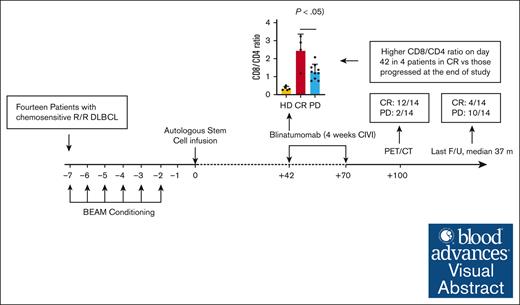
For patients with relapsed DLBCL or transformed follicular lymphoma, blinatumomab consolidation after auto-SCT is safe and well tolerated.
An increased CD8:CD4 T-cell ratio in the peripheral blood after auto-SCT before blinatumomab may predict for long-term remission.
Visual Abstract
Outcomes in patients with relapsed diffuse large B-cell lymphoma (DLBCL) who undergo autologous stem cell transplant (auto-SCT) are poor. Blinatumomab is a CD3/CD19 bispecific T-cell engager that directs cytotoxic T cells to CD19+ cells. Here, we performed a pilot study of blinatumomab consolidation after auto-SCT for 14 patients with DLBCL or transformed follicular lymphoma. All patients underwent standard-of-care auto-SCT with carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning followed by 1 cycle (4 weeks continuous infusion) of blinatumomab consolidation starting at day 42 after auto-SCT. All 14 patients treated on study completed BEAM auto-SCT and 1 cycle of posttransplant blinatumomab. Five patients developed grade 1 cytokine release syndrome (CRS), with no grade 2 or higher CRS. Immune effector cell–associated neurotoxicity syndrome was not observed. Patients were followed up for 3 years after auto-SCT, with median follow-up of 37 (range, 12-65) months. One-hundred days after auto-SCT (1 month after blinatumomab consolidation), 12 patients (86%) had achieved complete remission. At 1 year after auto-SCT, 7 patients (50%) remained in CR, and 1 patient had died of progressive disease. Patients who relapsed had a lower CD8:CD4 T-cell ratio before starting blinatumomab than patients who remained in remission. This pilot study demonstrates blinatumomab consolidation after auto-SCT is safe and well tolerated. Strategies to increase the CD8:CD4 ratio and use additional cycles of consolidation in a larger randomized trial are needed to confirm the efficacy of consolidation with blinatumomab after auto-SCT. This trial was registered at www.clinicaltrials.gov as #NCT03072771.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common histologic subtype of non-Hodgkin lymphoma (NHL), occurring at a rate of 7 per 100 000 persons annually.1 Five-year progression-free survival (PFS) remains 30% to 90%, depending on the risk category at diagnosis.2 Before the approvals for CD19-directed chimeric antigen receptor (CAR) T-cell therapies, high-dose chemotherapy and autologous stem cell transplantation (auto-SCT) was the standard treatment for all patients with relapsed or refractory (R/R) DLBCL.3,4 Auto-SCT remains the standard treatment for transplant-eligible patients who relapse more than 12 months after initial therapy. However, post–auto-SCT outcomes are poor, with 5-year PFS and overall survival (OS) rates of 40% and 50%, respectively.5
Blinatumomab is a bispecific T-cell engager molecule that transiently links CD3+ polyclonal T cells to CD19+ B cells, inducing T-cell activation followed by T-cell–mediated serial B-cell lysis and concomitant T-cell proliferation.6 Blinatumomab was first approved for use among patients with R/R B-cell acute lymphoblastic leukemia/lymphoma (B-ALL) after its phase 2 study demonstrated that 25 of 36 patients (69%) achieved a complete response (CR) or CR with incomplete hematologic recovery.7 The confirmatory randomized phase 3 trial TOWER upheld earlier results and demonstrated an improved median OS of 7.7 months in the blinatumomab group compared with 4.0 months in the standard chemotherapy group (hazard ratio for death with blinatumomab vs chemotherapy, 0.71; 95% confidence interval [CI], 0.55-0.93; P = .01) for patients with R/R B-ALL.8 In the study leading to its approval in B-ALL with detectable measurable residual disease (MRD) after induction, the status of 88 of 113 patients (78%) converted to undetectable MRD after 1 cycle of blinatumomab consolidation.9 The patients with undetectable MRD had significantly longer relapse free survival (23.6 vs 5.7 months; P = .002) and OS (38.9 vs 12.5 months; P = .002) than those who had detectable MRD. Clinically relevant adverse events (AEs) associated with blinatumomab include neurologic events and cytokine release syndrome (CRS), with their incidences correlating with both tumor burden and blinatumomab dose.
Although blinatumomab has proven long-term survival benefits for patients with B-ALL, data for its use in B-cell NHL (B-NHL) are less robust but remain encouraging. A phase 2 clinical trial of blinatumomab in 25 patients with R/R DLBCL demonstrated an overall response rate of 36% and a median duration of response of 11.6 months.10 Serious AEs related to treatment occurred in 8 patients (35%) with no treatment-related deaths. Grade 3 neurologic AEs were observed in 7 patients (28%) with no grade 4 or 5 neurologic AEs.
Blinatumomab cytotoxicity has been shown to be proportional to the ratio of effector to target (E/T) cells.11 An inverse relationship was observed between disease burden and CR rates in patients with R/R B-ALL treated with blinatumomab. Patients with bone marrow blasts ≥50% and <50% had CR rates of 73% and 29%, respectively, and patients with chemotherapy-refractory MRD had response rates of 80%.7,12 For the purpose of this study, disease burden should be minimal after treatment with salvage chemotherapy and auto-SCT, providing an opportunity for blinatumomab to effectively eradicate any residual disease.
