Advancements in orally bioavailable iron chelators and MRI methods have improved life expectancy and reproductive potential in thalassemia major (TM) and thalassemia intermedia (TI). Pregnancy is associated with adverse maternal and neonatal outcomes, frequency of which has not been well delineated. This systematic review aims to provide risk estimates of maternal and fetal outcomes in TM and TI and explore pregnancy's impact on iron homeostasis. Fifteen studies (429 participants, 684 pregnancies) were included. Meta-analysis revealed a higher thrombosis risk in TI (3.7%) compared to TM (0.92%), unchanged from prepregnancy. Heart failure risks in the earlier years appeared similar (TM 1.6% vs TI 1.1%), and maternal mortality in TM was 3.7%, but with current management, these risks are rare. Gestational diabetes and pre-eclampsia occurred in 3.9% and 11.3% of TM pregnancies, respectively. Caesarean section rates were 83.9% in TM and 67% in TI. No significant difference in stillbirth, small for gestational age neonates, or preterm birth incidence between TM and TI was observed. In TM pregnancies, red cell requirements significantly increased (from 102 to 139 ml/kg/year, P = 0.001), and 70% of TI pregnancies required blood transfusions. As expected, increased transfusion alongside chelation cessation led to a significant increase in serum ferritin during pregnancy (TM by 1005 ng/mL; TI by 332 ng/mL, P < 0.0001). Deterioration in iron status was further reflected by an increase in liver iron concentration (from 4.6 to 11.9 mg/g dry weight, P < 0.0001), and myocardial T2-star (T2∗) magnetic resonance imaging decreased (from 36.2 ± 2.5 ms to 31.1 ms) during pregnancy. These findings emphasize the elevated maternal risk of iron-related cardiomyopathy during pregnancy and labor, stressing the importance of cardiac monitoring and postpartum chelation therapy resumption.
Introduction
β-thalassemia syndromes can be classified as (1) transfusion dependent, including β-thalassemia major (TM) and β-thalassemia intermedia (TI) phenotypes; and (2) nontransfusion dependent, typically consisting of the TI phenotype.1 Both are manifestations of homozygous or compound heterozygous mutations affecting the β-globin genes, resulting in diminished or absent β-globin chain production, which leads to ineffective erythropoiesis and thereby chronic anemia, alongside other morbidities.1 Iron overload is a hallmark feature of these conditions, accruing as a result of chronic transfusions in the transfusion dependent group and, albeit at a slower pace, as a result of increased intestinal iron absorption owing to ineffective erythropoiesis and downregulation of hepcidin in the nontransfusion dependent group.2 The sequelae of iron overload are dependent on the organs affected by iron deposition, including (1) the pituitary (stunted growth and hypogonadotropic hypogonadism with impaired fertility), (2) islet cells of the pancreas (diabetes mellitus), (3) thyroid gland (hypothyroidism), (4) liver (hepatic fibrosis and hepatocellular carcinoma), and (5) heart (arrhythmias, heart failure, and pulmonary hypertension).3 Its presence is also an antecedent of early mortality.3
Before the availability of iron chelation therapy in the 1970s, pregnancy in individuals with TM was rarely encountered because ensuing hypogonadotropic hypogonadism rendered them infertile,4 and lifespan rarely extended beyond age 20 years.5 After the introduction of chelation regimens, and with improved fertility therapies, over the last 4 decades, pregnancy in those with TM has become more common.5 Prospective studies addressing management challenges associated with pregnancies in people affected by TM/TI are limited, and current management differs between centers. Individual small studies point to the high-risk nature of these pregnancies1,4,6-15; however, a comprehensive understanding of the magnitude of risk of specific complications and a clear delineation of the trajectory of iron loading through the duration of pregnancy is required. Thus, the objective of this systematic review was to assess the maternal and fetal outcomes in TM and TI and explore the impact of pregnancy on iron homeostasis.
Methods
The protocol of this systematic review and meta-analysis was registered on PROSPERO (CRD42020182475), and the study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses16 and Meta-Analysis of Observational Studies in Epidemiology17 guidelines. Studies were included if they (1) involved pregnant individuals with TM or TI, (2) examined pregnancy complications and pregnancy/neonatal outcomes, and (3) were controlled trials, cohort studies, or case series of >5 pregnancies.
