Key Points
Flow cytometry enhances morphological analysis to detect AML blasts with monocytic differentiation (monoblasts).
A high monoblasts/CD45+ proportion predicts poor response and reduced survival in newly diagnosed patients with AML receiving Ven-Aza.
Visual Abstract
The prognostic impact of monocytic differentiation in patients with acute myeloid leukemia (AML) receiving venetoclax (Ven) and azacitidine (Aza) remains unclear. In a prospective cohort of 86 newly diagnosed patients with AML treated with Ven-Aza, we used multiparametric flow cytometry (MFC) to define monoblasts as AML blasts coexpressing ≥2 monocytic markers (CD4, CD36, and CD64) per European LeukemiaNet (ELN) guidelines. Patients with higher monoblasts/CD45+ proportions had lower complete response rates (odds ratio, 0.24; P = .005) and significantly shorter overall survival (OS; 4.0 vs 14.9 months; P = .003). A ≥10% monoblasts/CD45+ threshold, identified via maximally selected rank statistics, stratified patients into monoblasthigh (≥10%) and monoblastlow (<10%) groups. MFC reclassified 20% of French-American-British (FAB) non-M4/5 and 15% of FAB M4/5 cases into monoblasthigh and monoblastlow groups, respectively. Multivariable analysis confirmed monoblasthigh status as an independent adverse prognostic factor for OS (hazard ratio [HR], 1.95; P = .023), with a particularly strong impact in ELN 2024 favorable-risk patients (HR, 2.81; P = .024). Our findings highlight monocytic differentiation, assessed via MFC, as a key predictor of Ven-Aza resistance and poor survival, independent of genetic classification. Given its availability in routine diagnostics, MFC-based monocytic assessment could improve AML risk stratification and treatment decisions in patients eligible for less intensive therapies. This trial was registered at www.clinicaltrials.gov as #NCT05326919.
Introduction
Venetoclax (Ven) combined with azacitidine (Aza) has become the standard of care for patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy owing to advanced age or comorbidities.1 Understanding the characteristics that influence response to Ven-Aza is critical, because 30% to 50% of newly diagnosed patients exhibit primary resistance.1
The recently published European LeukemiaNet (ELN) 2024 recommendations underscored the prognostic value of a 5-gene classifier (NPM1, TP53, N/KRAS, and FLT3-ITD) in predicting outcomes of patients with AML receiving Ven-Aza as frontline therapy.2 This classifier outperformed the ELN 2022 classification in predicting outcomes for patients treated with nonintensive strategies and was independently validated.3,4
Beyond genetic alterations, the differentiation state of AML blasts (ie, phenotype) may also influence their sensitivity to Ven-Aza.5,6 Although conflicting data have suggested that monocytic differentiation, as determined by morphology (French-American-British [FAB] M4/5 AMLs7) or multiparametric flow cytometry (MFC), contributes to primary resistance,8-10 its prognostic significance remains uncertain. In addition, whether monocytic differentiation affects outcomes independently of molecular alterations, particularly within the ELN 2024 classification framework, is unclear. In this study, we assess the prognostic relevance of monocytic differentiation, using both morphology and MFC in a single-center cohort of newly diagnosed patients with AML uniformly treated with Ven-Aza.
Patients and methods
Patients, treatment, and response assessment
Between January 2019 and January 2024, we enrolled 86 newly diagnosed, treatment-naïve, patients with nonpromyelocytic AML (aged ≥18 years) in our registry (Leukemia Institute Paris Saint-Louis). These patients received at least 1 cycle of Ven-Aza and underwent baseline morphological, cytogenetic, genomic, and MFC-based phenotypic assessments. Notably, our patient cohort accrues all consenting newly diagnosed patients with AML, regardless of clinical or molecular characteristics. Only those patients without health care coverage are ineligible. Due to national regulatory constraints, ethnicity data were not collected. The study was approved by an ethics committee (IDRCB 2021-A00940-41). Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Patients were treated according to the standard Ven-Aza regimen.1 During cycle 1, patients were hospitalized for Ven ramp-up and concomitant treatment of subcutaneous Aza. If a strong CYP3A inhibitor (ie, azole antifungals) was required after dose escalation, Ven was decreased from 400 to 100 mg; no adjustments were made for concomitant moderate CYP3A inhibitors or p-glycoprotein inhibitors. Dose modification/discontinuation of Ven-Aza during subsequent cycles occurred at the discretion of the treating physician.
We evaluated the best treatment responses to Ven-Aza based on the ELN 2022 criteria.4 Complete remission (CR) was defined as the absence of excess medullary or circulating blasts, accompanied by hematologic recovery (absolute neutrophil count ≥1 × 109/L and platelets ≥100 × 109/L). The overall response encompassed CR, CR with partial or incomplete hematologic recovery, and partial responses. The “per protocol” patient population includes only those patients who underwent bone marrow evaluation, whereas the “intent-to-treat” population encompasses all patients, with those lacking bone marrow evaluation classified as treatment failures.
