Consumption of whole-grain bread, oats, and rice (P < .05), and fruits and vegetables (P = .02) were associated with reduced risk of MGUS.
Intake of sugar-sweetened (P = .04) and artificially sweetened (P = .03) soft drinks was associated with increased risk of MGUS.
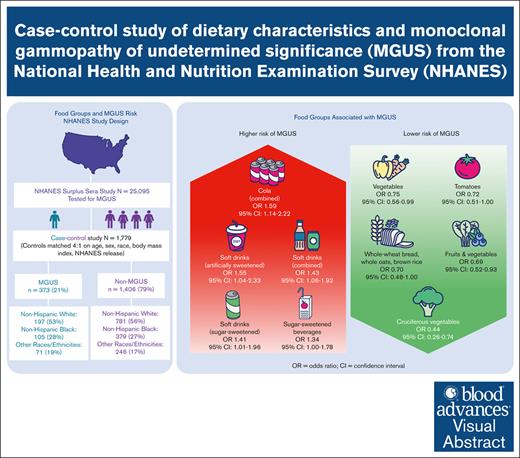
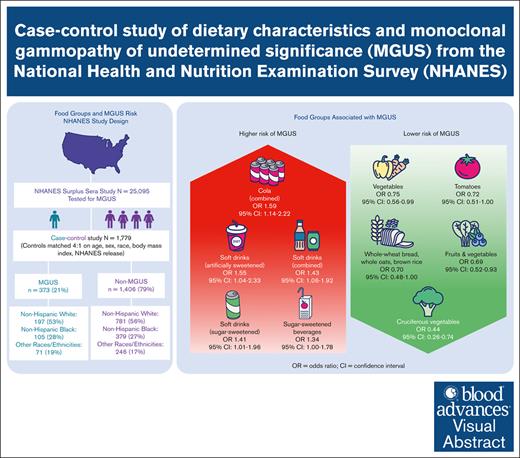
Visual Abstract
Monoclonal gammopathy of undetermined significance (MGUS), a precursor of multiple myeloma, is associated with shorter lifespan and cardiac, renal, neurologic, and immune-related comorbidities. There is little known about modifiable risk factors for this condition. To determine whether the risk of MGUS is associated with dietary factors in a racially diverse population, we conducted a US population–based case-control study from the National Health and Nutrition Examination Survey (1988-2004), which included 373 individuals with MGUS and 1406 matched controls. Diet was characterized by one 24-hour dietary recall, with gram intake of individual foods and beverages aggregated into groups. Unconditional multivariable logistic regressions were used to model associations between intake of several food groups and MGUS, with odds ratios (ORs) and 95% confidence intervals (CIs) reported for the highest relative to the lowest quantile of intake. Daily gram intake of several food and beverage groups were significantly associated with MGUS. MGUS was inversely associated with whole-grain bread, oats, and rice (OR, 0.70; 95% CI, 0.48-1.00; P < .05), fruits (excluding juice) and vegetables (OR, 0.69; 95% CI, 0.52-0.93; P = .02), vegetables (OR, 0.75; 95% CI, 0.56-0.99; P < .05), tomatoes (OR, 0.72; 95% CI, 0.51-1.00; P < .05), and cruciferous vegetables (OR, 0.44; 95% CI, 0.26-0.74; P < .01). Direct associations were observed for sugar-sweetened beverages (OR, 1.34; 95% CI, 1.00-1.78; P < .05), sugar-sweetened soft drinks (OR, 1.41; 95% CI, 1.01-1.96; P = .04), and artificially sweetened soft drinks (OR, 1.55; 95% CI, 1.04-2.33; P = .03). Our study shows that diet is potentially a modifiable risk factor for MGUS.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is characterized by elevated levels of abnormal serum proteins produced by clonal plasma cells. Although usually benign, MGUS progresses to multiple myeloma (MM) or a related disorder at a rate of ∼1% per year.1 Additionally, there is evidence that MGUS is associated with shorter lifespan1,2 and cardiac, renal, neurologic, and immune-related comorbidities.3 An estimated 3.0% to 3.6% of White and 5.9% to 8.4% of Black individuals have MGUS.4