The OvidSP search platform was used to carry out the literature search in the following databases: PubMed, EMBASE, LILACS, Web of Science, Scopus, and EBM Reviews (containing the Cochrane Library), including articles indexed from their inception to 31 December 2019. The search was limited to human data, without restrictions to language or year of publication. Additional articles were identified by scanning reference lists and searching the gray literature for the last 2 years for relevant abstracts from conference proceedings of the American Society of Hematology (ASH), the Canadian Hematology Society, the Canadian Hemoglobinopathy Association, the Society for Maternal-Fetal Medicine, the Royal College of Obstetricians and Gynaecologists, Thalassemia International Federation conference, Cooley's anemia foundation, British Society of Haematology, and European Hematology Association conferences. All references generated by the search were imported into Covidence.
Title and abstract screening, data extraction for studies meeting inclusion criteria, and risk of bias assessment were independently carried out by 2 reviewers (E.V. and A.K.M.). Disagreements were resolved by discussion and consensus. The risk of bias was assessed at the individual study level according to the Joanna Briggs Institute Checklist for Case Series18 and the Newcastle-Ottawa Scale (NOS)19 for nonrandomized studies (Table 1).
Primary maternal outcomes a priori included mortality, cardiac failure, and change in left ventricular ejection fraction (LVEF) percentage. Secondary maternal outcomes a priori included: pre-eclampsia; venous thromboembolism (VTE); gestational diabetes mellitus (GDM); cardiac arrhythmia, intrahepatic cholestasis of pregnancy, osteopenia/osteoporosis, alloimmunization, change in transfusion requirements during pregnancy, maternal pretransfusion hemoglobin target, transfusion-related viral infection, hemolytic transfusion reaction, mode of delivery, and postpartum hemorrhage. Details pertaining to parameters of iron status, such as ferritin, liver iron concentration, and cardiac magnetic resonance imaging (MRI) T2-star magnetic resonance imaging (T2∗), were also collected.
Primary fetal or neonatal outcomes a priori comprised mortality (intrauterine fetal demise or neonatal death). Secondary fetal or neonatal outcomes included spontaneous miscarriage, small for gestational age (SGA)/low birth weight, preterm birth (PTB), neonatal intensive care unit admission, and Apgar scores <7 at 5 minutes of life.
Data synthesis
For dichotomous variables, relative effect measures, primarily consisting of odds ratios (ORs) and relative risk and their 95% confidence intervals (CIs), were calculated. A random effects model was chosen given the diversity of individual studies, particularly concerning the study population, as well as protocols and targets of the intervention.20 For continuous variables, the mean difference was calculated. The degree of heterogeneity across the studies was examined using I2 values,21 classifying 50% as moderate heterogeneity and 75% as marked heterogeneity. Review Manager Software (version 5.3; the Cochrane Collaboration, Oxford, United Kingdom) was used to complete the meta-analysis. For prevalence proportional meta-analysis of events, the MedCalc software was used (Version 20.109; Belgium).
Results
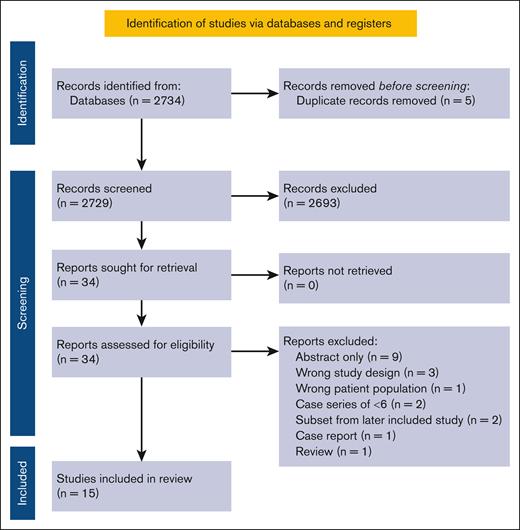
Figure 1 displays the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram itemizing study selection. A total of 15 studies met inclusion criteria: 14 case series and 1 cohort study with a total of 429 participants (684 pregnancies). Study characteristics are presented in Table 1. Five of 15 studies were multicenter. Seven studies reported on TM outcomes, 5 on TI outcomes, and 3 reported on both. A single study reported outcomes using low-risk pregnancy controls.