AML classification and genetic analysis
AML diagnoses were classified according to International Consensus Classification criteria.11 AML morphology was reviewed by 2 independent assessors (T.D.-R. and P.L.), who were blinded for MFC, and was classified based on the FAB classification.7
Targeted next-generation sequencing was performed using a panel of 86 genes recurrently mutated in myeloid malignancies (supplemental Table 1), following previously described methods.12 Patients were categorized according to the ELN 2024 classification as “adverse,” “intermediate,” or “favorable” based on their genetic profile.13 The “adverse” group included those with TP53 mutations, and the “intermediate” group comprised patients with FLT3-ITD and/or N/KRAS mutations without TP53 mutation, whereas all other genotypes were classified as “favorable.”
Assessment modalities in MFC
MFC was performed using a FACSCanto II (BD Biosciences) with 8-color panels (supplemental Table 2). The preanalytical and analytical procedures followed the ELN guidelines for MFC,14 ensuring standardized sample preparation, staining protocols, data acquisition, and analysis. Briefly, bone marrow samples were prepared through a bulk lysis process to eliminate red blood cells, ensuring optimal sample integrity. To maintain the consistency and quality of our flow cytometry analyses, all samples were collected in EDTA tubes, which enable prolonged preservation at ambient temperature without affecting antigen expression.15 This was followed by an initial wash to remove debris, a staining step to label specific cellular markers, and a final wash to eliminate unbound reagents.14 In addition, all analyses were conducted within 24 hours of sampling to ensure optimal data reliability.
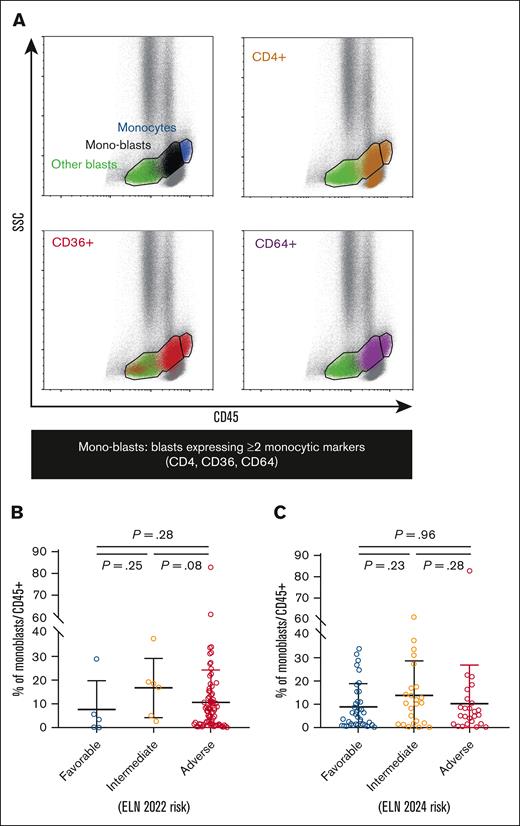
After excluding debris and doublets, live cells and mononuclear cells were selected based on size and structure using forward and side scatter (SSC) parameters. AML blasts were initially gated as CD45low/int/SSClow/int immature cells, after the exclusion of CD45high/SSCint mature monocytes. Among these, monocytic blasts (hereafter referred to as monoblasts) were identified using a Boolean gating strategy, requiring the coexpression of at least 2 differentiation markers from the monocytic lineage among CD4, CD36, and CD64, as recommended by ELN guidelines4 (Figure 1A). Throughout the study, the proportion of monoblasts relative to the total number of CD45+ cells (referred to as monoblasts/CD45+) was investigated as a potential biomarker of response to Ven-Aza.
Gating of mono-blasts and distribution according to ELN risk categories. (A) CD45/SSC scatterplots of an illustrative case with FAB M4 AML, with backgating of expression of monocytic markers CD4 (brown), CD36 (red), and CD64 (purple). After exclusion of CD45high/SSCint mature monocytes (blue population), CD45low/int/SSClow-int gated AML blasts are divided into monoblasts expressing ≥2 of these monocytic markers (black population) and other blasts (green population). The proportion of monoblasts among total CD45+ hematopoietic cells (monoblasts/CD45+) is inspected as a biomarker of Ven-Aza. (B-C) Scatterplots of monoblasts/CD45+ according to ELN 2022 (B) and ELN 2024 risk categories (C). Bars represent mean value with standard deviation.
Gating of mono-blasts and distribution according to ELN risk categories. (A) CD45/SSC scatterplots of an illustrative case with FAB M4 AML, with backgating of expression of monocytic markers CD4 (brown), CD36 (red), and CD64 (purple). After exclusion of CD45high/SSCint mature monocytes (blue population), CD45low/int/SSClow-int gated AML blasts are divided into monoblasts expressing ≥2 of these monocytic markers (black population) and other blasts (green population). The proportion of monoblasts among total CD45+ hematopoietic cells (monoblasts/CD45+) is inspected as a biomarker of Ven-Aza. (B-C) Scatterplots of monoblasts/CD45+ according to ELN 2022 (B) and ELN 2024 risk categories (C). Bars represent mean value with standard deviation.
