents as 1 or multiple cutaneous xanthogranulomas but may rarely involve extracutaneous tissues.
Targeted therapy with the TRK inhibitor larotrectinib is highly effective in patients with severe extracutaneous disease.
Visual Abstract
Non-Langerhans cell histiocytoses are a diverse group of histiocytic diseases. Different entities are defined based on clinical, histopathologic, and/or molecular characteristics. This study aimed to define NTRK-rearranged histiocytosis. Through international collaboration, we investigated 50 cases of histiocytosis with pan-tropomyosin receptor kinase (pan-TRK) expression and/or in-frame NTRK rearrangement. We also analyzed 45 control xanthogranulomas using pan-TRK immunohistochemistry and targeted RNA sequencing. Slides were centrally reviewed; clinical and molecular data were collected. The 50 cases comprised 30 children and 20 adults with a median age of 11.5 years (range, 0-73 years) and a male predominance (64%). Most patients (88%) had disease limited to the skin, including a single skin nodule in 41 patients and multiple skin lesions in 3 others. Four newborns presented with skin lesions, hepatomegaly, and thrombocytopenia that required transfusions. The 2 remaining patients had life-threatening lesions of the brain or bronchus. All cases displayed xanthogranuloma histology, often including foamy histiocytes and Touton giant cells. Histiocytes stained positive for pan-TRK in 50 of 50 cases, whereas all 45 control xanthogranulomas without in-frame NTRK fusions stained negative. NTRK1 fusion partners included IRF2BP2 (23/46), TPM3 (12/46), SQSTM1 (3/46), PRDX1 (3/46), NPM1 (2/46), LMNA (2/46), and ARHGEF2 (1/46). Clinical outcomes were favorable, including spontaneous disease regression in 3 of 4 newborns with systemic disease, and rapid clinical response in both patients with a brain or bronchial tumor treated with the TRK inhibitor larotrectinib. This study advances the molecular characterization of histiocytoses and may guide the diagnosis and personalized treatment of patients.
Introduction
The xanthogranuloma (XG) family of lesions are a type of non-Langerhans cell histiocytosis, mostly belonging to the cutaneous and mucocutaneous (C group) histiocytoses.1 Recent population-based data indicate that they are the most common histiocytic neoplasm.2 The XG family of lesions generally present during childhood when they are referred to as juvenile XG (JXG), but they may also appear during adulthood. JXG and adult XG often present in the skin as a solitary lesion but can also manifest as disseminated skin lesions and may rarely involve extracutaneous sites. Skin lesions may initially be pink and usually turn brown or yellowish over time. Dermoscopy often reveals a setting sun appearance that is characterized by a yellow-orange background, surrounding erythema, and telangiectasia.3-6 Extracutaneous disease is rare but may involve any organ, including the central nervous system (CNS), liver, and bones.7,8 The majority of JXG lesions regress spontaneously. However, extracutaneous/systemic XG can be more aggressive and even life threatening.8,9
On histologic examination, the XG family of lesions are characterized by the accumulation of histiocytes within the dermis, typically without epidermotropism. These histiocytes are often accompanied by variable amounts of lymphocytes, neutrophils, and eosinophils. So-called early lesions mainly contain small mononucleated histiocytes with eosinophilic cytoplasm, which may demonstrate spindled or epithelioid morphology without Touton giant cells. In contrast, mature lesions often include histiocytes with foamy (lipid-rich) cytoplasm10 and are commonly associated with the presence of multinucleated Touton giant cells. The histiocytes express CD163, CD68, CD4, Factor XIIIa, fascin, and PU.1 and are negative for CD1a and Langerin/CD207.10-12 Notably, the histology of XG is not specific, and Erdheim-Chester disease (ECD) and ALK-positive histiocytosis can have a similar morphology and immunophenotype.
The molecular characterization of histiocytic disorders began with the discovery of the BRAFV600E mutation in about half of patients with Langerhans cell histiocytosis (LCH) and ECD.13,14 Since then, somatic genetic alterations have been identified in several other genes,15,16 most of which activate the MAPK signaling pathway. Interestingly, the genomic driver is strongly correlated with the histiocytosis phenotype.17,18 For example, BRAF is mutated in half of LCH and ECD but is rarely mutated in cutaneous XG or Rosai-Dorfman-Destombes disease (RDD).15,16,19 The type of gene alteration may also differ between histiocytoses with MAP2K1 deletions being common in LCH and MAP2K1 single nucleotide variants prevailing in RDD.20 These insights in the genetic drivers of histiocytoses have not only improved our understanding of their biology but also enhanced the diagnosis and (sub)classification of these rare diseases and allowed for targeted therapy in many instances. BRAF inhibition is increasingly used in the treatment of patients with severe and/or relapsed/refractory histiocytosis that harbor BRAFV600E.21,22 Many patients without BRAFV600E respond to MEK inhibitors,23 and targeted therapy with inhibitors of receptor tyrosine kinases is highly efficient in patients with a gene fusion involving ALK,24RET,16 or NTRK1.25
NTRK fusions have been identified as drivers of various cancers, including secretory breast carcinoma, infantile fibrosarcoma, secretory salivary gland carcinoma, pediatric papillary thyroid carcinoma, and melanocytic tumors.26,27NTRK1, NTRK2, and NTRK3 encode the tropomyosin receptor kinase (TRK) proteins TRKA, TRKB, and TRKC, respectively. The efficacy of TRK inhibition in patients with NTRK-rearranged solid tumors is striking with an 80% overall response rate in a large cohort of patients with 17 tumor types treated with the pan-TRK inhibitor larotrectinib.26NTRK fusions have also been reported in some case reports of histiocytosis,15,25,27-35 often in the context of JXG. Moreover, a recent single-institutional study of 50 patients with JXG or adult XG revealed pan-TRK expression using immunohistochemistry in almost half of these cases, all with localized disease.34 Thus, NTRK fusions might be more common in histiocytosis than previously appreciated. However, only 2 of these 50 cases were molecularly analyzed and both harbored an NTRK1 fusion. Here, we provide a comprehensive study of NTRK-rearranged histiocytosis, describing clinicopathologic and molecular features of 50 cases of histiocytosis with pan-TRK expression, including 46 with confirmed NTRK1 fusions and 6 with extracutaneous disease, including 4 infants.