Risk factors for MGUS include older age,4 male sex,4 Black race,4 family history,4 chronic antigenic stimulation,5 infections and inflammatory conditions,6 certain pesticides,4,7 other chemicals,5 diabetes,8 and obesity.9 Obesity more than doubles the risk of progression from MGUS to MM.10
There are limited data on the association between diet and MGUS. In a population-based case-control study from Iceland, intake of fruit during adolescence and whole-wheat bread during middle age were inversely associated with MGUS.11 There is, however, evidence that diet is associated with MM risk or survival,12 including diets with higher inflammatory or insulinemic potential,13 vegetarian or vegan diets (inversely),14 and several foods or dietary compounds.15-18
There is an unmet need to identify additional risk factors related to MGUS that are potentially amenable to intervention because most risk factors for MGUS are nonmodifiable or not easily modifiable. Given that most MM cases are preceded by MGUS and dietary risk factors have been associated with MM, studying the evidence for dietary risk factors for MGUS is warranted.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) program is administrated by the National Center for Health Statistics within the Centers for Disease Control and Prevention. The goal of the program is to assess the health and nutritional status of the US population. To obtain reliable estimates of subgroups, certain populations, such as older persons, African Americans, Mexican Americans, and individuals with low income are oversampled. Further details are available on the Centers for Disease Control and Prevention website.19
Study sample
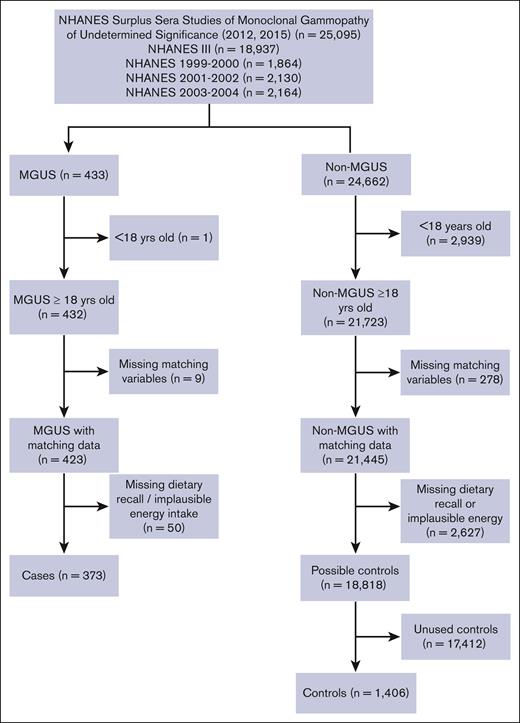
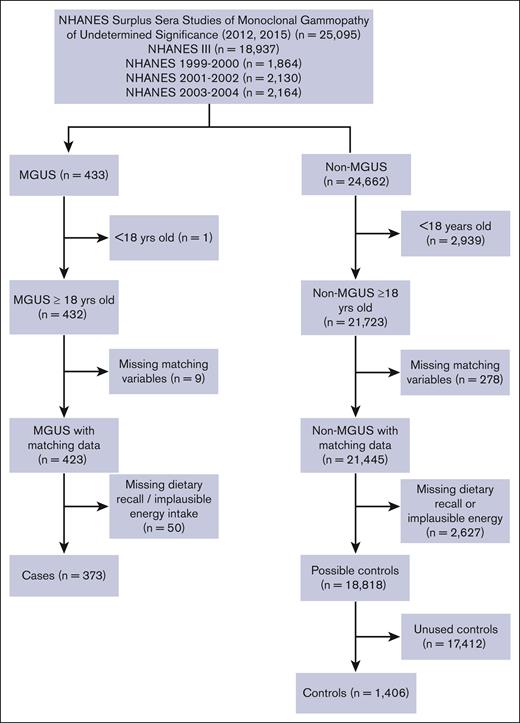
Data from this population-based, case-control study were obtained from 4 releases of NHANES (NHANES III, 1999-2000, 2001-2002, and 2003-2004), which included 77 772 individuals.20 The overall data set was restricted to 25 095 individuals whose sera were included in the surplus sera studies of MGUS released in 2012 for participants aged ≥50 years from NHANES III (n = 6557), 1999 to 2000 (n = 1864), 2001 to 2002 (n = 2130), and 2003 to 2004 (n = 2164) and in 2015 for participants aged <50 years from NHANES III (n = 12 380) (Figure 1), as described previously.21,22
Flowchart of participants included in the NHANES case-control study of dietary characteristics and MGUS.