The ideal timing of blinatumomab consolidation after auto-SCT is contingent on the timing of immune reconstitution after myeloablative conditioning to ensure an adequate pool of effector cells is present. CD8+ T cells and natural killer cells achieve healthy donor levels at 1 month after auto-SCT, whereas CD4+ T cells and B cells show delayed recovery. Four to 6 weeks after autologous transplantation, the CD8+ T-cell count is ∼700 cells per μL and the CD4+ T-cell count is ∼200 cells per μL, constituting a total CD3+ T-cell count of ∼900 cells per μL.13,14 Notably, this CD3+ T-cell count is higher than that reported for R/R ALL and ALL with detectable MRD (2 settings in which blinatumomab has already received Food and Drug Administration approval) before the first dose of blinatumomab.7,15
We hypothesized that blinatumomab consolidation after auto-SCT would help eradicate residual tumor cells, minimize relapse, and improve OS in patients with R/R DLBCL and transformed follicular lymphoma (FL). Additionally, the low tumor burden generally seen after auto-SCT may limit blinatumomab-related toxicities including neurologic AEs and CRS. The primary objective of this pilot study was to assess the feasibility and tolerability of blinatumomab consolidation therapy among patients with chemo-sensitive DLBCL and transformed FL who had undergone auto-SCT.
Methods
Study design and patient population
Adult patients (aged ≥18 years) with histologically confirmed CD19+ DLBCL or transformed FL were included. All patients had chemo-sensitive disease defined as having a CR or partial response (PR) to salvage chemoimmunotherapy based on pretransplant positron emission tomography/computed tomography (PET/CT) performed within 2 months of auto-SCT. All patients underwent auto-SCT. Patients with active central nervous system (CNS) disease involvement, clinically relevant CNS pathology, prior stem cell transplant, HIV, active hepatitis A, B, or C infection, or poor performance status (European Cooperative Oncology Group performance status ≥ 2 or Karnofsky score ≤ 60%) were excluded.
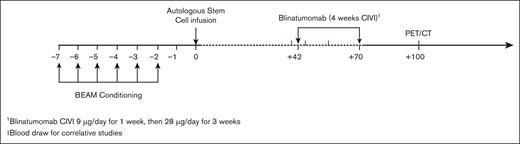
All eligible patients underwent standard-of-care auto-SCT with carmustine (BCNU), etoposide, cytarabine, and melphalan (BEAM) conditioning. After auto-SCT and before starting blinatumomab, all patients were assessed for secondary eligibility criteria and excluded for clinically relevant CNS pathology, poor performance status, and/or inadequate organ or marrow function (supplemental Methods). Patients meeting secondary eligibility criteria received 1 cycle of blinatumomab consolidation starting on day 42 after auto-SCT. Blinatumomab was administered as a continuous IV infusion at 9 μg per day for 1 week, then 28 μg per day for 3 weeks, for a total of 4 weeks (Figure 1). The primary end point was feasibility and tolerability, defined as the proportion of patients who completed a full course of blinatumomab. Secondary end points included PFS, OS, and CR rates. Exploratory end points included immunopharmacology data, rate of CRS, systemic toxicities, neurological complications, and persistent molecular disease. This study received institutional review board approval at Washington University School of Medicine and was conducted according to the Declaration of Helsinki.
Treatment schema. Auto-SCT: patients who underwent auto-SCT with BEAM conditioning per institutional guidelines. Conditioning regimens other than BEAM were allowed, per treating physician’s discretion. Consolidation: blinatumomab started 6 weeks (±1 week) after auto-SCT. Patients with CR or PR based on pretransplant PET/CT received blinatumomab as a continuous IV infusion (CIVI) at 9 μg/d for 1 week and then 28 μg/d for 3 weeks (total of 4 weeks).
Treatment schema. Auto-SCT: patients who underwent auto-SCT with BEAM conditioning per institutional guidelines. Conditioning regimens other than BEAM were allowed, per treating physician’s discretion. Consolidation: blinatumomab started 6 weeks (±1 week) after auto-SCT. Patients with CR or PR based on pretransplant PET/CT received blinatumomab as a continuous IV infusion (CIVI) at 9 μg/d for 1 week and then 28 μg/d for 3 weeks (total of 4 weeks).
Assessments
Response evaluation with PET/CT was performed at 100 days, 1 year, and 3 years after auto-SCT or when clinical progression was suspected. MRD was quantified by immunoglobulin high-throughput sequencing of plasma cell–free DNA on day 42 after auto-SCT (before blinatumomab) and on day 100 after auto-SCT (1 month after completion of blinatumomab) as previously described.16 Immunophenotyping of T cells in cryopreserved peripheral blood mononuclear cells (PBMCs) collected on days 42 (before blinatumomab), 56 (midpoint of blinatumomab treatment cycle), and 100 (1 month after blinatumomab) after auto-SCT was performed as detailed in this article.
Flow cytometry
PBMCs were isolated from whole blood by Ficoll-Paque density gradient centrifugation. Cryopreserved PBMCs were thawed, washed in phosphate-buffered saline, and stained 15 minutes at room temperature with a Live/Dead Fixable Blue Dead Cell Stain kit (Invitrogen, Carlsbad, CA). Cells were then washed in phosphate-buffered saline supplemented with 0.5% bovine serum albumin and 2 mM EDTA and incubated for 10 minutes at room temperature with human Fc Block (BD Biosciences, San Jose, CA). Pretitrated saturating dilutions of fluorochrome-labeled antibodies (supplemental Table 1) were added, and cells were incubated for 30 minutes at room temperature. Intracellular staining was performed using a transcription factor staining buffer set (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Fluorescence-minus-one controls were used to assess background fluorescence intensity and set gates for populations with negative status. Samples were analyzed on a ZE5 (Bio-Rad, Hercules, CA) flow cytometer. Single stain compensation controls were obtained using UltraComp eBeads (Thermo Fisher Scientific), and data were analyzed using FCS Express (DeNovo Software, Pasadena, CA). Absolute cell numbers were calculated by multiplying the adjusted white blood cell (WBC) count (WBC count minus the absolute neutrophil count to account for Ficoll-Paque purification before banking) by the percentage of each subset within the CD45+ cell population.