Statistical analysis
Continuous variables are reported as medians with interquartile ranges (IQR) and ranges, and categorial variables are reported as numbers with proportions (percent). Pearson χ2 or Fisher exact tests were used to compare categorial variables, depending on event size (≥5 or <5, respectively), to ensure accurate statistical inference.16 Wilcoxon rank sum tests were used to compare continuous variables.
Correlation between monoblasts/CD45+ as a continuous variable and continuous and dichotomic variables was estimated with Pearson and F statistics methods, as well as Mann-Whitney U and Fisher exact tests, respectively.
The follow-up period was estimated using the reverse Kaplan-Meier method. Survival was analyzed using the Kaplan-Meier method. Overall survival (OS) was defined from AML diagnosis to death or last follow-up. Event-free survival (EFS) was defined as the date from AML diagnosis to the date of treatment failure (defined as failure to achieve a response after 2 cycles of Ven-Aza), hematologic relapse from response, or death from any cause, whichever occurred first.4 Given the small number of patients who underwent transplantation (n = 6), allogeneic hematopoietic cell transplantation was not considered a censoring event. Monoblast statuses were compared using the log-rank test for OS and EFS.
Multivariable logistic regressions were performed for categorical variables. Multivariable analyses for survival were performed using Cox proportional hazard models, accounting for conventional prognostic factors, including log10-transformed white blood cell (WBC) count, age at AML diagnosis, and ELN 2024 (or ELN 2022 when indicated) risk classifications. The proportional hazard hypothesis was checked by visual inspection of Schoenfeld residuals. When assessed as a continuous variable, monoblasts/CD45+ value was divided by 10, indicating that the odds ratio (OR) reflects the effect of a 10% increase in the proportion of monoblasts among CD45+ cells. The ELN 2022 and ELN 2024 risk categories were analyzed as ordinal variables, with categories ranked as follows: favorable > intermediate > adverse. Consequently, the reported P values reflect a monotonic trend across these ordered groups. We incorporated all variables from the univariable analysis into the multivariable model, because they were deemed clinically and biologically relevant. Multicollinearity was assessed using the variance inflation factor, with a threshold of variance inflation factor >4 considered unacceptable.17
Maximally selected rank statistics were applied to select a cut point for the monoblasts/CD45+ variable in relation to OS.18 The cut point was determined by maximizing the difference in OS between the 2 groups, with higher values of the test statistic indicating a stronger separation in survival outcomes.
A k-fold crossvalidation approach was applied to a Cox proportional hazards model to assess the impact of monoblast status on OS. We chose a k of 5 to balance bias and variance while ensuring adequate data set representation.19 The model was trained on k-1 folds and tested on the remaining fold, with this process repeated across all folds. Model performance was evaluated using the concordance index, hazard ratio (HR), and P value, because these metrics are more suitable for assessing the goodness of fit of Cox proportional hazards models compared to traditional regression metrics, such as R2 or root mean squared error.20
All tests were 2-sided, and P values <.05 considered as significant. All statistical analyses were performed with R software (version 4.4.1.).
Results
Baseline clinical and biological characteristics
The median age of the cohort was 73 years (IQR, 69-77). Among the 86 study participants, 36 (42%) had AML with myelodysplasia-related gene mutations, whereas only 8 (9%) harbored NPM1 mutations (supplemental Figure 1). According to the ELN 2022 classification,4 75 patients (87%) were categorized as adverse risk. In contrast, the ELN 2024 classification stratified 36 patients (42%) as favorable risk, 24 (28%) as intermediate risk, and 26 (30%) as adverse risk (Table 1). In addition, 26 patients (30%) had a morphological diagnosis of monocytic AML (M4/5; patients with M4, n = 18; patients with M5, n = 8) based on FAB classification.7