Flowchart of participants included in the NHANES case-control study of dietary characteristics and MGUS.
Case and control definitions
Potential cases (n = 433) were defined as individuals with MGUS by established criteria.23-26 Potential controls (n = 24 662) were individuals whose sera did not meet the criteria for MGUS. We excluded individuals with age <18 years (1 case and 2939 controls), missing matching characteristics (9 cases and 278 controls), missing 24-hour dietary recall (DR), or implausible total energy intake (<500 or >3500 kilocalories per day [kcal/d]27; 50 cases and 2627 controls), leaving a total of 373 cases and 18 818 possible controls. Controls were frequency matched at a rate of 4 controls to 1 case on 5 criteria: NHANES release, sex, race/ethnicity, age at interview (5-year categories), and body mass index (BMI) category (<18.5, 18.5-24.9, 25.0-29.9, and ≥30.0 kg/m2; Figure 1).
Descriptive characteristics and dietary measures
Descriptive characteristics were available through NHANES interview questionnaires done before MGUS ascertainment.20 BMI was obtained by physical examination. Individuals with MGUS were risk stratified based on International Myeloma Working Group criteria (non–immunoglobulin G [IgG]–type MGUS, M protein concentration >1.5 g/dL, and/or abnormal serum free light chain ratio) into low-risk MGUS (LRMGUS; 0 risk factors) and intermediate/high-risk MGUS (IHRMGUS; ≥1 risk factors).28
Dietary practices were assessed with a DR that captured food and beverage intake for 24 hours through midnight before the interview. Based on the DR, NHANES provides individual estimates of total energy intake (kcal/d), macronutrients (g/d), and micronutrients. Additionally, the DR provided detail on individual foods consumed, classified by their 8-digit food code assigned by the US Department of Agriculture (USDA). The first digit identifies the food group as described in the USDA Food and Nutrient Database for Dietary Studies documentation29: (1) milk and milk products; (2) meat, poultry, fish, and mixtures; (3) eggs; (4) legumes, nuts, and seeds; (5) grain products; (6) fruits; (7) vegetables; (8) fats, oils, and salad dressings; and (9) sugars, sweets, and beverages. We divided (6) fruits into (6a) fruits (excluding juices) and (6b) fruit juices, and we created a combined “fruits (excluding juice) and vegetables” group.
Subsequent digits of the USDA food code allow for foods to be assigned to even more specific groups. We created 12 more custom food groups based on the second or third digit or, in a few instances, by selection of individual foods based on their 8-digit code. These custom groups provided insights into the drivers of an association for a primary food group or because of prior associations with MGUS or cancer (supplemental Table 1). For each primary and custom food group, we calculated each participant’s total grams consumed per day (g/d). Consumption of each food group (g/d) was divided into tertiles (Ts) or, for highly skewed distributions, quantiles (Qs), based on the distribution in the controls. When >90% of the controls had no intake, the sample was divided into “none” or “any” consumption.
The Healthy Eating Index (HEI), a measure of diet quality used to assess how well a set of foods aligns with the Dietary Guidelines for Americans, was available for NHANES III and calculated from DR data. Additionally, NHANES III administered an extensive food frequency questionnaire (FFQ), which was included as supplementary analyses for this study. This queried for the monthly intake of 60 items. Each item was mapped to 11 primary and 10 custom FFQ food groups, which largely aligned with the USDA-based DR food groups described earlier (supplemental Tables 2 and 3). Monthly consumption (times per month) was assigned into Ts or Qs, based on the distribution in controls.
Statistical methods
Key demographic and health-related characteristics of cases and controls were compared using Student t tests for differences in means or χ2 tests for differences in proportion.