Analysis of serum cytokines
A multiplexed bead assay was used to measure the serum concentrations of 17 cytokines or chemokines immediately before (day 42 after auto-SCT) and 24 hours after the initiation of blinatumomab treatment. For this portion of the study, peripheral blood was allowed to clot for 30 to 60 minutes before centrifugation at room temperature for 15 minutes and 2500 g. The serum was removed, centrifuged at 10 000 g for 10 minutes at 4°C, and stored at −80°C. Frozen samples were thawed completely, vortexed, and centrifuged before use to remove particulates. Levels of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF, interferon alfa 2, interferon gamma, interleukin-10 (IL-10), IL-12P70, IL-13, IL-15, IL-17A, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), and tumor necrosis factor α in the plasma were determined using a Milliplex Magnetic Bead Panel Kit (EMD Millipore, Billerica, MA) according to the manufacturer’s instructions. Briefly, fluorescently-labeled capture antibody-coated beads were mixed with standard or sample, incubated overnight at 4°C on an orbital shaker, washed, and then incubated with a biotinylated detection antibody. After washing, beads were incubated with a streptavidin phycoerythrin complex, and the mean fluorescent intensities quantified on a Bio-Plex 200 system (Bio-Rad). All samples were measured in duplicate, and data were analyzed using the Bio-Plex Manager software (Bio-Rad) and a 5-parameter logistic curve fit.
Statistical analyses
Clinical characteristics were summarized using descriptive statistics. OS was defined as the elapsed duration of time from auto-SCT to death. Alive patients were censored at the last follow-up. PFS was defined as the time from auto-SCT to either progression or death, whichever occurred first. Alive patients without progression were censored at the last follow-up. OS and PFS probabilities were calculated using Kaplan-Meier method.
Flow cytometry and cytokines data were summarized using means and standard deviations at each measurement time point. The over-time changes were assessed by 2-way analysis of variance for repeated measurement data, followed by post hoc multiple comparisons for between-group (CR vs progressive disease [PD]) differences at specific time points of interest. To better control the familywise type 1 (false positive) error, the post hoc comparisons were performed only to these biomarkers with an overall significant difference in time, response, or their interaction. Square-root (for cell subset percentages) or logarithm (for cell subset counts or cytokine data) transformation was also performed as necessary to reduce data dispersion and to better satisfy normality assumption. All data analyses were performed using SAS 9.4 (SAS Institutes, Cary, NC), and statistical significance was defined as 2-tailed P value < .05 for all analyses.
Results
Patient characteristics
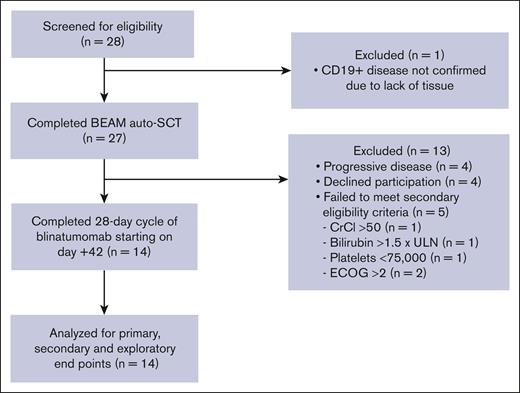
Twenty-eight patients were screened for study participation, and 27 ultimately underwent standard-of-care BEAM auto-SCT. After auto-SCT, 4 patients had PD and were treated off study. Additionally, 4 patients declined participation and 5 patients did not meet the secondary eligibility criteria, allowing for 14 patients for the final analysis (Figure 2). The median age was 64 years, and 10 patients (71%) were male. Seven patients (50%) had DLBCL, and 7 (50%) had transformed large cell lymphoma from low-grade disease. Ten patients (71%) had stage IV disease. Patients had received a median of 2 (range, 2-7) prior lines of therapy, and all received previous anti-CD20 directed therapy and anthracycline-based therapy. Four patients (29%) achieved PR and 10 patients (71%) achieved CR to salvage chemotherapy as determined by CT or PET/CT before auto-SCT. Patient characteristics and outcomes are summarized in Table 1.
CONSORT diagram. CrCl, creatine clearance; ECOG, Eastern Cooperative Oncology Group; ULN, upper limit of normal.
CONSORT diagram. CrCl, creatine clearance; ECOG, Eastern Cooperative Oncology Group; ULN, upper limit of normal.
Patient characteristics and outcomes
| ID . | Age at auto-SCT, y . | Sex . | Race . | Diagnosis, stage . | Prior lines of therapy . | Disease status prior to auto-SCT . | MRD detected after auto-SCT on day 42? . | MRD detected on day 100? . | Response on day 100 . | Relapse after auto-SCT and blin . | DOR after auto-SCT, mo . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | Yes | 33 | No |
| 2 | 64 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | Yes | 8 | No |
| 3 | 65 | M | Caucasian | DLBCL, II | 3 | CR | No | No | CR | Yes | 18 | No |
| 4 | 57 | F | Caucasian | DLBCL, IV | 3 | CR | No | No | CR | No | 49 | No |
| 5 | 64 | M | Caucasian | DLBCL, IV | 2 | PR | No | No | PD | Yes | 5 | Yes |
| 6 | 68 | F | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | No | 37 | No |
| 7 | 74 | M | Caucasian | Transformed FL, III | 2 | PR | Yes | No | CR | Yes | 10 | Yes |
| 8 | 74 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | No | 45 | No |
| 9 | 74 | M | Caucasian | Double hit lymphoma (Bcl-2/MYC), IV | 7 | CR | No | No | CR | No | 37 | No |
| 10 | 53 | F | Asian | Transformed FL, IV | 4 | PR | Yes, below LOD | No | CR | Yes | 24 | No |
| 11 | 61 | F | Caucasian | DLBCL, IV | 2 | CR | No | Yes | PD | Yes | 4 | No |
| 12 | 65 | M | Caucasian | Transformed FL, III | 2 | CR | No | No | CR | Yes | 18 | No |
| 13 | 34 | M | Caucasian | DLBCL, IV | 2 | CR | No | No | CR | Yes | 8 | Yes |
| 14 | 67 | M | Caucasian | DLBCL, III | 2 | PR | No | No | CR | Yes | 12 | Yes |
| ID . | Age at auto-SCT, y . | Sex . | Race . | Diagnosis, stage . | Prior lines of therapy . | Disease status prior to auto-SCT . | MRD detected after auto-SCT on day 42? . | MRD detected on day 100? . | Response on day 100 . | Relapse after auto-SCT and blin . | DOR after auto-SCT, mo . | Death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | Yes | 33 | No |
| 2 | 64 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | Yes | 8 | No |