Patients and methods
Cases were retrieved from the files of 2 international referral centers, namely Ambroise Paré Hospital (Paris, France) and Cincinnati Children’s Hospital Medical Center (Cincinnati, OH). In addition, relevant cases were identified in the Dutch Nationwide Pathology Databank (Palga).2,6 The inclusion criteria were (1) a diagnosis of histiocytosis confirmed by central pathology review (by S.F., J.L.P., and J.-F.E.); (2) expression of at least 1 of the histiocyte markers CD163, CD68, and/or CD14; and (3) positivity of histiocytes for pan-TRK immunostaining and/or in-frame rearrangement involving NRTK1, NRTK2, or NTRK3. Clinical information was collected retrospectively from the medical records. To assess the specificity of pan-TRK staining, we also analyzed 45 cases of XG in which targeted RNA sequencing did not detect an in-frame NTRK fusion. The study was approved by the French Ethics and Scientific Committee for Health Research, Studies and Evaluations (CESREES 2814848 bis), the Cincinnati Children’s Hospital Institutional Review Board (2019-1261), the Institutional Review Board of Leiden University Medical Center (B19.074), and the Palga Scientific Council and Privacy Committee (LZV-2016-183). Written informed consent was obtained from the patients and/or their legal representative(s) when required, or a waiver of consent was obtained from the relevant institutional review board for retrospective research.
Pan-TRK immunostaining with clone EPR17341 (Abcam) was performed for all cases during routine diagnostics or in the specific context of this study at Ambroise Paré Hospital and Cincinnati Children’s Hospital. The intensity of pan-TRK staining was assessed during central pathology review on a semi-quantitative scale as follows: 0 indicating no staining, 1 indicating very low staining, 2 indicating low staining, 3 indicating intermediate staining, and 4 indicating high intensity staining (supplemental Figure 1). Other immunostains such as CD163, CD68, CD14, CD4, S100, CD1a, CD207, Factor XIIIa, fascin, phosphorylated extracellular signal-regulated kinase (phosphoERK), ALK, and cyclin D1 were performed at the local pathologist’s discretion and were completed when required during central pathology review. Targeted RNA sequencing was performed for all cases except 4 for which the tissue sample was exhausted. RNA sequencing was performed using Archer FusionPlex technology, as previously described.24
Results
Fifty patients met the inclusion criteria, including 32 males and 18 females (Tables 1 and 2). The cohort comprised 30 children and 20 adults with a median age at diagnosis of 11.5 years (range, 0-73 years).
Clinical characteristics of patients with NTRK1-rearranged histiocytosis
| Patients . | N = 50 . |
|---|---|
| Sex, n (%) | |
| Male | 32 (64) |
| Female | 18 (36) |
| Age at diagnosis, median (range), y | 11.5 (0.0-73) |
| 0-10, n (%) | 23 (46) |
| 10-20, n (%) | 8 (16) |
| 20-50, n (%) | 10 (20) |
| >50, n (%) | 9 (18) |
| Disease extent, n (%) | |
| Skin-limited lesions | 44 (88) |
| Extracutaneous lesions | 6 (12) |
| Patients with skin-limited disease, n (%) | |
| No. of lesions | |
| 1 | 41 (93) |
| 2-5 | 2 (5) |
| >5 | 1 (2) |
| Location | |
| Head and neck | 21 (48) |
| Trunk | 12 (27) |
| Upper limbs | 10 (23) |
| Lower limbs | 6 (14) |
| Mucosa | 0 (0) |
| Patients . | N = 50 . |
|---|---|
| Sex, n (%) | |
| Male | 32 (64) |
| Female | 18 (36) |
| Age at diagnosis, median (range), y | 11.5 (0.0-73) |
| 0-10, n (%) | 23 (46) |
| 10-20, n (%) | 8 (16) |
| 20-50, n (%) | 10 (20) |
| >50, n (%) | 9 (18) |
| Disease extent, n (%) | |
| Skin-limited lesions | 44 (88) |
| Extracutaneous lesions | 6 (12) |
| Patients with skin-limited disease, n (%) | |
| No. of lesions | |
| 1 | 41 (93) |
| 2-5 | 2 (5) |
| >5 | 1 (2) |
| Location | |
| Head and neck | 21 (48) |
| Trunk | 12 (27) |
| Upper limbs | 10 (23) |
| Lower limbs | 6 (14) |
| Mucosa | 0 (0) |
Clinical and molecular features of patients with systemic and/or extracutaneous NTRK-rearranged histiocytosis
| Nr . | Gene fusion . | Sex . | Age at diagnosis . | Sites of disease . | First-line treatment . | Response . | Progression/relapse . | Second- and further-line treatment . | Outcome . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic | Infants with systemic disease with skin and liver involvement | ||||||||||
| PK 01 | SQSTM1:: NTRK1 | M | Newborn | Skin, hematopoietic system, liver, spleen, and kidney. Possible bone and GI involvement. | Chemotherapy: vinblastine and prednisone for 1 y | CR | No | No | Alive, NED | 8 y | This study |
| PK 02 | TPM3:: NTRK1 | F | Newborn | Skin, hematopoietic system, liver, and spleen. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 7 mo | This study and Kemps et al6 |
| AP 24 | PRDX1:: NTRK1 | M | Newborn | Skin, liver, and spleen. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 13 mo | This study |
| AP 27 | TPM3:: NTRK1 | F | Newborn | Skin, hematopoietic system, and liver. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 7 mo | This study |
| Isolated | Patients with isolated extracutaneous disease | ||||||||||
| AP 32 | IRF2BP2::NTRK1 | M | 8 mo | Right main bronchus | Bronchoscopy with partial resection (4×) | PD | Yes | TRK inhibition (larotrectinib) | Alive, NED | 8 mo | This study |
| US 13 | ARHGEF2::NTRK1 | F | 15 y | Left temporal lobe of the brain | TRK inhibition (larotrectinib) during 19 mo | PR | No | No | Alive, NED | 26 mo | This study & Newman et al33 |