Due to the frequency match between cases and controls, that is, not unique matching, unconditional multivariable logistic regression analyses were used to model the association between MGUS, LRMGUS, and IHRMGUS and daily intake of primary and custom food groups, with odds ratios (ORs) and 95% confidence intervals (CIs) as the measures of association. Firth’s corrections were applied to logistic regressions with referent groups representing ≥70% of the controls. Total daily energy intake (kcal), age in years, sex, race/ethnicity, and continuous BMI were included as covariates in all multivariable models. Variables associated with socioeconomic status (education and family income) were not included in the final models because they did not substantially affect the modeled measures of association. P values for food groups with ≥2 Qs of intake are stated as P for trend (Ptrend) between Q midpoints.
As secondary analyses, the association between MGUS, LRMGUS, and IHRMGUS and monthly intake of primary and custom FFQ food groups for 1079 participants in NHANES III was examined and modeled similar to the primary analyses.
In all models, a value of P ≤ .05 was considered significant. All analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC).
Results
Participant characteristics
There are 1779 total participants: 373 with MGUS and 1406 matched controls, included in the analysis. The mean age at interview is 67.3 years. The participants are predominantly overweight/obese (69.7%), from the NHANES III cycle (61.0%), male (57.6%), non-Hispanic White (55.0%), with no college education (69.5%), married (61.0%; data not shown), have a family income >$20 000 per year (52.1%), and are not current smokers (82.8%). The study was racially diverse with 45.0% minorities, including 27.2% non-Hispanic Blacks and 16.0% Mexican Americans. There are no statistically significant differences in these characteristics between cases and controls (Table 1).
Diet and MGUS association
Food groups (DR)
Participants report eating 1730 kcal per day on average. Participants from NHANES III have a mean HEI score of 64, indicating an average diet that needs improvement by USDA Center for Nutrition Policy and Promotion standards.30 This HEI score is roughly comparable with the US national average in 1994 among individuals aged ≥51 years; 64.0 for males and 67.1 for females.31 There are no significant differences in total energy, HEI, or macronutrient intake between cases and controls (data not shown).
Multivariable models of the association between daily food intake and MGUS, LRMGUS, and IHRMGUS, as defined by USDA primary or custom food groups, are shown for plant-based food groups (Table 2), animal-based food groups (Table 3), and fats, sweets, and beverages (Table 4).
Daily intakes of certain plant-based foods were inversely associated with MGUS, as shown in Table 2. Intake of whole-wheat bread, whole oats, and brown rice showed an inverse association with MGUS (Q2 OR, 0.70; 95% CI, 0.48-1.00; P < .05) and LRMGUS (Q2 OR, 0.51; 95% CI, 0.28-0.93; P = .03). Intake of fruits (excluding juice) and vegetables showed a dose-dependent (Ptrend = .02) inverse association with MGUS (T3 OR, 0.69; 95% CI, 0.52-0.93; P = .02). Taken alone, intake of fruits (excluding juice) was inversely associated with LRMGUS (Q2 OR, 0.62; 95% CI, 0.38-0.99; P < .05). Intake of vegetables was inversely associated with MGUS (T3 OR, 0.75; 95% CI, 0.56-0.99; P < .05). Intake of tomatoes showed an inverse association with MGUS (Q2 OR, 0.72; 95% CI, 0.51-1.00; P < .05). Intake of cruciferous vegetables showed an inverse association with both MGUS (Q2 OR, 0.44; 95% CI, 0.26-0.74; P < .01) and IHRMGUS (Q2 OR, 0.22; 95% CI, 0.09-0.57; P < .01).
Among animal products (Table 3), there was a suggestive dose-dependent (Ptrend = .08) positive association between consumption of milk and milk products and IHRMGUS (T3 OR, 1.53; 95% CI, 0.99-2.36; P = .05). Processed meat intake at intermediate levels was associated with LRMGUS (Q1 OR, 1.78; 95% CI, 1.19-2.67; P < .01), but there was a nonsignificant association at the highest level (Q2 OR, 1.31; 95% CI, 0.82-2.08; P = .26).