| 3 | 65 | M | Caucasian | DLBCL, II | 3 | CR | No | No | CR | Yes | 18 | No |
| 4 | 57 | F | Caucasian | DLBCL, IV | 3 | CR | No | No | CR | No | 49 | No |
| 5 | 64 | M | Caucasian | DLBCL, IV | 2 | PR | No | No | PD | Yes | 5 | Yes |
| 6 | 68 | F | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | No | 37 | No |
| 7 | 74 | M | Caucasian | Transformed FL, III | 2 | PR | Yes | No | CR | Yes | 10 | Yes |
| 8 | 74 | M | Caucasian | Transformed FL, IV | 2 | CR | No | No | CR | No | 45 | No |
| 9 | 74 | M | Caucasian | Double hit lymphoma (Bcl-2/MYC), IV | 7 | CR | No | No | CR | No | 37 | No |
| 10 | 53 | F | Asian | Transformed FL, IV | 4 | PR | Yes, below LOD | No | CR | Yes | 24 | No |
| 11 | 61 | F | Caucasian | DLBCL, IV | 2 | CR | No | Yes | PD | Yes | 4 | No |
| 12 | 65 | M | Caucasian | Transformed FL, III | 2 | CR | No | No | CR | Yes | 18 | No |
| 13 | 34 | M | Caucasian | DLBCL, IV | 2 | CR | No | No | CR | Yes | 8 | Yes |
| 14 | 67 | M | Caucasian | DLBCL, III | 2 | PR | No | No | CR | Yes | 12 | Yes |
Blin, blinatumomab; DOR, duration of response; LOD, level of detection.
Safety
Blinatumomab was well tolerated. All patients treated with blinatumomab after auto-SCT completed one 28-day cycle of blinatumomab administered by continuous IV infusion. Blinatumomab started 42 ± 8 days after auto-SCT for all patients. Five patients (36%) developed grade 1 CRS, with no grade ≥2 CRS. Immune effector cell–associated neurotoxicity syndrome was not observed. Six patients (43%) developed transient tremor (grade 1, n = 3; grade 2, n = 1; and grade 3, n = 2), and 1 patient developed grade 3 ataxia; all events completely resolved. One patient developed pneumonitis 28 days after starting blinatumomab (73 days after auto-SCT), which was considered related to BCNU and resolved with steroids. The same patient developed cytomegalovirus viremia that resolved with ganciclovir. Three patients (21%) required hospitalization, 2 for tremor and 1 for ataxia, and 1 patient (7%) had their hospitalization prolonged because of tremor. Other common AEs were fatigue, headache, and lymphopenia (Table 2).
Most common investigator-reported drug-related AEs in the study population
| Event . | Grade 1 to 2 . | Grade 3 . | Grade 4 . | Grade 5 . | Overall . |
|---|---|---|---|---|---|
| n (%) . | |||||
| Arthralgia/myalgia | 6 (43) | 0 | 0 | 0 | 6 (43) |
| Ataxia | 0 | 1 (7) | 0 | 0 | 1 (7) |
| Confusion | 1 (7) | 0 | 0 | 0 | 1 (7) |
| CRS | 5 (35) | 0 | 0 | 0 | 5 (35) |
| Diarrhea | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Dizziness | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Dyspepsia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Fatigue | 9 (63) | 0 | 0 | 0 | 9 (63) |
| Fever | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Flushing | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Headache | 4 (28) | 0 | 0 | 0 | 4 (28) |
| Hyponatremia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Hypotension | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Hypoxia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased aspartate aminotransferase | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased bilirubin | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased creatinine | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Infections | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Lymphopenia | 1 (7) | 7 (49) | 1 (7) | 0 | 9 (63) |
| Nausea | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Neuropathy | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Neutropenia | 1 (7) | 0 | 1 (7) | 0 | 2 (14) |
| Pruritus | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Thrombocytopenia | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Tremor | 4 (28) | 2 (14) | 0 | 0 | 6 (42) |
| Event . | Grade 1 to 2 . | Grade 3 . | Grade 4 . | Grade 5 . | Overall . |
|---|---|---|---|---|---|
| n (%) . | |||||
| Arthralgia/myalgia | 6 (43) | 0 | 0 | 0 | 6 (43) |
| Ataxia | 0 | 1 (7) | 0 | 0 | 1 (7) |
| Confusion | 1 (7) | 0 | 0 | 0 | 1 (7) |
| CRS | 5 (35) | 0 | 0 | 0 | 5 (35) |
| Diarrhea | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Dizziness | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Dyspepsia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Fatigue | 9 (63) | 0 | 0 | 0 | 9 (63) |
| Fever | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Flushing | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Headache | 4 (28) | 0 | 0 | 0 | 4 (28) |
| Hyponatremia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Hypotension | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Hypoxia | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased aspartate aminotransferase | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased bilirubin | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Increased creatinine | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Infections | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Lymphopenia | 1 (7) | 7 (49) | 1 (7) | 0 | 9 (63) |
| Nausea | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Neuropathy | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Neutropenia | 1 (7) | 0 | 1 (7) | 0 | 2 (14) |
| Pruritus | 1 (7) | 0 | 0 | 0 | 1 (7) |
| Thrombocytopenia | 2 (14) | 0 | 0 | 0 | 2 (14) |
| Tremor | 4 (28) | 2 (14) | 0 | 0 | 6 (42) |
Efficacy
The median follow-up duration for all patients was 37 months (range, 12-65). One-hundred days after auto-SCT (one month after blinatumomab consolidation), all patients were alive, with 12 patients (86%) in a complete remission as determined by imaging (CT or PET/CT). At 1 year after auto-SCT, 7 patients (50%) remained in a CR and 1 patient had died from disease progression. At 3 years after auto-SCT, 4 patients (29%) remained in a CR, 10 patients (71%) had PD, and 3 of those with PD had died. The median PFS was 18 months (95% CI, 6 months to not reached), and median OS was not reached. The 3-year PFS and OS were 28.6% (95% CI, 12.5-65.4) and 78.6% (95% CI, 59.8-100), respectively.