| Literature | Patients with systemic AND/OR extracutaneousNTRK-rearranged histiocytosis reported in the literature | ||||||||||
| L01 | IRF2BP2::NTRK1 | M | Newborn | Skin, ocular, and hematopoietic system. | Active monitoring | PD | Yes | TRK inhibition (larotrectinib) | Alive, NED | 18 months | Kim et al25 |
| L02 | TFG:: NTRK1 | M | 0.2 y | NR (ECD) | NR | NR | NR | NR | NR | NR | Taylor et al27 |
| L03 | TPR:: NTRK1 | M | 20 y | NR (IDCS) | NR | NR | NR | NR | NR | NR | Taylor et al27 |
| L04 | PLEKHA6::NTRK3 | M | 2.8 y | Bone, soft tissue (LCH) | Surgery and chemotherapy | CR | No | No | Alive, NED | 12 mo | Cai et al36 |
| Nr . | Gene fusion . | Sex . | Age at diagnosis . | Sites of disease . | First-line treatment . | Response . | Progression/relapse . | Second- and further-line treatment . | Outcome . | Follow-up . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic | Infants with systemic disease with skin and liver involvement | ||||||||||
| PK 01 | SQSTM1:: NTRK1 | M | Newborn | Skin, hematopoietic system, liver, spleen, and kidney. Possible bone and GI involvement. | Chemotherapy: vinblastine and prednisone for 1 y | CR | No | No | Alive, NED | 8 y | This study |
| PK 02 | TPM3:: NTRK1 | F | Newborn | Skin, hematopoietic system, liver, and spleen. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 7 mo | This study and Kemps et al6 |
| AP 24 | PRDX1:: NTRK1 | M | Newborn | Skin, liver, and spleen. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 13 mo | This study |
| AP 27 | TPM3:: NTRK1 | F | Newborn | Skin, hematopoietic system, and liver. | Supportive care (platelet transfusions) | CR | No | No | Alive, NED | 7 mo | This study |
| Isolated | Patients with isolated extracutaneous disease | ||||||||||
| AP 32 | IRF2BP2::NTRK1 | M | 8 mo | Right main bronchus | Bronchoscopy with partial resection (4×) | PD | Yes | TRK inhibition (larotrectinib) | Alive, NED | 8 mo | This study |
| US 13 | ARHGEF2::NTRK1 | F | 15 y | Left temporal lobe of the brain | TRK inhibition (larotrectinib) during 19 mo | PR | No | No | Alive, NED | 26 mo | This study & Newman et al33 |
| Literature | Patients with systemic AND/OR extracutaneousNTRK-rearranged histiocytosis reported in the literature | ||||||||||
| L01 | IRF2BP2::NTRK1 | M | Newborn | Skin, ocular, and hematopoietic system. | Active monitoring | PD | Yes | TRK inhibition (larotrectinib) | Alive, NED | 18 months | Kim et al25 |
| L02 | TFG:: NTRK1 | M | 0.2 y | NR (ECD) | NR | NR | NR | NR | NR | NR | Taylor et al27 |
| L03 | TPR:: NTRK1 | M | 20 y | NR (IDCS) | NR | NR | NR | NR | NR | NR | Taylor et al27 |
| L04 | PLEKHA6::NTRK3 | M | 2.8 y | Bone, soft tissue (LCH) | Surgery and chemotherapy | CR | No | No | Alive, NED | 12 mo | Cai et al36 |
CR, complete response; GI, gastrointestinal; IDCS, interdigitating dendritic cell sarcoma; NED, no evidence of disease; NR, not reported; PD, progressive disease; PR, partial response.
Disease characteristics
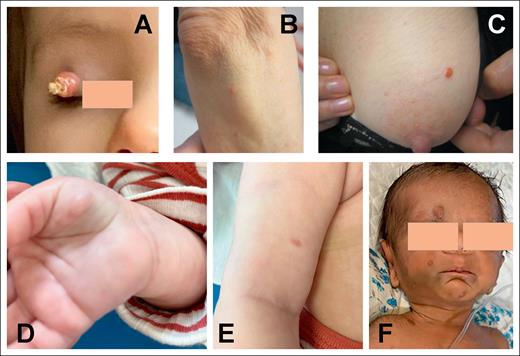
In 44 cases (88%), the disease was limited to the skin, including a single lesion in 41 of 44 cases and ≥2 lesions in the remaining 3 patients. Skin lesions were frequently located in the head and neck region (21/44; 48%), followed by the trunk and upper limbs (12/44 and 10/44 patients, respectively). Skin lesions often consisted of nodules (24/37; 65%) or papules (6/37; 16%). They measured 1 to 2 cm or less with a color ranging from yellow to red/brown (Figure 1) and with no notable difference between patients with 1 and those with multiple skin lesions.
Skin involvement by NTRK1-rearranged histiocytosis. (A) Single lesion on the eyelid of a 2-year-old girl (AP13). (B-C) Skin lesions of a 70-year-old female (AP11). (D-E) Multiple skin lesions of a female newborn (AP27). (F) Multiple skin nodules of a male newborn (AP24).
Skin involvement by NTRK1-rearranged histiocytosis. (A) Single lesion on the eyelid of a 2-year-old girl (AP13). (B-C) Skin lesions of a 70-year-old female (AP11). (D-E) Multiple skin lesions of a female newborn (AP27). (F) Multiple skin nodules of a male newborn (AP24).
Six patients had disease with extracutaneous involvement. Four newborns had systemic disease with skin lesions, hepatomegaly, and thrombocytopenia (Figure 2). Three of them also had splenomegaly, including 1 patient with additional kidney infiltration and probable bone and digestive tract involvement (Figure 2). Laboratory evaluation revealed abnormal liver blood tests in all 4 patients (supplemental Table 1) with occasional coagulopathy and anemia in 3 patients (supplemental Table 2). Although ferritin levels were universally increased (range, 846-2075 μg/L), none of the 4 cases had fever, hypertriglyceridemia, hypofibrinogenemia, or demonstrated hemophagocytosis. Thus, no patient fulfilled the diagnostic criteria for hemophagocytic lymphohistiocytosis.
Extracutaneous or systemic involvement by NTRK1-rearranged histiocytosis. (A) Liver involvement in a female newborn (AP27). (B-C) Kidney and bone involvement in a male newborn (PK1). (D-E) Large nodule obstructing the right main bronchus in a male infant (AP32). (F-G) Histology of liver and bronchia involvement (hematoxylin and eosin, original magnification ×20).
Extracutaneous or systemic involvement by NTRK1-rearranged histiocytosis. (A) Liver involvement in a female newborn (AP27). (B-C) Kidney and bone involvement in a male newborn (PK1). (D-E) Large nodule obstructing the right main bronchus in a male infant (AP32). (F-G) Histology of liver and bronchia involvement (hematoxylin and eosin, original magnification ×20).