Risk of bias assessment
Fourteen of the included studies were case series, and 1 was a cohort study. For the case series, the results have not been summarized with a score as not all questions are of ‘equal’ weighting. The results of the NOS and Joanna Briggs Institute assessment are shown in supplemental Table 1. All studies demonstrated clear criteria for inclusion, and the majority had clearly reported outcomes and appropriate statistical analysis. Some studies had unclear reporting of patient demographics and clinical information, as well as clinic demographics. Most had unclear reporting of TM/TI diagnosis and methods used for the identification of participants and consecutive/complete inclusion of participants. The sole cohort study was deemed to be at low risk of bias, with clear patient selection and clear outcome reporting.
Pregnancy outcomes in individuals with TM and TI
Table 2 contains a proportional meta-analysis of pregnancy outcomes in cohort studies of pregnant individuals with TM and TI. In the TM group, the risk of VTE was 0.92% (95% CI, 0.1-2.6), whereas in the TI group, it was 3.7% (95% CI, 1.2-7.5). This translates to a fourfold greater risk in the TI group compared with the TM group (P = .038). The risk of heart failure was similar for the TM (1.6%; 95% CI, 0.4-3.7) and TI (1.1%; 95% CI, 0.1-3.3) groups.
In the TM group, the mortality rate was 3.0% (95% CI, 0.6%-7.2%); however, 1 of the reported deaths was secondary to colon cancer, and excluding this, the mortality rate was 2.9% (95% CI, 0.56%-7.18%). The rate of pre-eclampsia in the TM group was 11.3% (95% CI, 6.2-15.3), whereas GDM was observed in 3.9% (95% CI, 1.3-8.0). Rates of these complications were not available for the TI group.
High proportions of cesarean delivery were noted in both groups: more pronounced in TM (83.9%; 95% CI, 65.2%-96.2%) than in TI (67%; 95% CI, 66.0-77.2; P < .0001). Data in either group were insufficient to quantify the risk of cardiac arrhythmias and changes in LVEF percentage.
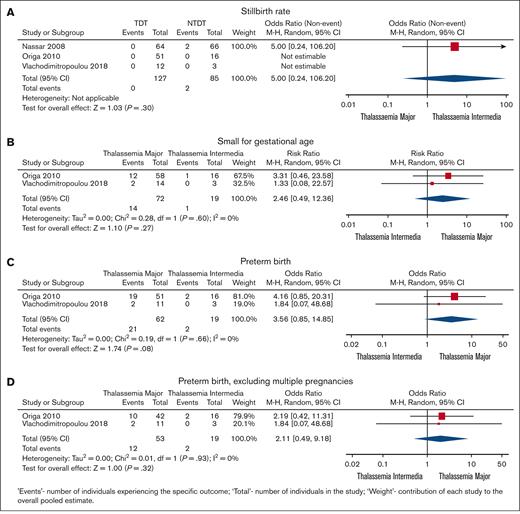
Pregnancy and neonatal outcomes are shown in Table 2 and Figure 2. Spontaneous miscarriage was similar in TM and TI (6.1%; 95% CI, 3.4-11.4 vs 9.5%; 95% CI, 3.8-17.5, respectively; P = .19). The proportion of stillbirths was similar for both groups (1.3% in TM and 1.2% in TI; OR, 5; 95% CI, 0.24-106.2; P = .3). Similarly, the proportion of SGA infants was similar for TM and TI (18.1%; 95% CI, 8.8-29.9 vs 14.5%; 95% CI, 2.3-34.8, respectively; OR, 2.46; 95% CI, 0.49-12.36; P = .27). In contrast, the proportion of PTB was significantly higher in TM than TI (25.2%; 95% CI, 12.3-41 vs 8.9%; 95% CI, 12.3-41, respectively; P < .0001; Table 1); however, in the random effects model, this difference did not reach significance (OR, 3.56; 95% CI, 0.85-14.85; P = .08). Data were insufficient to quantify the risk of a low neonatal Apgar score.