One patient’s status converted from detectable MRD on day 42 (after auto-SCT and before blinatumomab) to undetectable MRD on day 100 after receiving blinatumomab consolidation. Unfortunately, this patient subsequently relapsed 9.5 months after auto-SCT. Another patient had MRD present below the level of detection on day 42, and the MRD became undetectable on day 100; this patient was in remission for 24 months after auto-SCT before relapse. One patient had undetectable MRD on day 42; however, on day 100, after receiving blinatumomab, they had detectable MRD, and restaging PET/CT showed PD.
PBMC subsets and phenotype after auto-SCT
To explore whether outcomes were associated with a particular cell subset or phenotype, flow cytometry was performed on peripheral blood samples collected on day 42 (before blinatumomab), day 56 (midpoint of blinatumomab treatment cycle), and day 100 (1 month after the completion of blinatumomab treatment cycle) after auto-SCT. On day 42 after auto-SCT (before blinatumomab), patient WBC subsets, in median (range), were the following: WBC count, 4.8 (2.2-9.6); absolute neutrophile count, 2.9 (1.4-5.5); absolute lymphocyte count, 0.8 (0.2-3.2); CD3+ cells per μL, 691.5 (182.3-3391.3); CD4+ cells per μL, 308.8 (94.6-1921.1); and CD8+ cells per μL, 239.4 (74.3-1391.7) (Table 3). Samples from patients who remained in remission (CR; n = 4) were compared with those from patients with relapsed disease (PD; n = 10) and peripheral blood samples collected from 5 healthy untreated donor controls.
WBC subsets on day +42, before blinatumomab administration
| ID . | WBC per μL . | ANC per μL . | ALC per μL . | CD3+ cells per μL . | CD4+ cells per μL . | CD8+ cells per μL . | CD8:CD4 . |
|---|---|---|---|---|---|---|---|
| 1 | 5.5 | 4.2 | 0.7 | 357.8 | 212.3 | 130.3 | 0.61 |
| 2 | 5.1 | 3.7 | 0.8 | 533.3 | 269.9 | 229.5 | 0.85 |
| 3 | 4.3 | 2.7 | 1.1 | 928.5 | 189.2 | 249.4 | 1.32 |
| 4 | 4.4 | 3.2 | 0.6 | 849.6 | 442.7 | 371.1 | 0.84 |
| 5 | 9.6 | 5.5 | 3.2 | 3391.3 | 1921.1 | 1391.7 | 0.72 |
| 6 | 6.9 | 1.9 | 1.8 | 1622.7 | 1227.5 | 378.3 | 0.31 |
| 7 | 4.3 | 3 | 0.6 | 443.2 | 256.0 | 170.1 | 0.66 |
| 8 | 6 | 3.9 | 0.2 | 482.6 | 347.7 | 120.4 | 0.35 |
| 9 | 4.7 | 2.7 | 0.9 | 1458.7 | 1032.1 | 411.1 | 0.40 |
| 10 | 5.6 | 3.4 | 1.5 | 1415.5 | 883.6 | 423.7 | 0.48 |
| 11 | 4.9 | 2.7 | 1.4 | 1481.6 | 763.5 | 658.1 | 0.86 |
| 12 | 3.5 | 2.7 | 0.3 | 182.3 | 94.6 | 74.3 | 0.79 |
| 13 | 2.2 | 1.4 | 0.5 | 431.9 | 195.0 | 218.9 | 1.12 |
| 14 | 3.5 | 2.3 | 0.3 | 283.5 | 121.3 | 157.8 | 1.30 |
| ID . | WBC per μL . | ANC per μL . | ALC per μL . | CD3+ cells per μL . | CD4+ cells per μL . | CD8+ cells per μL . | CD8:CD4 . |
|---|---|---|---|---|---|---|---|
| 1 | 5.5 | 4.2 | 0.7 | 357.8 | 212.3 | 130.3 | 0.61 |
| 2 | 5.1 | 3.7 | 0.8 | 533.3 | 269.9 | 229.5 | 0.85 |
| 3 | 4.3 | 2.7 | 1.1 | 928.5 | 189.2 | 249.4 | 1.32 |
| 4 | 4.4 | 3.2 | 0.6 | 849.6 | 442.7 | 371.1 | 0.84 |
| 5 | 9.6 | 5.5 | 3.2 | 3391.3 | 1921.1 | 1391.7 | 0.72 |
| 6 | 6.9 | 1.9 | 1.8 | 1622.7 | 1227.5 | 378.3 | 0.31 |
| 7 | 4.3 | 3 | 0.6 | 443.2 | 256.0 | 170.1 | 0.66 |
| 8 | 6 | 3.9 | 0.2 | 482.6 | 347.7 | 120.4 | 0.35 |
| 9 | 4.7 | 2.7 | 0.9 | 1458.7 | 1032.1 | 411.1 | 0.40 |
| 10 | 5.6 | 3.4 | 1.5 | 1415.5 | 883.6 | 423.7 | 0.48 |
| 11 | 4.9 | 2.7 | 1.4 | 1481.6 | 763.5 | 658.1 | 0.86 |
| 12 | 3.5 | 2.7 | 0.3 | 182.3 | 94.6 | 74.3 | 0.79 |
| 13 | 2.2 | 1.4 | 0.5 | 431.9 | 195.0 | 218.9 | 1.12 |
| 14 | 3.5 | 2.3 | 0.3 | 283.5 | 121.3 | 157.8 | 1.30 |
ALC, absolute lymphocyte count; ANC, absolute neutrophile count.