Of the remaining 2 patients, 1 was a male infant with a single nodule that obstructed the right main bronchus who was diagnosed at 8 months of age after presenting with recurrent bronchiolitis and dyspnea (Figure 2). The other patient was a 15-year-old female who presented with a single brain nodule while in complete remission for previous B-cell acute lymphoblastic leukemia (B-ALL). This teenager had a germline pathogenic variant of ETV6 and had received blinatumomab (a CD19/CD3 bispecific T cell engager) and undergone brain irradiation for her B-ALL, as previously reported.33
Clinical outcomes
Among patients with isolated skin lesions, R0 (complete resection) or R1 (positive margin) excisions were performed in 23 and 21 cases, respectively. No relapse was observed in any of the 24 patients for whom clinical follow-up data were available (median follow-up, 31.1 months; range, 0.5-164.4 months). Two patients with multiple skin lesions experienced spontaneous regression of the nodules that were not resected; these lesions were not present anymore at 11 and 40 months after diagnosis.
The 4 newborns with systemic disease were all hospitalized and required platelet transfusions. Three patients received only supportive care with subsequent spontaneous regression of their histiocytosis within 7 months. The other case received chemotherapy with vinblastine and prednisone for 1 year with complete resolution of disease within this period.
Finally, the 2 patients with isolated, extracutaneous, life-threatening tumors in the right main bronchus or brain were treated with the pan-TRK inhibitor larotrectinib. The 8-month-old infant with the bronchial NTRK1-rearranged JXG had previously undergone 4 bronchoscopies with partial resection of the endoluminal tumor. He subsequently received 40 mg twice daily of larotrectinib, with rapid clinical response and discharge from the hospital 1 week after start of treatment. At a control bronchoscopy 3 months after treatment initiation, the tumor had completely regressed, and the child remains without clinical symptoms 8 months after starting targeted therapy. The teenager with the NTRK1-rearranged CNS-JXG received 200 mg/day of larotrectinib. She had significant clinical improvement at 2 months, and a very good partial response by magnetic resonance imaging at 10 months of treatment.33 Larotrectinib was stopped after 19 months without evidence of relapse 7 months later.
Histopathologic features and immunophenotype
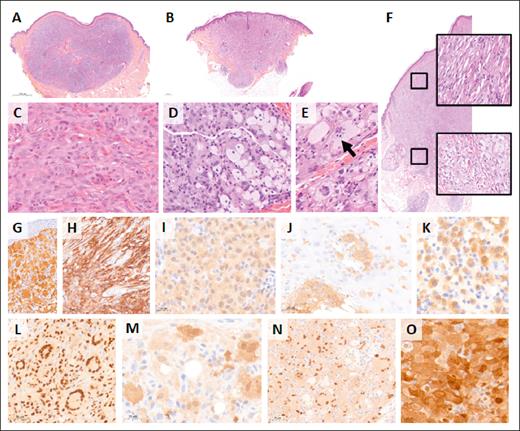
Skin lesions mostly comprised well-circumscribed nodules (33/38; 87%) in the dermis (28/40; 70%) with pushing borders (33/41; 80%) and without epidermotropism (37/47; 79%) (Table 3). However, 8 of 41 cases (20%) displayed a more infiltrative aspect with finger-like borders. The cytological features were rather uniform; histiocytes were frequently spindle shaped or epithelioid. Among the 50 cases, 39 were classified as mature (81%) lesions with typical lipidization and Touton giant cells and 9 were classified as early lesions without lipidization. One case had an early aspect of the superficial part of the XG with typical mature aspect of the deeper part of the excision (Figure 3). Another case (US7) had an early aspect on the first shave biopsy, initially misdiagnosed as myeloid sarcoma, but mature histology on excision with typical Touton giant cells. The cytoplasm in our cases was always eosinophilic with frequent xanthomization (foamy cells) (34/50; 68%) and never granular. The nuclei were often folded or indented (45/50; 90%) and hypochromatic (49/50; 98%) with mild anisokaryosis (39/49; 80%). The nucleoli were generally small when noted. Numerous mitoses were present in early lesions, and one of these displayed a high proliferation index of >50%. Multinucleated histiocytes were common (37/50; 74%), some of which were characteristic of Touton giant cells (26/50, 52%). Emperipolesis was observed in half of the cases (23/50; 46%) and always involved multinucleated cells The stroma always contained an inflammatory infiltrate, primarily composed of lymphocytes (46/50; 92%). None of the cases contained typical RDD cells, which are defined as large cells that harbor a large nucleus with clear chromatin and a prominent nucleolus and with abundant clear cytoplasm with emperipolesis.