Characteristics of the study population at AML diagnosis
| Characteristic . | Overall . | Monoblastshigh . | Monoblastslow . | P value . |
|---|---|---|---|---|
| N = 86 . | n = 34 . | n = 52 . | ||
| Sex at birth | .8 | |||
| Female | 39 (45) | 16 (47) | 23 (44) | |
| Male | 47 (55) | 18 (53) | 29 (56) | |
| Age | 73 [69-77] (20-85) | 73 [69-78] (20-85) | 73 [68-76] (25-85) | .8 |
| WBC, ×109/L | 5 [2-18] (0-229) | 11 [4-23] (1-229) | 4 [2-10] (0-69) | .014 |
| FAB category | <.001 | |||
| M4/5 | 26 (30) | 22 (65) | 4 (8) | |
| Non-M4/5 | 60 (70) | 12 (35) | 48 (92) | |
| Performance status | .3 | |||
| 0-2 | 74 (86) | 31 (91) | 43 (83) | |
| >2 | 12 (14) | 3 (9) | 9 (17) | |
| Clinical ontogeny | ||||
| De novo | 63 (73) | 24 (70) | 39 (75) | .7 |
| Secondary AML | 23 (27) | 10 (30) | 13 (25) | .7 |
| Therapy related | 18 (21) | 7 (21) | 11 (21) | >.9 |
| ELN 2022 classification | .3 | |||
| Favorable | 5 (6) | 1 (3) | 4 (8) | |
| Intermediate | 6 (7) | 4 (12) | 2 (4) | |
| Adverse | 75 (87) | 29 (85) | 46 (88) | |
| ELN 2024 classification | .084 | |||
| Favorable | 36 (42) | 12 (35) | 24 (46) | |
| Intermediate | 24 (28) | 14 (41) | 10 (19) | |
| Adverse | 26 (30) | 8 (24) | 18 (35) | |
| Follow-up, mo | 23.7 [14.9-35.8] (0.5-54.2) | 26.6 [8.1-27.6] (0.5-31.0) | 21.5 [14.9-30] (0.5-54.2) | .01 |
| No. of Ven-Aza cycles | 3 [2-7] (1-25) | 2 [2-4] (1-18) | 5 [2-9] (1-25) | .032 |
| Allogeneic hematopoietic cell transplantation | 6/86 (7) | 0/34 (0) | 6/52 (12) | .077 |
| Characteristic . | Overall . | Monoblastshigh . | Monoblastslow . | P value . |
|---|---|---|---|---|
| N = 86 . | n = 34 . | n = 52 . | ||
| Sex at birth | .8 | |||
| Female | 39 (45) | 16 (47) | 23 (44) | |
| Male | 47 (55) | 18 (53) | 29 (56) | |
| Age | 73 [69-77] (20-85) | 73 [69-78] (20-85) | 73 [68-76] (25-85) | .8 |
| WBC, ×109/L | 5 [2-18] (0-229) | 11 [4-23] (1-229) | 4 [2-10] (0-69) | .014 |
| FAB category | <.001 | |||
| M4/5 | 26 (30) | 22 (65) | 4 (8) | |
| Non-M4/5 | 60 (70) | 12 (35) | 48 (92) | |
| Performance status | .3 | |||
| 0-2 | 74 (86) | 31 (91) | 43 (83) | |
| >2 | 12 (14) | 3 (9) | 9 (17) | |
| Clinical ontogeny | ||||
| De novo | 63 (73) | 24 (70) | 39 (75) | .7 |
| Secondary AML | 23 (27) | 10 (30) | 13 (25) | .7 |
| Therapy related | 18 (21) | 7 (21) | 11 (21) | >.9 |
| ELN 2022 classification | .3 | |||
| Favorable | 5 (6) | 1 (3) | 4 (8) | |
| Intermediate | 6 (7) | 4 (12) | 2 (4) | |
| Adverse | 75 (87) | 29 (85) | 46 (88) | |
| ELN 2024 classification | .084 | |||
| Favorable | 36 (42) | 12 (35) | 24 (46) | |
| Intermediate | 24 (28) | 14 (41) | 10 (19) | |
| Adverse | 26 (30) | 8 (24) | 18 (35) | |
| Follow-up, mo | 23.7 [14.9-35.8] (0.5-54.2) | 26.6 [8.1-27.6] (0.5-31.0) | 21.5 [14.9-30] (0.5-54.2) | .01 |
| No. of Ven-Aza cycles | 3 [2-7] (1-25) | 2 [2-4] (1-18) | 5 [2-9] (1-25) | .032 |
| Allogeneic hematopoietic cell transplantation | 6/86 (7) | 0/34 (0) | 6/52 (12) | .077 |
Frequency (%) and median with (IQR) and ranges [minimum to maximum] are shown for categorial and continuous variables, respectively.
We analyzed the surface expression of monocytic markers on AML blasts, identified as CD45low/int/SSClow-int immature cells, after excluding CD45high/SSCint mature monocytes (Figure 1A). Notably, a significant proportion of AML blasts from FAB non-M4/5 patients expressed monocytic markers, although, as expected, at significantly lower levels than in FAB-M4/5 patients with AML (supplemental Figure 2A).
Following the guidelines,4 we identified monocytic blasts (monoblasts) using a Boolean gating strategy, requiring the coexpression of at least 2 monocytic differentiation markers (among CD4, CD36, and CD64; Figure 1A). Their proportion in CD45+ cells, referred to as monoblasts/CD45+, was quantified for subsequent analyses. The median monoblasts/CD45+ proportion in the entire cohort was 7% (IQR, 2%-14%; supplemental Figure 3). As expected, this proportion was significantly higher in FAB-M4/5 AMLs than non-M4/5 AMLs (17% [IQR, 12%-27%] vs 3% [IQR, 1%-7%]; P < .001; supplemental Figure 2B), although some overlap was observed, with monoblasts detected in certain non-M4/5 AML cases. In contrast, monoblasts/CD45+ proportions were comparable across ELN 2022 (Figure 1B) and ELN 2024 (Figure 1C) risk categories.