Comparison between pregnancy outcomes in TM and TI. (A) Stillbirth rate. (B) SGA babies. (C) Preterm birth rate. (D) Preterm birth rate excluding multiple pregnancies.
Comparison between pregnancy outcomes in TM and TI. (A) Stillbirth rate. (B) SGA babies. (C) Preterm birth rate. (D) Preterm birth rate excluding multiple pregnancies.
Impact of pregnancy on transfusion requirements and iron status of persons with TM and TI
There appeared to be a consensus of a pretransfusion hemoglobin target of 10 g/dL in patients dependent on transfusions.
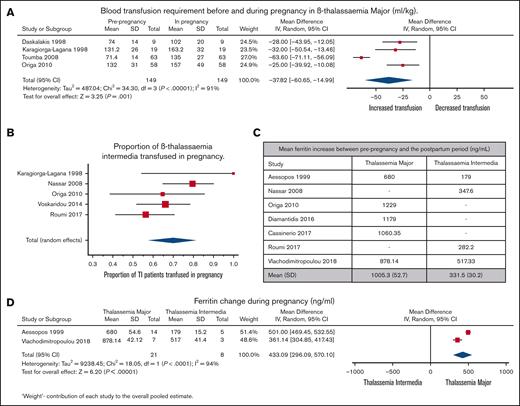
Figure 3 depicts the impact of pregnancy on transfusion requirements and iron status in individuals with TM and TI. Transfusion requirements in TM in the 9-month prepregnancy period compared with the transfusion requirements during pregnancy are depicted in Figure 3A. A significant increase in transfusion requirements was observed in this population during pregnancy (4 studies and 149 pregnancies). Blood consumption increased from 102 to 139 mL of red cells per kg per year in the year that included the pregnancy. Proportional meta-analysis in individuals with TI who did not receive regular blood transfusions (5 studies and 168 pregnancies; Figure 3B) demonstrated a transfusion proportion of 70% (95% CI, 57.7%-81.3%; I2, 62%; P = .03).
Transusion and ferritin outcomes in TM and TI. (A) Forest plot comparing transfusion requirements in the 9-month prepregnancy period to pregnancy (mL/kg) in TM. (B) Forest plot comparing ferritin increase between prepregnancy and the postpartum period in TM and TI. (C) Comparison between change in the ferritin level before pregnancy and post partum in TM and TI. (D) Forest plot comparing changes in ferritin level during pregnancy in TM and TI.
Transusion and ferritin outcomes in TM and TI. (A) Forest plot comparing transfusion requirements in the 9-month prepregnancy period to pregnancy (mL/kg) in TM. (B) Forest plot comparing ferritin increase between prepregnancy and the postpartum period in TM and TI. (C) Comparison between change in the ferritin level before pregnancy and post partum in TM and TI. (D) Forest plot comparing changes in ferritin level during pregnancy in TM and TI.
The pooled mean serum ferritin concentration increase from prepregnancy to the postpartum period was 1005 ng/mL in TM and 332 ng/mL in TI (Figure 3C). Serum ferritin concentration (2 studies and 21 pregnancies) increased significantly more in TM than in TI (mean difference, 433 ng/mL; P < .00001; Figure 3D).
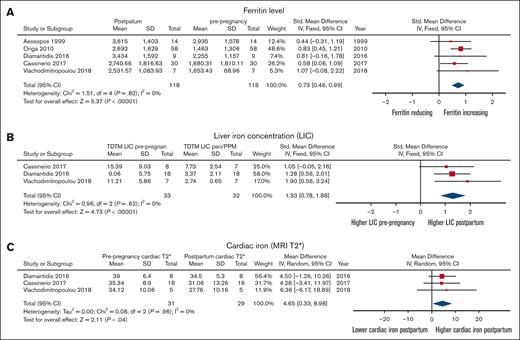
The accrual of body iron during pregnancy in individuals with TM is shown in Figure 4. Mean serum ferritin concentration increased significantly from 1762 (± 342) to 2850 (± 400) ng/mL (P < .00001). This was accompanied by a significant increase in liver iron concertation from 4.6 (± 2.7) to 11.9 (± 3.2) mg/g dry weight (P < .00001) and a significant decrease in myocardial T2∗ 36.2 ± 2.5 ms to 31.1 ± 3.4 ms (P = .04). There were insufficient data concerning alloimmunization, transfusion-related viral infections, and hemolytic transfusion reactions in individuals dependent on transfusion during pregnancy. There were similarly insufficient data concerning intrahepatic cholestasis of pregnancy and postpartum hemorrhage.