Immunochemistry and histologic findings
| Patients . | N = 50 . |
|---|---|
| Peripheral limitation, n (%) | |
| Well circumscribed | 33 (87) |
| Irregular | 2 (5) |
| Invasive | 3 (8) |
| N/A | 12 |
| Pushing borders, yes/no, n (%) | 33/8 (80/20) |
| N/A | 9 |
| Epidermotropism, n (%) | |
| Absent | 37 (79) |
| Present, occasional | 9 (19) |
| Present, frequent | 1 (2) |
| N/A | 3 |
| Deepness of infiltration, n (%) | |
| Only upper- and mid-dermis | 1 (2) |
| Deep dermis | 27 (68) |
| Hypodermis | 12 (30) |
| N/A | 10 |
| Foamy histiocytes, n (%) | |
| Absent | 16 (32) |
| <50% of histiocytes | 27 (54) |
| >50% of histiocytes | 7 (14) |
| Touton giant cells/non-Touton giant cells, n (%) | |
| Absent | 24/13 (48/26) |
| Few | 16/14 (32/28) |
| Numerous | 10/23 (20/46) |
| Emperipolesis in multinucleated cells/nonmultinucleated cells, n (%) | |
| Absent | 27/35 (54/70) |
| Few | 12/10 (24/20) |
| Moderate | 6/4 (12/8) |
| Numerous | 5/1 (10/2) |
| Nucleus size, n (%) | |
| <2× lymphocyte’s size | 8 (16) |
| 2-3× lymphocyte’s size | 37 (74) |
| >3× lymphocyte’s size | 5 (10) |
| Chromatin, hypochromatism/hyperchromatism, n (%) | 49/1 (98/2) |
| Folded/indented nuclei, n (%) | |
| Absent | 5 (10) |
| Rare | 37 (74) |
| Frequent | 8 (16) |
| Anisocaryosis, n (%) | |
| Absent | 4 (8) |
| Rare | 40 (80) |
| Frequent | 6 (12) |
| Nucleoli, n (%) | |
| Conspicuous at 40× magnification | 42 (84) |
| Conspicuous at 10× magnification | 7 (14) |
| Conspicuous at 2.5× magnification | 1 (2) |
| Cytoplasm eosinophilic/basophilic, n (%) | 50/0 (100/0) |
| Cytoplasmic abundance, n (%) | |
| Scant cytoplasm | 9 (18) |
| Moderate abundance | 35 (70) |
| Abundant cytoplasm | 6 (12) |
| Granular cytoplasm, n (%) | 0 (0) |
| Stroma, n (%) | |
| Neutrophils | 4 (8) |
| Eosinophils | 22 (44) |
| Lymphocytes | 46 (92) |
| Plasma cells | 1 (2) |
| Siderophages, n (%) | 2 (4) |
| Early-like lesions, yes/no, n (%) | 10/39 (20/80) |
| Pan-TRK immunochemistry, n (%) | |
| 4+ | 15 (30) |
| 3+ | 14 (28) |
| 2+ | 12 (24) |
| 1+ | 7 (14) |
| Positive but intensity N/A | 2 (4) |
| Patients . | N = 50 . |
|---|---|
| Peripheral limitation, n (%) | |
| Well circumscribed | 33 (87) |
| Irregular | 2 (5) |
| Invasive | 3 (8) |
| N/A | 12 |
| Pushing borders, yes/no, n (%) | 33/8 (80/20) |
| N/A | 9 |
| Epidermotropism, n (%) | |
| Absent | 37 (79) |
| Present, occasional | 9 (19) |
| Present, frequent | 1 (2) |
| N/A | 3 |
| Deepness of infiltration, n (%) | |
| Only upper- and mid-dermis | 1 (2) |
| Deep dermis | 27 (68) |
| Hypodermis | 12 (30) |
| N/A | 10 |
| Foamy histiocytes, n (%) | |
| Absent | 16 (32) |
| <50% of histiocytes | 27 (54) |
| >50% of histiocytes | 7 (14) |
| Touton giant cells/non-Touton giant cells, n (%) | |
| Absent | 24/13 (48/26) |
| Few | 16/14 (32/28) |
| Numerous | 10/23 (20/46) |
| Emperipolesis in multinucleated cells/nonmultinucleated cells, n (%) | |
| Absent | 27/35 (54/70) |
| Few | 12/10 (24/20) |
| Moderate | 6/4 (12/8) |
| Numerous | 5/1 (10/2) |
| Nucleus size, n (%) | |
| <2× lymphocyte’s size | 8 (16) |
| 2-3× lymphocyte’s size | 37 (74) |
| >3× lymphocyte’s size | 5 (10) |
| Chromatin, hypochromatism/hyperchromatism, n (%) | 49/1 (98/2) |
| Folded/indented nuclei, n (%) | |
| Absent | 5 (10) |
| Rare | 37 (74) |
| Frequent | 8 (16) |
| Anisocaryosis, n (%) | |
| Absent | 4 (8) |
| Rare | 40 (80) |
| Frequent | 6 (12) |
| Nucleoli, n (%) | |
| Conspicuous at 40× magnification | 42 (84) |
| Conspicuous at 10× magnification | 7 (14) |
| Conspicuous at 2.5× magnification | 1 (2) |
| Cytoplasm eosinophilic/basophilic, n (%) | 50/0 (100/0) |
| Cytoplasmic abundance, n (%) | |
| Scant cytoplasm | 9 (18) |
| Moderate abundance | 35 (70) |
| Abundant cytoplasm | 6 (12) |
| Granular cytoplasm, n (%) | 0 (0) |
| Stroma, n (%) | |
| Neutrophils | 4 (8) |
| Eosinophils | 22 (44) |
| Lymphocytes | 46 (92) |
| Plasma cells | 1 (2) |
| Siderophages, n (%) | 2 (4) |
| Early-like lesions, yes/no, n (%) | 10/39 (20/80) |
| Pan-TRK immunochemistry, n (%) | |
| 4+ | 15 (30) |
| 3+ | 14 (28) |
| 2+ | 12 (24) |
| 1+ | 7 (14) |
| Positive but intensity N/A | 2 (4) |
Histology and immunophenotype of NTRK1-rearranged histiocytosis. Low magnification photomicrographs of 2 exemplary skin lesions with either a well-limited pushing nodule in the dermis (A; AP30) or a more infiltrative lesion with finger-like borders (B; AP16). Histiocytes were medium sized with eosinophilic cytoplasm in early type lesions (C; AP11), whereas mature lesions contained larger mononucleated cells with a foamy cytoplasm (D; AP30) and multinucleated Touton giant cells (E; AP17). Some multinucleated cells demonstrated emperipolesis (arrow). Case AP25 was early type in the upper part and mature in the deep dermis (F). (A-F) Hematoxylin and eosin staining. Immunohistochemistry with pan-TRK (G-K) showing strong expression of multinucleated cells (G; AP19) and mononucleated spindle cells (H; AP31) and milder expression in an early type lesion (I; AP32). Positive cells were detectable within the epidermis in a few cases (J; AP12). In this early type lesion, only a fraction of the mononucleated histiocytes expressed pan-TRK (K; AP09). Cells were positive with histiocyte markers, such as PU.1 (L; AP10), and some also expressed S100 (M; AP24). Frequent positivity for cyclin D1 (N; AP30) and phosphorylated extracellular signal-regulated kinase (phophoERK) (O; AP32) confirmed the activation of the MAPK pathway in these neoplasms with NRTK1 fusions.