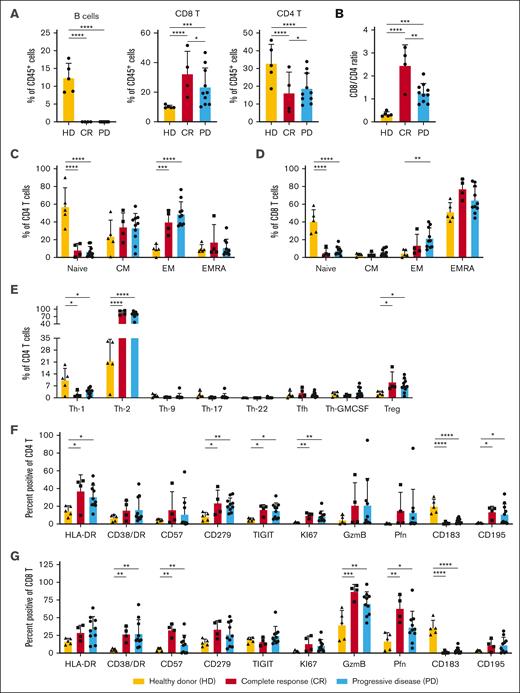
On day 42 after auto-SCT (before blinatumomab), patients who underwent transplantation (both CR and PD) exhibited no significant numbers of circulating CD19+ B cells, with significantly reduced percentages of CD4+ T cells and more CD8+ T cells compared with the healthy donors (Figure 3A). Interestingly, the 10 patients with relapse had a significantly lower CD8:CD4 T-cell ratio before starting blinatumomab than patients who remained in remission (t test, P = .017; Figure 3B). Extended immunophenotyping of the T-cell subsets before blinatumomab treatment revealed that the patients who underwent transplantation (both CR and PD) had significantly lower percentages of naïve CD4 and CD8 T cells, with increased percentages of CD4 effector memory (EM) cells and T helper type 1 and T helper type 2 cells, than the healthy controls (Figure 3C-E). There were significantly increased percentages of CD4 T cells expressing HLA-DR, CD279 (PD1), TIGIT, and KI67 and CD8 T cells expressing granzyme B, perforin, CD57, and CD38/HLA-DR (T-cell activation phenotpye17) in the patients who underwent auto-SCT compared with those in the healthy donors (Figure 3F-G). Increased percentages, but not absolute numbers, of intermediate (CD14+CD16−) monocytes and plasmacytoid dendritic cells were observed before blinatumomab in patients with PD vs CR (supplemental Figure 1A-B).
Flow cytometric evaluation of PBMCs collected immediately before blinatumomab treatment on day 42 after auto-HCT. Flow cytometry was performed on cryopreserved PBMCs collected on day 42 (before blinatumomab) after auto-HCT. Samples from patients who remained in remission (CR; n = 4) were compared with those from patients with relapsed disease (PD; n = 10) and peripheral blood samples collected from 5 healthy untreated donor controls (HDs). (A) Percentage of CD19+CD20+ B cells, CD3+CD56−CD8+ T cells (CD8 T), and CD3+CD56−CD4+ T cells (CD4 T) within the total CD45+ hematopoietic cells. (B) The ratio of CD8 to CD4 T cells within the CD3+CD56− T-cell subset. (C-D) Relative percentages of naïve (CD45RA+CD197+), central memory (CM; CD45RA−CD197+), EM (CD45RA−CD197-), and EMRA (CD45RA+CD197−) CD4 (C) or CD8 (D) T-cell subsets. (E) Percentage of CD4 T helper subsets within the CD3+CD56−CD4+CD45RA− T memory population or regulatory T-cell (Treg; CD3+CD56−CD4+FoxP3+CD25+) subsets. Various chemokine receptors were used to distinguish Th-1 (CD185−CD196−CCR10−CD183+), Th-2 (CD185−CD196−CCR10−CD183−), Th-9 (CD185−CD194−CD196+), Th-17 (CD185−CD196+CCR10−CD183−CD194+), Th-22 (CD185−CD196+CCR10+CD183−CD194+), T follicular helper cells (T-fh; CD185+CCR10−), and T GM-CSF–secreting (Th-GMCSF; CD185−CD196−CCR10+CD183−) cells. (F-G) The percentage of CD4 (F) or CD8 (G) T cells expressing antigens that are altered during T-cell activation, proliferation, exhaustion, and trafficking. P values were calculated using a 2-way analysis of variance for repeated measurement data as described in “Methods.” ∗P < .05; ∗∗P < .001; ∗∗∗P < .0001; ∗∗∗∗P < .00001. EMRA, effector memory re-expressing CD45RA; Th-1, T helper type 1 cell.
Flow cytometric evaluation of PBMCs collected immediately before blinatumomab treatment on day 42 after auto-HCT. Flow cytometry was performed on cryopreserved PBMCs collected on day 42 (before blinatumomab) after auto-HCT. Samples from patients who remained in remission (CR; n = 4) were compared with those from patients with relapsed disease (PD; n = 10) and peripheral blood samples collected from 5 healthy untreated donor controls (HDs). (A) Percentage of CD19+CD20+ B cells, CD3+CD56−CD8+ T cells (CD8 T), and CD3+CD56−CD4+ T cells (CD4 T) within the total CD45+ hematopoietic cells. (B) The ratio of CD8 to CD4 T cells within the CD3+CD56− T-cell subset. (C-D) Relative percentages of naïve (CD45RA+CD197+), central memory (CM; CD45RA−CD197+), EM (CD45RA−CD197-), and EMRA (CD45RA+CD197−) CD4 (C) or CD8 (D) T-cell subsets. (E) Percentage of CD4 T helper subsets within the CD3+CD56−CD4+CD45RA− T memory population or regulatory T-cell (Treg; CD3+CD56−CD4+FoxP3+CD25+) subsets. Various chemokine receptors were used to distinguish Th-1 (CD185−CD196−CCR10−CD183+), Th-2 (CD185−CD196−CCR10−CD183−), Th-9 (CD185−CD194−CD196+), Th-17 (CD185−CD196+CCR10−CD183−CD194+), Th-22 (CD185−CD196+CCR10+CD183−CD194+), T follicular helper cells (T-fh; CD185+CCR10−), and T GM-CSF–secreting (Th-GMCSF; CD185−CD196−CCR10+CD183−) cells. (F-G) The percentage of CD4 (F) or CD8 (G) T cells expressing antigens that are altered during T-cell activation, proliferation, exhaustion, and trafficking. P values were calculated using a 2-way analysis of variance for repeated measurement data as described in “Methods.” ∗P < .05; ∗∗P < .001; ∗∗∗P < .0001; ∗∗∗∗P < .00001. EMRA, effector memory re-expressing CD45RA; Th-1, T helper type 1 cell.