Clinical and genetic features associated with monoblasts/CD45+
We then examined the correlation between monoblasts/CD45+ as a continuous variable and baseline clinical and biological covariates. In univariable analyses, higher WBC count (P = .001) and TET2 (P = .003), N/KRAS (P = .026), and ASXL1 (P = .035) mutations were significantly associated with an increased proportion of monoblasts/CD45+ (Figure 2), whereas in multivariable analysis, only higher WBC count (P = .04) but not TET2 (P = .09), N/KRAS (P = .178), and ASXL1 (P = .22) status independently correlated with a higher proportion of monoblasts/CD45+. Neither age (P = .48), sex at birth (P = .55), ELN 2022 (P = .64) or ELN 2024 (P = .84) risk classifications, nor the presence of cytogenetic abnormalities (P = .88) influenced monoblasts/CD45+ distribution.
Clinical and genetic correlates of mono-blasts proportion. Volcano plot representing the association between monoblasts/CD45+ (as a continuous variable) and covariates (x-axis, estimate of Pearson correlation or F statistics method for continuous or dichotomous variables, respectively; the y-axis, P value expression on an inverted log scale for Mann-Whitney U tests and Fisher exact tests for continuous and dichotomous variables, respectively). P values <.05 are highlighted in red. Dot size is proportional to the percentage of patients at risk.
Clinical and genetic correlates of mono-blasts proportion. Volcano plot representing the association between monoblasts/CD45+ (as a continuous variable) and covariates (x-axis, estimate of Pearson correlation or F statistics method for continuous or dichotomous variables, respectively; the y-axis, P value expression on an inverted log scale for Mann-Whitney U tests and Fisher exact tests for continuous and dichotomous variables, respectively). P values <.05 are highlighted in red. Dot size is proportional to the percentage of patients at risk.
Impact of monoblasts/CD45+ on response to treatment
Patients received a median of 3 cycles (IQR, 2-7) of Ven-Aza (Table 1). Fifteen patients could not be evaluated for response: 7 due to early death before the first-cycle assessment, 4 lost to follow-up, 3 not evaluated due to poor patient performance status, and 1 due to bone marrow evaluation failure. Among the 71 evaluable patients for response (excluding those with missing response data), 46 (65%) achieved an overall response according to ELN 2022 criteria,4 including 23 (32%) who achieved CR (supplemental Table 3).
As a continuous variable, a higher monoblasts/CD45+ proportion significantly and negatively influenced the odds of achieving CR in both univariable analysis (OR, 0.87; 95% confidence interval [CI], 0.80-0.96; P = .004) and multivariable modeling (OR, 0.24; 95% CI, 0.07-0.56; P = .005). The multivariable model accounted for log10(WBC) (OR, 0.07; 95% CI, 0.01-0.43; P = .011), ELN 2024 risk classification (adverse risk [OR, 0.17; 95% CI, 0.03-0.76; P = .028] and intermediate risk [OR, 8.03; 95% CI, 0.94-117; P = .085]), and age (OR, 1.05; 95% CI, 1.00-1.13; P = .12; Table 2).
Univariable and multivariable logistic regressions for complete response after Ven-Aza treatment
| Variable . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Monoblasts/CD45+ | 0.87 | 0.80-0.96 | .004 | 0.24 | 0.07-0.56 | .005 |
| Age, y | 1.01 | 1.00-1.01 | .2 | 1.05 | 1.00-1.13 | .12 |
| Log10(WBC) | 0.83 | 0.69-0.99 | .041 | 0.07 | 0.01-0.43 | .011 |
| ELN 2024 | ||||||
| Favorable | — | — | — | — | ||
| Intermediate | 0.90 | 0.69-1.19 | .5 | 8.03 | 0.94-117 | .085 |
| Adverse | 0.77 | 0.60-0.99 | .048 | 0.17 | 0.03-0.76 | .028 |
| Variable . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Monoblasts/CD45+ | 0.87 | 0.80-0.96 | .004 | 0.24 | 0.07-0.56 | .005 |
| Age, y | 1.01 | 1.00-1.01 | .2 | 1.05 | 1.00-1.13 | .12 |
| Log10(WBC) | 0.83 | 0.69-0.99 | .041 | 0.07 | 0.01-0.43 | .011 |
| ELN 2024 | ||||||
| Favorable | — | — | — | — | ||
| Intermediate | 0.90 | 0.69-1.19 | .5 | 8.03 | 0.94-117 | .085 |
| Adverse | 0.77 | 0.60-0.99 | .048 | 0.17 | 0.03-0.76 | .028 |
Bold face values point to significant (p<0.05) values in univariable and/or multivariables analyses.
Monoblasts/CD45+ remained an independent adverse predictor of response (OR, 0.27; 95% CI, 0.08-0.64; P = .011) when substituting ELN 2024 for ELN 2022 classification (adverse risk [OR, 0.04; 95% CI, 0.00-0.72; P = .078] and intermediate risk [OR, 0.39; 95% CI, 0.01-18.8; P = .6]; supplemental Table 4). Conversely, morphological classification as FAB M4/5 AML had no significant impact on the response to Ven-Aza (OR, 0.37; 95% CI, 0.08-1.38; P = .2; supplemental Table 5). A separate analysis considering separately FAB M4 (n = 20) and M5 cases (n = 11) yielded similar results (not shown). Finally, an “intent-to-treat” analysis, considering all nonevaluable patients as treatment failures, produced similar results (n = 86; OR, 0.90; 95% CI, 0.82-0.97; P = .019 for monoblasts/CD45+ in multivariable analysis; supplemental Table 6).