Histology and immunophenotype of NTRK1-rearranged histiocytosis. Low magnification photomicrographs of 2 exemplary skin lesions with either a well-limited pushing nodule in the dermis (A; AP30) or a more infiltrative lesion with finger-like borders (B; AP16). Histiocytes were medium sized with eosinophilic cytoplasm in early type lesions (C; AP11), whereas mature lesions contained larger mononucleated cells with a foamy cytoplasm (D; AP30) and multinucleated Touton giant cells (E; AP17). Some multinucleated cells demonstrated emperipolesis (arrow). Case AP25 was early type in the upper part and mature in the deep dermis (F). (A-F) Hematoxylin and eosin staining. Immunohistochemistry with pan-TRK (G-K) showing strong expression of multinucleated cells (G; AP19) and mononucleated spindle cells (H; AP31) and milder expression in an early type lesion (I; AP32). Positive cells were detectable within the epidermis in a few cases (J; AP12). In this early type lesion, only a fraction of the mononucleated histiocytes expressed pan-TRK (K; AP09). Cells were positive with histiocyte markers, such as PU.1 (L; AP10), and some also expressed S100 (M; AP24). Frequent positivity for cyclin D1 (N; AP30) and phosphorylated extracellular signal-regulated kinase (phophoERK) (O; AP32) confirmed the activation of the MAPK pathway in these neoplasms with NRTK1 fusions.
The bronchial (AP32) and brain (US13) lesions had histology similar to the skin lesions of other patients but with mild nuclear atypia (US13). In one of the newborns with systemic disease (AP24), a liver biopsy showed preserved architecture without fibrosis. Intrasinusoidal hematopoiesis and marked lobular cholestasis were observed. One portal tract was enlarged and infiltrated by mononucleated histiocytes with a pale cytoplasm, which is associated with fibrosis (Figure 2).
All cases were positive for CD163 and/or CD68. Most expressed Factor XIIIa (13/15 cases; 87%) and phosphorylated extracellular signal-regulated kinase (25/28 cases; 89%). S100 was positive in 16 of 23 cases (70%) with variable light to moderate staining.
Pan-TRK immunostaining
Altogether, 95 samples of histiocytosis were studied by both pan-TRK immunohistochemistry and targeted RNA sequencing. All 46 samples that stained positive for pan-TRK and that had a molecular analysis performed had an in-frame fusion of NTRK1 detected by RNA sequencing. The cytoplasmic staining intensity with pan-TRK was variable; 29 were 3 to ≥4 strong; 12 were moderate, and 7 were weak. All 45 cases that stained negative for pan-TRK did not have an NTRK gene fusion, except for 1 that had a KCTD5::NTRK1 fusion that was not in-frame, explaining the absence of pan-TRK expression. Thus, pan-TRK immunostaining with clone EPR17341 was 100% sensitive and 100% specific for the presence of in-frame NTRK fusions in histiocytosis, albeit with variable cytoplasmic staining intensity.
Pan-TRK immunostaining was cytoplasmic and often homogenous within the lesion (35/50; 70%). The intensity of the staining was variable between cases. Expression also varied within some lesions by cell type. Touton-type giant cells and other multinucleated cells had the strongest expression, whereas mononucleated histiocytes had less expression, and foamy mononucleated cells had the weakest expression, presumably because of the lipid-rich cytoplasm. The case with an early lesion at the surface and a mature aspect in the deeper part also displayed this variation with strong expression in the superficial part and weaker expression in the lower part that was characterized by many foamy cells.
NTRK fusions
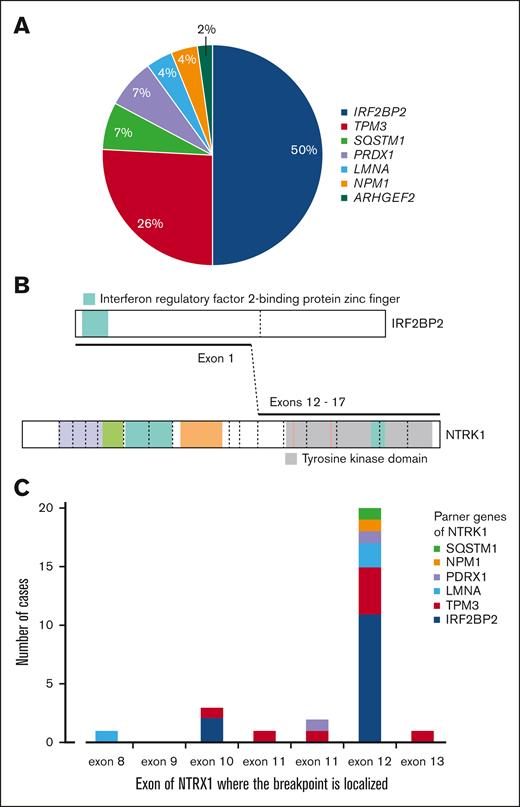
All in-frame fusions involved NTRK1. There were 7 different partner genes with IRF2BP2 being the fusion partner in about half of the cases, followed by TPM3 in one-quarter of cases (Figure 4A-B). The IRF2BP2::NTRK1 fusion was confirmed at the complimentary DNA level in 3 cases by polymerase chain reaction (supplemental Data). There was no correlation between partner genes and the intensity of pan-TRK immunostaining, other histopathologic features, or clinical characteristics.
NTRK1 rearrangements. (A) Frequencies of fusion partner genes. (B) Illustration of the most frequent fusion. (C) Localizations of the fusion breakpoints at the NTRK1 locus.
NTRK1 rearrangements. (A) Frequencies of fusion partner genes. (B) Illustration of the most frequent fusion. (C) Localizations of the fusion breakpoints at the NTRK1 locus.
Each sample with IRF2BP2::NTRK1 had at least 2 variants of fusion, which prompted us to analyze the next-generation sequencing data of complimentary DNA in detail. On IRF2BP2, 2 different breakpoints were identified at the end of exon 1; breakpoint chr1:234744241 was always more abundant than breakpoint chr1:234744193. These breakpoints corresponded to known splice sites of IRF2BP2 (NM_001077397.1 and NM_182972.2, respectively). On NTRK1, the most frequent splice site was chr1:156845312 on exon 12 (NM_002529.3). However, this breakpoint was not detected in 2 of 13 cases (AP25 and AP29). These 2 cases contained a major breakpoint in exon 10 (chr1:156844363) and a minor breakpoint in exon 9 (chr1:156844175). One case with the common NTRK1 exon 12 breakpoint also contained a minor breakpoint in exon 13 of NTRK1 (chr1:156845872).
Cases with NTRK1 fusions to other partner genes were less abundant, but some of them also contained >1 fusion variant. On NTRK1, most cases had a major breakpoint in exon 12 or within intron 11 just upstream of exon 12 (Figure 4C).