Comparison of iron parameters before pregnancy and post partum in women with TM. (A) Ferritin level. (B) Liver iron concentration. (C) Cardiac iron assessed by cardiac MRI T2∗.
Comparison of iron parameters before pregnancy and post partum in women with TM. (A) Ferritin level. (B) Liver iron concentration. (C) Cardiac iron assessed by cardiac MRI T2∗.
Discussion
This meta-analysis examines the pregnancy outcomes of individuals with TM and TI and is primarily based on 14 case series and a single cohort study. Our findings suggest that pregnant individuals with TM face a risk of maternal mortality, if not optimally treated, and development of pre-eclampsia, whereas data on the proportion of these conditions in patients with TI are not available. Rates of heart failure in the earlier years appear to be similar between the 2 groups, with all reported events predating the widespread adoption of cardiac MRI T2∗ for cardiac iron assessment. Furthermore, pregnant individuals with TI appear to have a significantly higher risk of VTE in pregnancy than individuals with TM, unchanged from prepregnancy frequency. Although cesarean delivery rates are high in both groups, the chance of this is more pronounced in those with TM. Despite these increased risks, both TM and TI pregnancies demonstrate a high rate of live births, with the chance of SGA and PTB potentially more frequent in the TM group.
Individuals with β-thalassemia have an overall increased incidence of VTE in view of their chronic hypercoagulable state, having often undergone a splenectomy, as well as the presence of increased red blood cell P-selectin–derived microparticles.22 These factors, combined with the known physiological changes of pregnancy (increased levels of clotting factors, decreased fibrinolysis, decreased free protein S, and acquired resistance to activated protein C),23 would lead to an assumption of an even higher risk of VTE in pregnant patients with thalassemia. Yet, despite the chronic hypercoagulable state in these patients, there is no apparent increase in the incidence of VTE in TM during pregnancy, compared with the baseline in the nonpregnant state.24,25 Although the literature on VTE in the nonpregnant β-thalassemia population is scarce, in the 2006 study by Taher et al, which included 8860 patients with TM and 2190 with TI, the incidence of VTE was 0.9% and 3.9%, respectively. The incidence of VTE in our meta-analysis was strikingly similar: 0.92% in the TM group and 3.7% in the TI group.24 The ASH guidelines for management of VTE in the context of pregnancy suggest a threshold of 2% VTE risk for initiation of antepartum thromboprophylaxis.26 Thus, unless there is a history of VTE or additional risk factors of thrombosis, routine thromboprophylaxis has not routinely been advised in TM pregnancies.27 In keeping with this observation, our proportional meta-analysis in the TM group did not show any additional risk of developing thromboembolism in pregnancy, with a proportion of 0.92%, which is similar to the described incidence of VTE in pregnancy of 1 to 2 per 1000.28,29 This may be secondary to the abrogation of some of the physiological changes by the widely adopted transfusion protocols.30
Our meta-analysis, however, does highlight a fourfold greater VTE risk in patients with TI (3.7%) than patients with TM (0.9%), reflecting the findings in the nonpregnant β-thalassemia population, in which VTE risk was 4.38-fold higher in patients with TI than those with TM.24 Increased thrombotic tendency in this group includes the presence of abnormal red cells, expressing phosphatidylserine on their cell surface,31 that shed prothrombotic microvesicles.22 Various laboratory changes are evident, including increased urinary thromboxane A2 metabolites, enhanced expression of P-selectin in intact thalassemic platelets, elevated plasma level of thrombin-antithrombin III complexes, elevated levels of endothelial adhesion protein (ICAM-1, ELAM-1, VCAM-1, von Willebrand factor, and thrombomodulin31), and low plasma levels of the natural anticoagulants, protein C and protein S, and heparin cofactor II.32,33 Splenectomy is a clear risk factor of thrombosis in TI,24,30 as is a high nucleated red cell count >300 × 106/L, a platelet count >500 × 109/L, evidence of pulmonary hypertension, or being transfusion naïve.24,30 Median time to thrombosis after splenectomy is 8 years in Middle Eastern and Italian