Impact of monoblasts/CD45+ on survival
With a median follow-up of 23.7 months (IQR, 14.9-35.8), a total of 54 patients (63%) had died at the time of the last follow-up. The median OS for the cohort was 8.1 months (IQR, 3.6-26.8). During the study period, 6 patients (7%) underwent allogeneic hematopoietic cell transplantation.
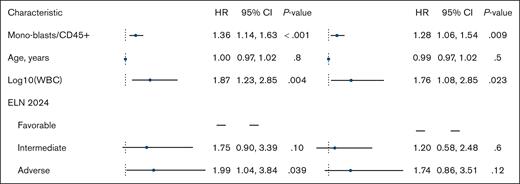
When analyzed as a continuous variable, a higher proportion of monoblasts/CD45+ was associated with worse OS in both univariable (HR per 10% increase, 1.36; 95% CI, 1.14-1.63; P < .001) and multivariable analyses (HR, 1.28; 95% CI, 1.06-1.54; P = .009). The multivariable model accounted for log10(WBC) (HR, 1.76; 95% CI, 1.08-2.85; P = .023), age (HR, 0.99; 95% CI, 0.97-1.02; P = .5), and ELN 2024 classification (adverse risk [HR, 1.74; 95% CI, 0.86-3.51; P = .12] and intermediate risk [HR, 1.20; 95% CI, 0.58-2.48; P = .6]; reference, favorable risk; Figure 3). A separate multivariable analysis censoring patients at allogeneic hematopoietic cell transplantation (n = 6) yielded similar results (not shown).
Forest plot showing Cox univariable and multivariable proportional hazards regression analysis for OS.
Forest plot showing Cox univariable and multivariable proportional hazards regression analysis for OS.
Similarly, monoblasts/CD45+ negatively affected EFS in both univariable (HR per 10% increase, 1.26; 95% CI, 1.06-1.49; P = .009) and multivariable analyses (HR, 1.21; 95% CI, 1.01-1.45; P = .041). The multivariable model included log10(WBC) (HR, 1.61; 95% CI, 1.02-2.54; P = .042), age (HR, 0.98; 95% CI, 0.96-1.00; P = .11), and ELN 2024 classification (adverse risk [HR, 1.61; 95% CI, 0.86-3.03; P = .14] and intermediate risk [HR, 1.13; 95% CI, 0.58-2.20; P = .7]; supplemental Table 7).
Definition and evaluation of the prognostic impact of monoblast status
By applying maximally selected rank statistics, a method that determines the optimal cutoff by maximizing the separation between survival curves,18 we established that a threshold of ≥10% monoblasts/CD45+ optimally predicts OS (supplemental Figure 4). This cutoff stratified the cohort into 34 patients (40%) classified as monoblastshigh and 52 patients (60%) as monoblastslow.
Patients classified as monoblastshigh had significantly higher WBC counts (11 × 109/L [IQR, 4 × 109 to 23 × 109/L] vs 4 × 109/L [IQR, 2 × 109 to 10 × 109/L]; P = .014). However, no other significant differences were observed between the 2 groups regarding demographics, clinical presentation, or risk classification based on either ELN 2022 (P = .3) or ELN 2024 (P = .084; Table 1). Notably, 12 of 60 patients with non-M4/5 AML (20%) were reclassified as monoblastshigh, whereas 4 of 26 patients with M4/5 AML (15%) were reclassified as monoblastslow. Patients in the monoblastshigh group had lower CR rates in both the “per protocol” patient population (patients with bone marrow response evaluation, n = 71; 4/28 [14%] vs 19/43 [44%]; P = .01) and in the intent-to-treat analysis (n = 86; 4/34 [12%] vs 19/52 [37%]; P = .02).