Discussion
Through international collaboration, we present a comprehensive study of NTRK-rearranged histiocytosis with detailed clinicopathologic data of 50 cases with pan-TRK expression, including 46 cases with confirmed NTRK1 rearrangements. Our study demonstrates that NTRK1 fusions predominantly occur in isolated cutaneous XG family lesions but may occasionally drive more severe extracutaneous and/or systemic histiocytosis in young children, albeit often with a more indolent course than systemic LCH. Histologic features were always those of the XG family, ranging from early-phase lesions composed of small mononucleated, nonlipidized histiocytes to more mature lesions rich in xanthomatous histiocytes and Touton giant cells. Although only NTRK1 fusions were detected, gene fusion partners included various genes, including IRF2BP2 and TPM3. Immunohistochemistry with the pan-TRK clone EPR17341 was 100% sensitive and specific for the detection of in-frame NTRK fusions with a range of cytoplasmic intensity, which facilitated early screening for the diagnosis of NTRK-rearranged histiocytosis. Finally, although systemic disease in newborns may resolve spontaneously with supportive therapy, targeted therapy is highly effective in the treatment of extracutaneous manifestations of NTRK1-rearranged histiocytosis as evidenced by 2 patients with life-threatening tumors in the bronchus or brain who were successfully treated with the TRK inhibitor larotrectinib.
In our series, NTRK-rearranged histiocytosis was twice as frequent in males than in females, which is in accordance with previous findings in JXG with a reported male to female ratio of 1.3:1 overall and 12:1 when considering the disseminated form.7 This male predominance was also noted when reviewing published NTRK-rearranged histiocytosis cases (1.62:1)15,25,27-35 and is in accordance with findings in other histiocytoses, including LCH and ECD.2 In contrast, ALK-positive histiocytosis, which may also exhibit XG morphology, is characterized by a female predilection (0.63:1).24 The median age at the time of diagnosis in our series was 11.5 years as opposed to 13 years15,25,27-35 in previously published cases of NTRK-rearranged histiocytosis. All but 2 of our cases had skin involvement, demonstrating a strong predilection for the skin of NTRK-rearranged histiocytosis, even in the setting of systemic manifestation. Cutaneous involvement was also noted in 32 of 33 published cases of histiocytosis with pan-TRK expression and/or NTRK rearrangement.15,25,27-35 Although diverse histiocytoses can involve the skin, including LCH, ECD, and RDD,36 such a strong predilection for this organ is a distinguishing feature. Interestingly, the skin is involved in <1% of solid tumors with NTRK fusions in adults and in <4% of infants.26 Predilections for specific organs have also been observed in other histiocytoses that are characterized by particular genetic alterations, such as the predilection for neurologic involvement in ALK-positive histiocytosis,24 testicular involvement in mixed ECD/RDD with MAP2K1 mutations,37 and pulmonary (children)18 or hepatic and vulvar (adults)38 involvement in LCH driven by BRAF exon 12 deletions. These findings highlight the intimate relationship between molecular pathogenesis and the clinicopathologic histiocytosis phenotype.
The clinical outcomes of patients with NTRK-rearranged histiocytosis confined to the skin were highly favorable with no relapse in any of the 26 patients with available follow-up. Moreover, spontaneous regression of unresected skin nodules was observed in 2 patients with multifocal cutaneous involvement. Similarly, melanomas with NTRK fusions, which generally occur in young patients and often manifest on the face, are also associated with indolent behavior.39NTRK-rearranged histiocytosis in infants with systemic disease was associated with favorable outcomes, which is further supported by a recently published case with cutaneous and hepatic involvement.25 Despite abnormal laboratory tests with anemia, thrombocytopenia and liver dysfunction with hyperbilirubinemia and coagulopathy, these infantile systemic disease presentations do surprisingly well with supportive care or standard LCH-based chemotherapy, which is reminiscent of the infantile manifestation of ALK-positive histiocytosis.24,40 Although children with ALK-positive histiocytosis often did not have skin lesions and fulfilled more hemophagocytic lymphohistiocytosis criteria (suggestive of more profound systemic inflammation) (supplemental Table 2), spontaneous disease regression was regularly observed in both groups of patients with non-LCH despite so-called risk-organ involvement according to LCH criteria (defined as liver, spleen, and/or bone marrow involvement). Notably, some infants with ALK-positive histiocytosis had progressive disease despite vinblastine/prednisolone chemotherapy, including 1 who died of progressive disease24; none had received ALK inhibitor therapy. Interestingly, the infant with systemic NTRK1-rearranged histiocytosis reported by Kim et al was successfully treated with larotrectinib.25 As in our 2 patients treated with this agent, response to larotrectinib was rapid and occurred within 2 weeks of treatment. Although targeted therapies are highly efficient in the treatment of diverse histiocytic neoplasms,21-23 stopping inhibitors can quickly lead to relapse of the disease.22,41,42 However, because JXG lesions are known to frequently regress spontaneously, this particular disease may be less prone to relapse when targeted therapy is stopped. Supporting this notion, patient US13 still has no evidence of disease at 5 months after discontinuation of larotrectinib.
Only one of our patients had NTRK-rearranged histiocytosis and another hematologic neoplasm (US13).33 In contrast, other histiocytoses are frequently associated with other hematologic neoplasms, which occur in up to 40% of patients with indeterminate dendritic cell histiocytosis, in 28% of patients with malignant histiocytosis, 10% of patients with ECD, and 5% of adults with LCH.43-47 This patient was in complete remission for her B-ALL at diagnosis of CNS-JXG.33 Although histiocytic neoplasms that occur after ALL are generally associated with a poor prognosis,48 our patient had a complete response to the pan-TRK inhibitor larotrectinib. Thus, the adoption of targeted therapy may represent a turning point in the prognosis of patients with secondary non-malignant histiocytic neoplasms that are not considered malignant histiocytosis.