Among fats, sweets, and beverages (Table 4), the daily gram intake of the primary USDA food group sugars, sweets, and beverages was associated with MGUS (T3 OR, 1.39; 95% CI, 1.02-1.89; P = .04) and LRMGUS (T3 OR, 1.68; 95% CI, 1.08-2.60; P = .02). This overall association is attributed to sugar- and artificially sweetened soft drinks, as described further, with no associations seen for the other major contributors of this primary USDA food group, such as desserts, candy, coffee, or alcoholic beverages (data not shown).
Intake of sugar-sweetened beverages was associated with both MGUS (Q2 OR, 1.34; 95% CI, 1.00-1.78; P < .05) and LRMGUS (Q2 OR, 1.68; 95% CI, 1.13-2.49; P = .01) but not with IHRMGUS. Intake of soft drinks, including both sugar-sweetened and artificially sweetened, was associated with MGUS (Q2 OR, 1.43; 95% CI, 1.06-1.92; P = .02) and LRMGUS (Q2 OR, 1.68; 95% CI, 1.11-2.53; P = .01). Similarly, intake of soft drinks (sugar-sweetened) was associated with MGUS (Q2 OR, 1.41; 95% CI, 1.01-1.96; P = .04). There are even more pronounced associations between intake of soft drinks (artificially sweetened) and MGUS (Q2 OR, 1.55; 95% CI, 1.04-2.33; P = .03) and LRMGUS (Q2 OR, 2.13; 95% CI, 1.28-3.56; P < .01). Similarly, daily intake of cola (regular and diet) was associated with MGUS (Q2 OR, 1.59; 95% CI, 1.14-2.22; P < .01) and LRMGUS (Q2 OR, 1.77; 95% CI, 1.13-2.78; P = .01) but not IHRMGUS. Lastly, any daily intake of any tea (sugar-sweetened) was associated with LRMGUS (OR, 1.88; 95% CI, 1.07-3.28; P = .03). By comparison, tea (unsweetened) exhibited nonsignificant inverse associations with MGUS, LRMGUS, and IHRMGUS, suggesting that added sugar is relevant to MGUS.
Usual intake (FFQ)
Our secondary analyses of the association in NHANES III between usual monthly frequency of intake of 60 items aggregated into primary (supplemental Table 4) and custom (supplemental Table 5) food groups and MGUS, LRMGUS, and IHRMGUS provides support for some of the associations seen in the primary analysis of daily intake. There are dose-dependent associations between fruit juice and MGUS and IHRMGUS and between meat, poultry, seafood, and stews and MGUS. There was also an association between soft drinks (artificially sweetened) and MGUS and a borderline association with LRMGUS. Lastly, monthly intake of tea is inversely related to LRMGUS.
Discussion
In this study, there were associations between MGUS and daily intake of certain food groups, suggesting that lower diet quality is associated with MGUS. Notably, low daily intake of whole-wheat bread, whole oats, brown rice, fruits (excluding juices) and vegetables, tomatoes, and cruciferous vegetables and high daily intake of processed meat, sugars, sweets, and beverages, specifically sugar-sweetened and artificially sweetened beverages, were associated with MGUS. Most findings were consistent with the American Institute of Cancer Research and World Cancer Research Fund cancer prevention recommendations, which recommend eating a diet rich in whole grains, vegetables, fruits, and beans and limiting the consumption of sugar-sweetened drinks, fast and processed foods, and red and processed meat.