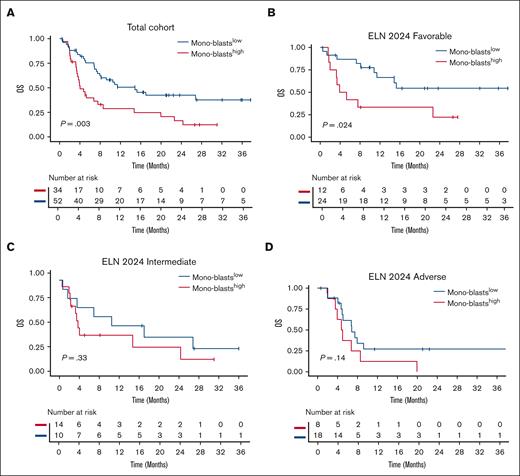
Monoblast status adversely affected OS, with a 1-year OS of 29% (95% CI, 16-50) in the monoblastshigh group compared to 51% (95% CI, 38-68) in the monoblastslow group (HR, 2.23; 95% CI, 1.30-3.83; P = .004; Figure 4A). The monoblastshigh status remained an adverse prognostic factor (HR, 1.95; 95% CI, 1.10-3.47; P = .023) in a multivariable Cox model accounting for log10(WBC) (HR, 1.68; 95% CI, 1.06-2.65; P = .026), ELN 2024 risk classification (adverse risk [HR, 1.75; 95% CI, 0.88-3.46; P = .11] and intermediate risk [HR, 1.15; 95% CI, 0.57-2.34; P = .7]), and age (HR, 0.99; 95% CI, 0.97-1.01; P = .4; Table 3). Morphological FAB M4/5 classification did not influence OS when substituting monoblasts status in the same model (HR, 1.82; 95% CI, 1.01-3.26; P = .052; supplemental Table 8). In a subgroup analysis, monoblast status was most predictive within the ELN 2024 favorable group, with a 1-year OS of 33% (95% CI, 15-74) in the monoblastshigh group compared to 66% (95% CI, 49-90) in the monoblastslow group (HR, 2.81; 95% CI, 1.10-7.19; P = .024; Figure 4B). In contrast, monoblast status did not significantly discriminate OS in the ELN 2024 intermediate (HR, 1.63; 95% CI, 0.60-4.38; P = .33; Figure 4C) or adverse subgroups (HR, 1.98; 95% CI, 0.79-4.95; P = .14; Figure 4D).
Overall Survival according to mono-blasts status. OS in the whole cohort (A) and in ELN 2024 risk categories (B-D) according to the dichotomic monoblast status (P value from log-rank test).
Overall Survival according to mono-blasts status. OS in the whole cohort (A) and in ELN 2024 risk categories (B-D) according to the dichotomic monoblast status (P value from log-rank test).
Cox univariable and multivariable proportional hazards regression analyses for OS
| Characteristic . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Monoblastshigh | 2.23 | 1.30-3.83 | .004 | 1.95 | 1.09-3.47 | .023 |
| Age, y | 1.00 | 0.97-1.02 | .8 | 0.99 | 0.97-1.01 | .4 |
| Log10(WBC) | 1.87 | 1.23-2.85 | .004 | 1.68 | 1.06-2.65 | .026 |
| ELN 2024 | ||||||
| Favorable | — | — | — | — | ||
| Intermediate | 1.75 | 0.90-3.39 | .10 | 1.15 | 0.57-2.34 | .7 |
| Adverse | 1.99 | 1.04-3.84 | .039 | 1.75 | 0.88-3.46 | .11 |
| Characteristic . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Monoblastshigh | 2.23 | 1.30-3.83 | .004 | 1.95 | 1.09-3.47 | .023 |
| Age, y | 1.00 | 0.97-1.02 | .8 | 0.99 | 0.97-1.01 | .4 |
| Log10(WBC) | 1.87 | 1.23-2.85 | .004 | 1.68 | 1.06-2.65 | .026 |
| ELN 2024 | ||||||
| Favorable | — | — | — | — | ||
| Intermediate | 1.75 | 0.90-3.39 | .10 | 1.15 | 0.57-2.34 | .7 |
| Adverse | 1.99 | 1.04-3.84 | .039 | 1.75 | 0.88-3.46 | .11 |
Bold face values point to significant (p<0.05) values in univariable and/or multivariables analyses.
Finally, we conducted a k-fold (k = 5) crossvalidation of the Cox proportional hazards model to internally validate the impact of monoblast status on OS. The Cox model was iteratively trained on k-1 folds and tested on the remaining fold, repeating this process across all folds. The mean concordance index (0.612), HR (2.236), and P value (.011) metrics confirmed the statistical significance of this association across multiple data subsets (supplemental Figure 5), indicating that monoblastshigh status consistently and adversely affected OS.
Discussion
Identifying factors that influence sensitivity to Ven-Aza remains a key challenge to improve prognostication and tailor therapeutic strategies. In our single-center cohort of patients with AML treated with frontline Ven-Aza, the presence of ≥10% monoblasts among CD45+ cells, as detected by MFC, was associated with lower CR rates and shorter OS, independently of gene mutations. Notably, in the ELN 2024 favorable subgroup, monoblast status identified a subset of patients with markedly worse outcomes, despite their anticipated benefit based on genetics alone.
Our cohort of 86 newly diagnosed patients with AML was prospectively included in our registry and demonstrated overall response rates and median OS comparable to those reported in other real-world settings.21,22 Our findings, based on a simple and affordable 8-color flow cytometry panel, emphasize the role of the monocytic phenotype in intrinsic resistance to the Ven-Aza regimen, in line with emerging evidence. Pei et al showed that monocytic subclones drive resistance to Ven-based therapies, with AML blasts at this differentiation stage relying primarily on MCL1 rather than BCL2 for oxidative phosphorylation and survival, leading to intrinsic resistance to Ven-Aza.8 Expanding on these insights, our study demonstrates that a quantifiable monocytic signature, assessed by MFC, is an independent adverse prognostic factor in patients with AML receiving Ven-Aza.