Although all cases had histologic features within the XG family, some findings were atypical. Whereas XG family lesions are usually characterized by the absence of epidermotropism with a grenz zone between the dermal histiocytic lesion and epidermis, we observed epidermotropism in 10 of 47 skin lesions (21%). Importantly, histiocytes within the epidermis were highlighted by pan-TRK immunostaining, confirming their neoplastic origin (Figure 3). Most of these lesions (6/10 cases) demonstrated excoriation or ulceration with reactive changes of the epidermis (Figure 3). Thus, scratching may trigger migration of NTRK1-rearranged histiocytes into the epidermis. In addition, we hypothesize that histiocyte maturation may be influenced by interactions with the accompanying inflammatory infiltrate, because maturation was generally observed in the deeper parts at the lesional borders. Finally, emperipolesis, which is a hallmark of RDD, can be present (n = 23/50; 46%) or even abundant (5/50; 10%), and some of these cases also expressed S100. However, none of our NTRK-rearranged histiocytosis cases contained typical RDD cells. S100 expression has already been reported in some XG.49
Consistent with the recommendation by the European Society for Medical Oncology to use immunohistochemistry as a screening tool,50 we demonstrated that pan-TRK staining with clone EPR17341 can be used to screen for NTRK fusions in histiocytosis. Pathologists have to be aware that the staining is sometimes of low/light intensity (1+) and that the intensity of staining may be particularly limited when tissue sections were cut from the formalin-fixed paraffin-embedded block more than 1 month before staining (J.-F.E., personal experience, data not shown). Because the specificity of pan-TRK immunohistochemistry was 100% in our study and others,51 fluorescence in situ hybridization or RNA sequencing may not be necessary to confirm the diagnosis of NTRK-rearranged histiocytosis; however, we recommend molecular confirmation in cases with atypical clinical and/or pathologic features or when systemic therapy is in consideration. NTRK-rearranged histiocytosis accounted for 13.4% of the 194 C-group histiocytoses evaluated in the Ambroise Paré Hospital between June 2018 and December 2024 (supplemental Figure 2); thus, we recommend systematic screening of cutaneous XG with pan-TRK immunohistochemistry.
Although different NTRK genes (1/2/3) are involved in solid tumors, NTRK2 and NTRK3 were not rearranged in our cases. We detected 7 different genes with an in-frame fusion with NTRK1 with IRF2BP2 presenting in almost half of our cases and TPM3 presenting in one-quarter. In solid tumors, ETV6::NTRK3 is the most common NTRK fusion (27.2%), followed by TPM3::NTRK1 (21.5%) and LMNA::NTRK1 (9.5%).52 Interestingly, IRF2BP2 represents less than 2% of fusion partner genes in solid tumors.52 Thus, the landscape of fusion partners seems to differ by tumor type. Accordingly, KIF5B::ALK fusions are highly prevalent in ALK-positive histiocytosis but are rarely detected in other ALK-rearranged tumors.24,53 Moreover, ETV3::NCOA2 and CLTC::SYK fusions have exclusively been reported in histiocytic neoplasms.6,43,47,54,55
Our study has several limitations. As a retrospective study performed in referral centers, it may be limited by selection bias. Moreover, staging was performed according to standard-of-care practices in different institutions and countries and based on clinician judgement. In addition, some tissue samples were exhausted or badly fixed, preventing the successful identification of NTRK fusions and/or gene fusion partners through RNA sequencing. Finally, clinical information and/or follow-up data could not be retrieved for all cases. Nevertheless, our study represents the largest series, to our knowledge, of NTRK-rearranged histiocytosis to date with central pathology review by expert pathologists and molecular confirmation of NTRK fusions in 46 of 50 cases.
Conclusion
We describe a large series of molecularly confirmed NTRK1-rearranged histiocytosis. Although the most frequent manifestation was that of isolated or multiple cutaneous XGs, systemic disease of newborns with skin lesions, together with hepatomegaly and thrombocytopenia, may occur. In addition, NTRK1 fusions may sometimes be associated with extracutaneous histiocytic tumors with XG histology, such as tumors in the bronchus or brain and rarely after a previous leukemia. Spontaneous regression of NTRK1-rearranged histiocytosis is frequent, even for the infantile systemic form, which may initially seem clinically aggressive. Moreover, targeted therapy with the TRK inhibitor larotrectinib is highly effective in patients with serious extracutaneous manifestations. NTRK fusions only involved NTRK1 and various gene partners; pan-TRK immunohistochemistry can be used to screen for the presence of these oncogenic in-frame fusions. Together, this study advances the molecular understanding of histiocytic neoplasms and may provide guidance for the diagnosis and clinical management of patients with these rare diseases.
Acknowledgments
The authors thank all the technicians of the Histopathology and Molecular Pathology Laboratory and Biobank of Ambroise Paré Hospital, and the Pathology Laboratory of Cincinnati Children’s Hospital, including Chris Woods and Margot J.A.M. Tegelberg-Stassen (Department of Dermatology, Diakonessenhuis Hospital, Utrecht, The Netherlands), for providing clinical information for one of the cases.
This study was funded, in part, by grants from Programme de Recherche Translationnelle sur le Cancer PRTK 19-143 and Association pour la Recherche et l’Enseignement en Pathologie.
Authorship
Contribution: R.F., S.F., P.G.K., J.L.P., and J.-F.E. designed the research, reviewed the slides, collected and analyzed the data, and wrote the manuscript; Z.H.-R. and J.-F.E. performed the molecular analyses; S.R., B.B., A.L.B., S.D., M.J., J.Z., K.S., L.K., A.C.H.d.V., R.M.V., J.A.M.v.L., R.J.L., P.D., E.D.C., J.H., A.D.K., M.S., M.-L.J., F.B., R.L., A.G.S.v.H., S.H., E.L.D., B.H.D., A. Bhattacharya, J.H., J.D., and A. Borkhardt provided data for patients, contributed to the analysis of data, and corrected the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer L. Picarsic, Department of Pathology, University of Pittsburgh School of Medicine, UPMC Children’s Hospital, 4401 Penn Ave, Main Hospital-Lab-B 260, Pittsburgh, PA 15224; email: jenpicarsic@gmail.com; and Jean-François Emile, Service de Pathologie, Hôpital Ambroise-Paré, 9 Ave Charles de Gaulle, 92104 Boulogne, France; email: jean-francois.emile@uvsq.fr.
References
Author notes
R.F., S.F., and P.G.K. contributed equally to this study.
Data are available on reasonable request from the corresponding authors, Jennifer L. Picarsic (jenpicarsic@gmail.com) and Jean-François Emile (jean-francois.emile@uvsq.fr).
The full-text version of this article contains a data supplement.