TI populations.24 Transfusions mitigate thrombotic risk by reducing thrombogenic red cells and microparticles22; moreover, transfusion also corrects deficiency of natural anticoagulants in TI.33 Given the risk of VTE during pregnancy in TI is 3.7%, which exceeds the 2% threshold for thromboprophylaxis adopted in the ASH guidelines, it may be worthwhile to consider prophylactic measures against thrombosis in pregnant patients with TI, particularly those with additional risk factors for VTE, such as splenectomy and high platelet and high nucleated red blood cell counts. Options could include prophylactic anticoagulation34 and/or transfusion. However, transfusions should be weighed against risks such as alloimmunization35,36 and hemolytic disease of the fetus or newborn.37
Compared with the antepartum risk amortized over ∼40 weeks, the 6-week postpartum period has a greater daily VTE risk, which is 15- to 35-fold higher than age-matched nonpregnant controls and gradually returns to baseline over 6 to 12 weeks post partum.38-41 We are unable to comment on the true magnitude of postpartum risk in individuals with TM or TI, given the majority of patients received prophylactic subcutaneous low molecular weight heparin for 6 weeks post partum, as per routine practice.27
Diabetes is a recognized complication of hemosiderosis in adults with transfusion-dependent thalassemias42,43 and may manifest itself for the first time during pregnancy in the form of GDM. The proportion of patients with TM who developed GDM was 3.9%, with no documented GDM in the TI group. The incidence of GDM in a European population was reported to range from 3.8% to 7.8%,44 which is comparable with the aforementioned risk.
In TM, the risk of pre-eclampsia was noted to be 11.3%, more than double the estimated 4.6% global prevalence.45 Anemia and hypoxia lead to a reduction in soluble fms-like tyrosine kinase-1, an antiangiogenic protein that interferes with vascularization, which could impede the function of vascular endothelial growth factors and placental growth factor,46 leading to endothelial dysfunction and an imbalance in angiogenesis, possibly increasing the risk of pre-eclampsia in these patients.47-49 In view of this finding, consideration of low-dose aspirin 150-162 mg nightly, starting before 16-week gestation and continuing to 36-week gestation, would be reasonable for primary pre-eclampsia prevention.50
The mode of delivery of pregnant individuals with TM remains unsettled. The proportion of cesarean delivery in our meta-analysis was 83.9% in the TM group and 67% in the TI group (P < .001). Both rates are substantially higher than the overall 21% rate worldwide, reported by the World Health Organization in June 2021.51 In TM, 1 study documented a notably lower rate of cesarean births (27.8%) than others, in which the rates ranged from 72.7% to 100%. Excluding this outlier, the longitudinal analysis revealed no significant temporal variation in the cesarean delivery rates, with 86.9% occurring before 2010 and 92.7% subsequent to 2010 (P = .22). In contrast, in TI, the cesarean delivery rate was significantly higher in studies reporting data before 2010, at 86.4%, than in those reported thereafter at 58.9% (P = .001). A commonly speculated indication for cesarean delivery for those with β-thalassemia is cephalopelvic disproportion due to short stature or skeletal deformities spurred by bone-altering marrow expansion and extramedullary hematopoiesis.27 However, elective cesarean delivery is not a universal approach in this setting and in the absence of other maternal or fetal indications, there is no absolute contraindication to trial of labour and vaginal delivery.27
With respect to fetal and neonatal complications, there was no significant difference between the stillbirth rate in TM and TI, with the absolute proportion of stillbirths at 1.3% in TM and 1.2% in TI, higher than the 0.47% stillbirth rate reported by BORN (Better Outcomes Registry & Network) Ontario52 between March 2015 and February 2020. Considering the reported stillbirth rates are driven by the results of 1 study,36 with no reported stillbirths in either group in 2 other smaller studies,15,53 conclusions with respect to the relative risk of stillbirth in individuals with β-thalassemia in comparison with the general population remain speculative, and indeed, the high rates of live births are overall reassuring.