As noted, intakes of whole grains, fruits, and vegetables show inverse associations with the risk of MGUS, LRMGUS, and/or IHRMGUS, with a particularly strong inverse association between cruciferous vegetables and IHRMGUS. This is consistent with large epidemiologic studies that have shown a reduced cancer risk associated with intake of fruits and vegetables, especially cruciferous and green-yellow vegetables32 and whole grains.33 Plant-based foods reduce cancer risk through multiple mechanisms. They are a rich source of dietary flavonoids and fiber, which decrease levels of insulin, insulin-like growth factor 1, and inflammation.34 Dietary flavonoids and fiber also have beneficial effects on the immune system and microbiome, through increased levels of butyrate producers.34,35 Higher stool butyrate producers and butyrate concentrations have been associated with sustained minimal residual disease negativity in patients with MM.36 Cruciferous vegetables (a group of vegetables from the Brassica genus of plants37) are unique dietary sources of glucosinolates, which hydrolyze in vivo to isothiocyanates (eg, sulforaphane) and indoles, with known anticancer properties.38 Tomatoes are a major source of carotenoids such as lycopene and beta-carotene that have anticancer properties39 and are a good source of vitamin C.40
Among animal-based foods, the direct association between MGUS and intake of processed meat is consistent with its classification by the International Agency for Research on Cancer as a group 1 carcinogen, although this classification was based on associations with colorectal and gastric cancer.41
The association between MGUS and high daily intake of sugar-sweetened and artificially sweetened beverages could be due to multiple underlying mechanisms. They are a concentrated source of calories without other nutrients, such as fiber, to mitigate these effects and thus contribute to obesity, which is linked with MGUS.9 Obesity can promote systemic inflammation and lower the production of adiponectin,42 an adipokine with anti-inflammatory, insulin-sensitizing, and antitumor effects, including in MM cells.43 High intake of sugar is linked to chronic inflammation,44 which can facilitate tumorigenesis.45 Sugar intake can also lead to increased insulin and insulin-like growth factor 1, a potent mitogen in myeloma cells.46 Soft drinks include phosphoric acid, which may dysregulate the calcium/phosphorus balance, leading to decreased bone density and fractures47 and possibly tumorigenesis.48 Sugary drinks and/or fruit juices have been previously associated with the risk of overall, breast, biliary tract, colorectal, prostate, thyroid, skin, and pancreatic cancers.49,50 Thus, it is important to differentiate refined carbohydrates, such as those found in sugary drinks, from complex carbohydrates, such as found in whole grains, because they have opposite associations with MGUS risk and risk of other cancers.
Associations between dietary intake and MGUS differ by risk of progression, with no food groups or nutrients significant in both LRMGUS and IHRMGUS, possibly due to the small subgroup sample sizes. Most notably, the associations between sugar-sweetened and artificially sweetened beverages and LRMGUS, the most marked associations observed in this study, are null in IHRMGUS, suggesting that there could be etiologic differences between LRMGUS and IHRMGUS; the etiology of IHRMGUS could be overwhelmed by genetics or environmental exposure, obscuring associations between diet and the condition.
Strengths of the study include the comprehensive dietary assessment, using both quantitative (DR) and nonquantitative (FFQ) tools, capturing dietary practices in a racially diverse cohort that included minority populations. The large number of participants without MGUS allowed for us to match multiple characteristics associated with MGUS. Blood samples were randomly evaluated for MGUS without any pre-existing suspicion of MGUS, resulting in an unbiased method used to ascertain case-control status. All participants were subject to the dietary evaluations before their blood being tested for MGUS, reducing the risk of recall bias.
A weakness is that certain food groups combine heterogenous foods and beverages with possibly different physiologic effects. This might have obscured associations that would have been more apparent if it were possible to analyze intake of individual food items in relation to MGUS, although that would be problematic from a statistical power standpoint. Additionally, the representation by both solid foods and beverages in certain categories complicate the interpretation, because the total gram weight consumption of the category is largely driven by intake of beverages, which tend to be consumed in larger quantities (by gram weight) and are highly variable from person to person.
Another limitation is that the assessment tools (DR and FFQ) provide a snapshot of a person’s diet that might not be connected to the biologically relevant exposure window. Longer-term dietary data, such as those used in the Iceland study,11 would benefit future analyses. Additionally, this study did not assess the overall dietary patterns in relation to MGUS and can be addressed in future studies. Furthermore, the study population had a low-quality diet overall, with the majority of the population being below the recommended daily allowances of many micronutrients studied. This limited distribution made it difficult to detect an association between healthful dietary intakes and MGUS.
Conclusions
To the best of our knowledge, this is the most comprehensive study of dietary risk factors in MGUS in a racially diverse US population. This study provides evidence to support that diet may be a risk factor for MGUS. Given the case-control study design, it is not possible to determine a causal relationship between diet and MGUS. Future studies should focus on dietary patterns and examine dietary composition at multiple timepoints throughout life to interrogate the biologically relevant exposure window. There is also a need to examine whether the observed associations differ by race, sex, or obesity status, which are established risk factors for MGUS,4,9 and physical activity, an emerging risk factor for several cancers, including MM.51 Nevertheless, this study provides insight into how diet, a modifiable risk factor, could be related to MGUS, a condition for which very little is known regarding risk-reduction strategies.