We then compared PBMC subsets in patients who sustained remissions with those with PD after blinatumomab treatment on day 100 after auto-SCT. Similar to day 42 (before blinatumomab), patients with sustained CRs exhibited a statistically greater CD8:CD4 ratio with significantly increased percentages of CD8 T cells and reduced percentages of CD4 T cells on day 100 (1 month after completion of blinatumomab treatment cycle) after auto-SCT (supplemental Figure 2A-C). Neither particular CD4 or CD8 T-cell subset (naïve, central memory, effector memory, or effector memory re-expressing CD45RA) nor phenotype was significantly associated with the sustained remission phenotype (Figure 3C-G; supplemental Figures 2D-E, 3, and 4). Additional analyses of immune cell subtypes on day 42 (before blinatumomab treatment), day 56 (midway through blinatumomab treatment), and day 100 after SCT are reported in the supplemental Methods.
Serum cytokine levels
A multiplexed bead assay was used to measure the serum concentrations of 17 cytokines or chemokines immediately before (day 42 after auto-SCT) and 24 hours after the initiation of blinatumomab treatment. Consistent with the lack of severe clinical CRS, we observed that only G-CSF and MCP-1 were significantly altered after blinatumomab administration (supplemental Figure 5). Serum levels of G-CSF increased twofold (P = .03; day 42 vs day 43), whereas MCP-1 decreased 1.6-fold (P = .02; day 42 vs day 43).
Discussion
This pilot study demonstrates that blinatumomab consolidation after auto-SCT for patients with R/R DLBCL and transformed FL is safe and well tolerated. Our results suggest that 6 weeks after auto-SCT represents the ideal time for initiating T-cell engagers such as blinatumomab, with the goal of eradicating residual lymphoma cells. At this time point, not only have the T-cell compartments recovered, as characterized by an increase in effector memory phenotypes and activated T cells, but also the lowest disease burden early after auto-SCT is present.
Blinatumomab is approved by the Food and Drug Administration currently only for use among patients with R/R B-ALL and B-ALL with detectable MRD after induction. For both indications, blinatumomab is administered continuously at doses up to 28 μg per day, with dosing for R/R B-ALL done stepwise, as in this study, to mitigate the risk of CRS. However, the initial phase 1 dose-escalation and expansion trial of blinatumomab was performed for patients with R/R B-NHL (NCT00274742) and defined the maximally tolerated dose to be 60 μg/m2 per day.18 For patients treated at 60 μg/m2 per day (n = 35), the overall response rate was 69% across NHL subtypes and 55% for DLBCL (n = 11); median duration of response was 404 days (95% CI, 207-1129 days). Longer follow-up of a subset of 38 patients from the original phase 1 cohort found that patients treated with blinatumomab doses ≥60 μg/m2 per day achieved a median PFS of 1.5 years (range, 0-10.3 years) compared with 32 days (range, 0-8 months; hazard ratio, 0.2; 95% CI, 0.1-0.4; P < .001) for patients treated at lower dose levels.19 This suggests that a dose of ≥60 μg/m2 per day is required for durable remissions in patients with R/R B-NHL. In this pilot study, the maximum dose used was 28 μg per day, which is approximately one-fourth of the established maximally tolerated dose for patients with R/R B-NHL. Higher doses of blinatumomab are associated with greater risk of serious AEs, however, potentially it could have provided longer response durations. The main rationale for using a lower dose of blinatumomab assumed that the burden of disease after auto-SCT should be minimal. Additionally, early studies of T-cell engagers demonstrate high potency of blinatumomab at low doses, because engagement of only a few CD3 receptor subunits leads to activation, serial lysis, and proliferation.20
However, blinatumomab consolidation in patients with DLBCL has been investigated previously in a frontline setting. In a phase 2 trial, patients with high-risk DLBCL received induction with standard rituximab-chemotherapy combinations followed by 1 to 2 cycles of blinatumomab consolidation in patients who did not progress by end of treatment PET/CT.21 Using a step-up dosing approach, 28 patients received continuous infusion blinatumomab at a max dose of 112 μg per day for 56 days over the course of a 84-day cycle. After 1 cycle of blinatumomab consolidation, 7 of 8 patients with persistent disease (PR or stable disease) after induction achieved a CR. Although these results are encouraging, ∼60% of patients with newly diagnosed DLBCL are cured with upfront therapy, and for many patients, consolidation strategies after induction will be unnecessary. In contrast, patients with R/R DLBCL are at high risk of poor outcomes and, therefore, are more likely to benefit from consolidative strategies.
Interestingly, in this study, the 4 patients who remained in remission had a significantly higher CD8:CD4 T-cell ratio both before (day 42) and after (day 100) blinatumomab administration than the 10 patients who relapsed. This finding provides mechanistic support for the mode of action of blinatumomab, which uses CD3+ CD8+ effector T cells to kill tumor cells. In a post hoc biomarker analysis of the TOWER study, investigators similarly found a higher CD45+ CD3+ CD8+ T-cell percentage at baseline to be predictive and associated with hematologic remission in the blinatumomab group (odds ratio, 1.44; 95% CI, 1.22-1.70) but not in the chemotherapy group (odds ratio, 1.06; 95% CI, 0.82-1.38).22 A different phase 2 study of blinatumomab maintenance after allogeneic SCT for B-ALL has also demonstrated that patients who maintained a CR had higher proportions of CD8 T cells after therapy.23
Prior in vitro studies have demonstrated that the cytotoxic effect of blinatumomab is mediated through T cells via the perforin pathway rather than through a direct (eg, apoptosis-inducing) effect of the antibody itself.11 Furthermore, isolated CD8 T cells containing preformed cytolytic granules accounted for the cytotoxic effect, whereas CD4 T cells remained largely inactive. Because the cytotoxic effect of blinatumomab is known to correlate with the E:T ratio, strategies to date have focused on decreasing the target (tumor) cells through chemoimmunotherapeutic approaches. However, a higher number of effector cells can also increase the E:T ratio and appears to also predict for patients who will have durable responses with blinatumomab after auto-SCT. Finally, the unusually high activation immunophenotype T effector/granzyme B positive (Teff/GB+) seen in CD8+ T cells of patients with DLBCL after auto-SCT (compared with those seen in resting peripheral blood) may both affect the response to blinatumomab and provide key insights into the optimal timing for administration after auto-SCT.