The proportion of SGA infants was similar between the TM (18.1%) and TI (14.5%) groups and higher than the documented incidence of 11% in the general population.54 The overall proportion of PTB was significantly different in TM (25.2%) compared with TI (8.9%) and higher than the global incidence of 5% to 18% reported by the World Health Organization in November 2022.55 These differences could possibly be attributed to imbalances in angiogenic factors impairing placentation, as noted above for pre-eclampsia. There is no clarity in the literature as to the extent to which PTB is iatrogenic.
There are a number of physiological changes in pregnancy, including a ∼50% increase in blood volume,56,57 that could increase cardiac stress, with the peripartum period posing the most burden.58 Despite this, the risk of heart failure in our meta-analysis was similar for the TM and TI groups and not higher than the overall prevalence of heart failure of 2.5% in TM59 and 2.7% to 5.4% in TI,60,61 outside of pregnancy. Nevertheless, it is significantly higher than the overall rate of heart failure in unaffected mothers, estimated to be <0.1%.62 Overall, 3 cases of heart failure were reported in the TM group4,14 and only 1 case in the TI group, although limited clinical details were provided.36 It is not possible to determine whether the individual in the TI group who experienced heart failure exhibited clinical characteristics more akin to those of a patient with TM. Our proportional meta-analysis placed the risk of maternal death at 2.9%. Maternal deaths in TM have been reported in the literature, the majority of which have been secondary to cardiac complications.4 It is worth noting that there were no reports of heart failure or maternal death during pregnancy after the introduction of cardiac MRI T2∗ assessment in the early 2000s.63,64 These observations highlight the historical context in which heart failure and maternal death were documented and underscore the utility and effectiveness of cardiac MRI T2∗ in monitoring and risk mitigation of cardiac complications in patients with thalassaemia during and after pregnancy. Thus, to achieve healthy pregnancy outcomes, cardiac optimization is of utmost importance.
Our meta-analysis also demonstrates a significant increase in transfusion requirements in this population during pregnancy. Despite variation in transfusion practices, blood transfusions were needed at more frequent intervals to maintain hemoglobin levels and minimize major fluctuations in cardiac workload. Similarly, although individuals with TI do not typically receive regular blood transfusions, 70% in this group required their initiation during pregnancy, a finding of relevance for counseling and management/resource planning.
Our meta-analysis suggests significant deterioration in iron parameters and amplified hepatic and cardiac iron loading in TM during pregnancy. This is reflected by an elevation in serum ferritin levels, increased liver iron concentration, and decrease in the myocardial MRI T2∗ during pregnancy, attributed to an increase in blood transfusion volume/frequency and cessation of chelation. Although transfusion practices vary globally, hemoglobin is often maintained >10 g/dL for patients with TM. The suspension of iron chelation therapy during pregnancy is common due to concerns about potential teratogenic risks.27 However, in cases of developing cardiac iron overload, even with preserved cardiac function, consideration of reintroducing desferrioxamine (DFO) at 1 g per day in the late second or third trimester may be warranted. Over 3 decades, >45 pregnancies in patients overloaded with iron receiving DFO at various stages of the pregnancy have shown no adverse fetal/neonatal effects.10,65-73
Patients with normal cardiac performance and adequate prepregnancy chelation treatment typically progress without complications; whereas those with impaired cardiac function or with myocardial hemosiderosis may be at risk of developing heart failure. There have been at least 2 case reports of fatal heart failure during pregnancy in patients with TM.4 Both occurred before the availability of cardiac MRI T2∗, which may have hindered the degree of myocardial iron loading, precluding cardiac optimization. Myocardial iron loading can rapidly develop during pregnancy when chelation is withheld, possibly increasing the risk of iron-mediated cardiomyopathy74 during pregnancy and peri partum.