Previous studies have reported conflicting results regarding the prognostic significance of monocytic differentiation, assessed solely by morphology, in patients with AML receiving nonintensive treatment.23,24 Sango et al24 found no significant difference in OS between patients with morphologically defined monocytic AML. However, patients with N/KRAS mutations had significantly shorter OS. These findings align with a post hoc analysis of the M14-358/VIALE-A trials, which reported no significant OS difference based on FAB classification.23 Multiple factors could contribute to the divergence between our findings and those of previous studies. The use of MFC-based evaluation in our study reclassified 20% of FAB non-M4/5 and 15% of FAB M4/5 AML cases into the monoblasthigh and monoblastlow groups, respectively. This highlights the added value of MFC in refining the identification of monocytic phenotypes beyond conventional morphology, leading to improved prognostic stratification. Unlike FAB classification, which infers monocytic differentiation based on morphology alone, MFC enables precise quantification of key monocytic markers (CD4, CD36, and CD64) on AML blasts, revealing antigen expression heterogeneity and better accounting for intrapatient phenotypic heterogeneity.25 Moreover, differences in the approach to assessing the prognostic impact of RAS pathway mutations may also contribute to the variation in findings. The study by Sango et al24 relied on univariable analysis without incorporating established molecular classifiers. In contrast, our findings demonstrate that monoblast status predicts OS and EFS independently of genetic factors, suggesting a broader role for MFC in risk stratification beyond molecular profiling.
Ongoing research aims to identify early predictive biomarkers for Ven-Aza response in newly diagnosed, unfit patients with AML. Waclawiczek et al showed that the differential expression patterns of BCL2 family members in AML stem cells correlate with treatment response.9 Although valuable, these assays are technically complex and not widely accessible in routine clinical practice. Similarly, standardizing BH3 profiling in a prospective setting remains challenging.26 In contrast, our MFC-based screening approach, which assesses 3 monocytic markers (CD4, CD36, and CD64), is both accessible and already integrated into standard AML diagnostic workflows. This makes it a practical and feasible tool for real-time risk stratification in clinical settings.
Integrating MFC-based monocytic differentiation with the ELN 2024 molecular classification improved prognostic stratification in patients with AML receiving Ven-Aza. Notably, this approach was particularly relevant for patients within the heterogeneous ELN 2024 favorable-risk group. Despite a favorable genetic profile, a subset of these patients exhibited a high proportion of monoblasts/CD45+ cells, which was associated with adverse outcomes. These findings highlight the potential of MFC-based monocytic differentiation assessment as an additional prognostic tool to refine risk stratification and optimize treatment strategies.
The main limitation of this study is the lack of validation in an independent cohort due to the unavailability of MFC data in a separate prospective data set. To further address this limitation and strengthen the reliability of our findings, we performed an internal validation using the k-fold crossvalidation method. This analysis consistently confirmed that monoblasthigh status was associated with poor prognosis, reinforcing the robustness of our results.
In conclusion, our study highlights the prognostic significance of monoblasts/CD45+ quantification by MFC, providing additional value beyond traditional morphological assessment. This approach allowed for a more accurate reclassification of patients based on monocytic differentiation, effectively identifying those at higher risk of treatment resistance and poor survival. Although independent validation is required, these findings could improve risk stratification and inform the design of future clinical trials exploring alternative frontline treatment strategies.
Acknowledgments
The authors thank Anne Boussen, Caroline Bardon, Mélanie De Gouveia, Danièle Liechtenauer, and Laetitia Louarn for help with flow cytometry experiments; Lila Nessah, Manel Benamrouche, Sylia Benhaddad, and Laure Gilles for clinical data collection; and Valérie Bardet for helpful discussions.
This work was supported by funding from the integrated cancer research center SiRIC InsiTu (Insights Into Cancer: From Inflammation to Tumor; grant number INCa-DGOS-INSERM-ITMO Cancer_18008); and a grant managed by the French National Research Agency under the France 2030 program (reference ANR-23-IAHU-0005), Leukemia Institute Paris Saint-Louis.
Authorship
Contribution: L.-P.Z., T.D.-R., L. Adès, and R.I. designed the study and drafted the manuscript; L.-P.Z. and L.B. performed clinical data collection; T.D.-R., C.C., P.L., and S.M. performed morphology and flow cytometry analyses; L.-P.Z., L.B., L. Aguinaga, A.C., R.D.B., E.R., P.F., M.S., R.I., and L. Adès accrued patients; E.C. and M.D. performed molecular genetics; L.-P.Z. and R.I. performed statistical analyses; and all authors reviewed the manuscript and approved its final version.
Conflict-of-interest disclosure: R.I. has consulted and received research funding from AbbVie, unrelated to this work. The remaining authors declare no competing financial interests.
Correspondence: Raphaël Itzykson, Service Hématologie Adultes, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, F-75010 Paris, France; email: raphael.itzykson@aphp.fr.
References
Author notes
L.-P.Z. and T.D.-R. contributed equally as first authors to this study.
L. Adès and R.I. contributed equally as senior authors to this study.
Data regarding this study are available upon reasonable request from the corresponding author, Raphaël Itzykson (raphael.itzykson@aphp.fr).
The full-text version of this article contains a data supplement.