This phase 1 study was designed to evaluate the safety and tolerability of blinatumomab after auto-SCT. Therefore, the small sample size limits our ability to make conclusions regarding efficacy or compare with alternative chemoimmunotherapy approaches. Considering historical controls, patients with R/R DLBCL treated with BEAM auto-SCT have a 1-year PFS of 50% to 60%, approximating the results presented herein with blinatumomab consolidation.24 The benefit of blinatumomab after auto-SCT could potentially be augmented through the use of additional cycles of therapy or higher doses of IV blinatumomab. Furthermore, future strategies of blinatumomab consolidation should consider initiating treatment at a prespecified CD8 threshold, when the ideal proportion of effector T cells are present to optimize response.
The landscape of treatment options for R/R BCL is rapidly evolving. Although CAR T-cell therapy has the most long-term data, other platforms including antibody-drug conjugates, bispecific T-cell engagers, and targeted therapies, such as Bruton tyrosine kinase inhibitors and PI3 kinase inhibitors, have shown encouraging results in R/R B-NHL. The success of blinatumomab in B-ALL has paved the way for the next generation of bispecific T-cell redirectors in B-NHL that target CD20, including epcoritamab, mosunetuzumab, glofitamab, odronextamab, and plamotamab.25 T-cell engagers have the advantage over autologous CAR T-cell therapies of being immediately available (“off the shelf”) and having lower rates of CRS and neurotoxicity. In addition to the promise of next generation T-cell engagers, the subcutaneous formulation of blinatumomab is being investigated that may improve efficacy, patient convenience, and reduce costs.26,27
When this study was initiated, CAR T-cell therapy for R/R DLBCL had not yet been approved. Even now, patients with DLBCL who relapse more than 1 year after induction receive salvage chemotherapy and, if they respond adequately, auto-SCT. Patients who relapse within 1 year of induction chemotherapy or are refractory to initial treatment are eligible for CAR T-cell therapy. However, those who relapse after secondline CAR T-cell therapy are eligible for innovative salvage therapies followed by auto-SCT and, if still CD19+, may benefit from strategies such as blinatumomab consolidation. Strategies to increase the CD8:CD4 ratio and use additional cycles of consolidation in a larger randomized trial are needed to confirm the efficacy of consolidation with T-cell engagers after auto-SCT.
Acknowledgments
This study was supported by a National Cancer Institute (NCI) Outstanding Investigator Award (R35 CA210084; J.F.D.), an NCI Research Specialist Award (R50 CA211466; M.P.R.), and the Siteman Cancer Center (P30CA91842 Lymphoma Team Science Award; T.A.F. and B.K.).
Authorship
Contribution: N.C.F. and J.C. drafted the manuscript; S.C. performed cytokine analyses; L.G. performed fluorescence-activated cell sorting; E.S. performed sample processing; N.W. and J.R. performed sample processing and fluorescence-activated cell sorting; M.P.R. created the figures for the manuscript and supplemental Methods; and all authors collected and analyzed the data, revised the manuscript critically, and gave final approval to submit the manuscript for publication.
Conflict-of-interest disclosure: A.G. reports honoraria and/or consulting/advisory role and/or speaker's bureau fee and/or research funding from Kite, Celgene, Amgen, and Wugen. N.M.-S. reports consulting/advisory role with Kyowa Kirin, Daiichi Sankyo/UCB Japan, Secura Bio, AstraZeneca, and Genentech/Roche, and research funding from Bristol Myers Squibb, Genentech/Roche, Celgene, Verastem, Innate Pharma, Corvus Pharmaceuticals, AstraZeneca, C4 Therapeutics, Daiichi Sankyo, Yingli Pharma, and Dizal Pharma. T.A.F. reports stock/ownership in Kiadis Pharma, Indapta Therapeutics, and Orca Bio; honoraria from CytoSen; consulting fees from Nkarta and Nektar; and research funding from Altor BioScience, Affimed Therapeutics, and Compass Therapeutics. B.K. reports consulting/advisory role fee from AbbVie, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, MEI Pharma, Kite/Gilead, Janssen, Bristol Myers Squibb, Incyte, TG Therapeutics, and Seattle Genetics/Astellas, and research funding from Genentech, BeiGene, and AstraZeneca. N.L.B. reports research funding from ADC Therapeutics, Autolus, Bristol Myers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, and Seattle Genetics, and advisory role in ADC Therapeutics, Roche/Genentech, and Seattle Genetics. J.F.D. reports equity stock/ownership in Magenta Therapeutics and Wugen; consulting fees from Incyte and RiverVest Venture Partners; board or advisory committee membership in Cellworks Group, RiverVest Venture Partners, and Magenta; research funding from Amphivena Therapeutics, NeoImmune Tech, Macrogenics, Incyte, BioLineRx, and Wugen; speaker fees from Incyte; and has patents in Wugen. The remaining authors declare no competing financial interests.
Correspondence: Armin Ghobadi, Division of Medical Oncology, Section of Stem Cell Transplant and Leukemia, Center for Gene and Cellular Immunotherapy, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007-29, St. Louis, MO 63110; email: arminghobadi@wustl.edu.
References
Author notes
Data are available on request from the corresponding author, Armin Ghobadi (arminghobadi@wustl.edu).
The full-text version of this article contains a data supplement.