Patients with a history of increased myocardial iron loading (T2∗ < 20 ms) may be at risk of increased loading during pregnancy, even if the myocardial T2∗ has recently normalized before pregnancy. In such cases, close monitoring during pregnancy is recommended (including cardiology consultation possibly including myocardial T2∗ assessment in late pregnancy). If LVEF or myocardial T2∗ falls, initiating low-dose DFO in the later pregnancy weeks may be prudent, despite the absence of sanctioned drug labeling. Intrapartum DFO infusion has been proposed by some27 and requires further investigation. DFO, known for its low levels in breast milk and negligible oral absorption, is considered safe during breastfeeding.75
Optimally treated and chelated patients with TM are at low risk of complications during pregnancy. As chelation is withheld during pregnancy, the main concern is the herein documented deterioration in iron status and, particularly, the cardiac risks associated with iron overload, which are not trivial. Individuals with TM can sometimes develop rapid myocardial iron loading during pregnancy in the absence of iron chelation, with a possibility of cardiac dysfunction resulting from myocardial hemosiderosis, causing both ventricular pump failure and arrhythmias.74 These risks can be decreased by recommending pregnancy to patients with the lowest cardiac risk and by intensive chelation before pregnancy, when appropriate. Prepregnancy assessment is pivotal and should include a detailed assessment of cardiac function by electrocardiogram, echocardiogram, Holter monitoring, a cardiac MRI scan, and a detailed history of previous heart failure and/or arrhythmias as well as review by a cardiologist. When this is not possible, close multidisciplinary care, including hematology, cardiology, maternal-fetal medicine, and anesthesia, can help reduce maternal risks. Future research should investigate iron chelation in pregnancy and potentially intrapartum in those with evidence of myocardial iron loading.
Strengths and limitations
A notable strength of this review is the comprehensive, objective appraisal of the available scientific literature and its meta-analysis. The main limitation is the suboptimal methodologic quality of the included studies, with the majority having a moderate-to-high risk of bias and the absence and/or heterogeneity of definitions for exposures and outcomes of interest.
Conclusion
This systematic review and meta-analysis highlights the challenges faced by individuals with TM and TI during pregnancy. Our findings indicate that pregnant patients with TM and TI are at risk of adverse maternal outcomes, including heart failure and mortality, if not managed using modern technologies such as MRI T2∗, as well as adverse obstetric and fetal outcomes such as caesarean delivery and SGA size. Individuals with TM have a higher risk of pre-eclampsia. Additionally, in TI, there is a disproportionately higher risk of VTE, whereas in TM, there is a disproportionately higher risk of PTB. Additionally, the results suggest that red cell requirements and indices of iron loading (serum ferritin concentration, liver iron concentration, and myocardial T2∗ values) worsen significantly during pregnancy in these individuals because iron chelation therapy is discontinued, possibly putting them at risk of iron-mediated cardiomyopathy, underscoring the need for cardiac monitoring and optimization during pregnancy and the importance of resuming chelation therapy post partum. The results of this review (1) highlight the importance of multidisciplinary care and the need for close monitoring in TM and TI during pregnancy to ensure the best possible outcomes for both mother and infant; (2) encourage re-evaluation of the need for antenatal thromboprophylaxis for TI pregnancies; and (3) highlight the need for further study concerning optimal approach to iron chelation antenatally and intrapartum in individuals with TM.
Acknowledgments
The authors thank the researchers and institutions for making this systematic review possible. The authors are grateful to their colleagues who provided insightful feedback and support throughout the course of this project.
Authorship
Contribution: M.H. and A.K.M. developed the search strategy, which was performed by M.H., E.V., A.K.M., and H.M., and extracted data for the meta-analysis; E.V., A.K.M., and N.S. analyzed the data and wrote the manuscript; and all authors provided input into the research design and protocol, and provided critical feedback toward manuscript revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evangelia Vlachodimitropoulou, Fetal Medicine Unit, Mount Sinai Hospital, OPG-3203, 700 University Ave, Toronto, ON M5G 1X5, Canada; email: elinakv@doctors.net.uk.
References
Author notes
Data are available upon request to the corresponding author, Evangelia Vlachodimitropoulou (elinakv@doctors.net.uk).
The full-text version of this article contains a data supplement.