Acknowledgments
The authors are grateful to Jeanne A. Joseph for her careful editing of the manuscript.
This study is funded in part through the National Institutes of Health/National Cancer Institute Cancer Center support grant at Memorial Sloan Kettering P30 CA008748; received research and salary support from the National Cancer Institute MSK Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746 and American Society of Hematology Scholar Award (U.A.S.); also research funding from the Paula and Rodger Riney Foundation and HealthTree Foundation (U.A.S.); and is partially supported by the Roswell Park Alliance Foundation (J.M.J.).
Authorship
Contribution: J.M.J., S.E.M., and U.A.S. designed the study and initiated this work; J.M.J. developed the statistical analysis plans and analyzed the data; J.M.J. and U.A.S. wrote the manuscript; and all authors made substantial contributions to design of the study, interpretation of results, revised the manuscript critically, and gave final approval of the manuscript to be submitted.
Conflict-of-interest disclosure: J.H. serves on the advisory boards of Adaptive, Amgen, Angitia, Axxess Networks, Bristol Myers Squibb (BMS), GlaxoSmithKline, Janssen Biotech, Oncopeptides, ONCOtracker, Prothena, Sanofi, and Skyline; has given talks at Amgen, BeiGene, Beijing Medical Award Foundation, Curio Science, and Janssen Biotech; and serves on the data and safety monitoring board of Janssen Biotech. U.A.S. reports support from National Institutes of Health/National Cancer Institute Cancer Center grant P30CA008748, MSK Paul Calabresi Career Development Award for Clinical Oncology K12CA184746, Paula and Rodger Riney Foundation, Allen Foundation Inc, Parker Institute for Cancer Immunotherapy at MSK, HealthTree Foundation, and International Myeloma Society as well as nonfinancial support from American Society of Hematology Clinical Research Training Institute, Transdisciplinary Research in Energetics and Cancer training workshop R25CA203650 (principal investigator: Melinda Irwin); research funding support from Celgene/BMS and Janssen to the institution, nonfinancial research support from Plantable, Sabinsa pharmaceuticals, and M&M Labs to the institution; personal fees from Association of Community Cancer Centers, MashUp MD, Janssen Biotech, Sanofi, BMS, MJH LifeSciences, Intellisphere, Phillips Gilmore Oncology Communications, RedMedEd, and i3Health outside the submitted work. A.M.L. reports a grant from Novartis, during the conduct of the study; grants from BMS; personal fees from Trillium Therapeutics; grants, personal fees, and nonfinancial support from Pfizer; grants and personal fees from Janssen, outside the submitted work; and has a patent US20150037346A1, with royalties paid. O.L. reports personal fees from Amgen, Celgene, Janssen, Karyopharm, Adaptive Biotech, Binding Site, and BMS; other support from Takeda, Janssen, and Merck; and grants from Tow Foundation, Perelman Family Foundation, National Cancer Institute, and Riney Foundation outside the submitted work. S.Z.U. reports grants and personal fees from AbbVie, 404 Amgen, BMS, Celgene, GlaxoSmithKline, Janssen, Merck, MundiPharma, Oncopeptides, 405 Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, and Takeda. The remaining authors declare no potential competing interests.
Susan E. McCann died on 5 May 2022.
Correspondence: Janine M. Joseph, Department of Cancer Prevention and Control, Roswell Park Comprehensive Cancer Center, Elm and Carlton Sts, Buffalo, NY 14263; email: janine.joseph@roswellpark.org; and Urvi A. Shah, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: shahu@mskcc.org.
References
Author notes
Original data sets are publicly available from the Centers for Disease Control and Prevention (https://wwwn.cdc.gov/nchs/nhanes/).
The data sets generated during this study are available on reasonable request from the corresponding author, Janine M. Joseph (janine.joseph@roswellpark.org).
The full-text version of this article contains a data supplement.