Key Points
Activating PK improved red cell integrity in SCD by reducing Tyr-p-bd3.
ATP plays a critical role in preventing the membrane-destabilizing effects of Tyr-p-bd3 by improving activity of constitutive phosphatase.
Visual Abstract
In a phase 1 study (NCT04000165), we established proof of concept for activating pyruvate kinase (PK) in sickle cell disease (SCD) as a viable antisickling therapy. AG-348 (mitapivat), a PK activator, increased adenosine triphosphate (ATP) and decreased 2,3-diphosphoglycerate levels while patients were on treatment, in line with the mechanism of the drug. We noted that the increased hemoglobin (Hb) persisted for 4 weeks after stopping AG-348 until the end of study (EOS). Here, we investigated the pathways modulated by activating PK that may contribute to the improved red blood cell (RBC) survival after AG-348 cessation. We evaluated frozen whole blood samples taken at multiple time points from patients in the phase 1 study, from which RBC ghosts were isolated and analyzed by western blotting for tyrosine phosphorylation of band 3 (Tyr-p-bd3), ankyrin-1, and intact (active) protein tyrosine phosphatase 1B (PTP1B) levels. We observed a significant dose-dependent decrease in mean Tyr-p-bd3 from baseline in the patients, accompanied by an increase in the levels of membrane-associated ankyrin-1 and intact PTP1B, all of which returned to near baseline by EOS. Because PTP1B is cleaved (inactivated) by intracellular Ca2+-dependent calpain, we next measured the effect of AG-348 on ATP production and calpain activity and the plasma membrane Ca2+ ATPase pump–mediated efflux kinetics in HbAA and HbSS erythrocytes. AG-348 treatment increased ATP levels, decreased calpain activity, and increased Ca2+ efflux. Altogether, our data indicate that ATP increase is a key mechanism underlying the increase in hemoglobin levels upon PK activation in SCD. This trial was registered at www.clinicaltrials.gov as #NCT04000165.
Introduction
A prime target in sickle cell disease (SCD) therapeutics is hemoglobin S (HbS) polymerization, which causes stiffening and distortion (“sickling”) of red blood cells (RBCs); these events initiate hemolysis and microvascular occlusion, leading to episodes of severe pain, hemolytic anemia, and progressive organ damage.1-4 HbS polymerizes only when deoxygenated, and intracellular concentration of 2,3-diphosphoglycerate (2,3-DPG) is a key factor influencing HbS oxygenation.5 Increased 2,3-DPG promotes HbS polymerization by shifting the oxygen dissociation curve to the right, increasing the fraction of the polymerizing T conformation,6 and stabilizing the HbS fiber.7-9 Additionally, 2,3-DPG decreases intracellular pH, which further promotes HbS polymerization.10 2,3-DPG levels in RBCs of patients with SCD are significantly higher than those of healthy patients11; indeed, reducing 2,3-DPG had been proposed as an approach to antisickling therapy.9,10
Pyruvate kinase (PK) is a key enzyme in glycolysis; its activity regulates the rate of the final adenosine triphosphate (ATP)–generating step in the glycolytic pathway. Increasing PK activity increases glycolytic flux, elevates ATP, and decreases intracellular levels of the glycolytic intermediate 2,3-DPG. In a phase 1 dose escalation study in adults with HbSS (NCT04000165),12 AG-348 (mitapivat), a PK activator, improved hematologic parameters, increased oxygen affinity, and reduced sickling kinetics. In keeping with its mechanism of action, we observed a dose-dependent increase in ATP and a decrease in 2,3-DPG levels that returned to baseline at the end of drug taper. The increase in Hb levels were sustained after stopping AG-348 at the end of taper until follow-up 4 weeks later (end of the study [EOS]), suggesting an improvement in RBC survival.
We hypothesized that AG-348 treatment affects the state of tyrosine phosphorylation of band 3 (Tyr-p-bd3), which is a critical determinant of RBC integrity.13,14 We performed western blotting on the stored frozen RBC samples from participants treated with AG-348 in the phase 1 study12 and confirmed a significant dose-dependent reduction in RBC Tyr-p-bd3 that was accompanied by increased red cell membrane ankyrin-1 and a significant dose-dependent increase in red cell membrane–associated intact protein tyrosine phosphatase 1B (PTP1B).
PTP1B can be cleaved into an inactive form by calpain, which in turn must be activated by intracellular Ca2+,15 which is known to be elevated in RBCs from patients with SCD.16-18 To further elucidate the mechanics of how AG-348 therapy restores the activity of PTP1B, we performed a series of ex vivo experiments and showed that AG-348 treatment prevented calpain-mediated cleavage and inactivation of PTP1B that is, in turn, caused by the lower intracellular Ca2+ ions from the increased activity of the plasma membrane Ca2+ ATPase (PMCA) pump.
Our study provides evidence for another mechanistic role for ATP in RBC metabolism, in which it prevents the membrane-destabilizing effects of Tyr-p-bd3 by suppressing the Ca2+-mediated cleavage of PTP1B.
Methods and materials
Details on the study cohort and methodology are provided in the supplemental Material.
Human participants and preparation of blood samples
Whole blood samples were evaluable in 15 patients enrolled in a phase 1 AG-348 dose escalation study (NCT04000165),12 from which RBC ghosts were isolated at multiple time points (baseline, after 2 weeks of 5, 20, 50, and 100 mg twice daily, end of drug taper, and EOS; supplemental Figure 1) and analyzed by western blotting for Tyr-p-bd3, ankyrin-1, and intact PTP1B levels. Blood samples for ex vivo studies were obtained from ethnic-matched healthy controls (HbAA) and HbSS patients enrolled under protocol 03H015 (#NCT00047996), approved by the National Heart, Lung, and Blood Institute Institutional Review Board and in accordance with the Declaration of Helsinki.19 Patients were excluded if they were aged <18 or >80 years, were pregnant, or had a history of blood transfusion in the previous 8 weeks. For ex vivo experiments, peripheral whole blood was collected in EDTA tubes; 300-μL blood samples were centrifuged at 1000g for 10 minutes at 4°C; and plasma and buffy coat were removed. RBC pellets were washed twice with phosphate-buffered saline (PBS) containing 1% glucose, 170 mg/L adenine, and 5.25 g/L mannitol and used fresh or frozen at –80°C until needed.
Antibodies and reagents
Specific antibody against Tyr-8 and Tyr-21 of band 3 was provided by Philip Low (Purdue University) and antibodies against band 3, ankyrin-1, and α-spectrin by Xiuli An (New York Blood Center). Mouse antibodies against band 3 (for immunoprecipitation) and PTP1B were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit antibodies against PTP1B (for immunoprecipitation) were purchased from Cell Signaling (Danvers, MA), against ankyrin-1 from Bethyl Laboratories (Montgomery, TX), and against actin from Sigma-Aldrich (St. Louis, MO). AG-348 and AG-946 (an investigational, small-molecule, oral allosteric activator of PK) were provided by Agios Pharmaceuticals Inc (Cambridge, MA) and dissolved in dimethyl sulfoxide (DMSO). R406 was purchased from MedChemExpress (Monmouth Junction, NJ) and dissolved in DMSO.
Preparation of red cell ghosts (membranes), whole cell lysates, and western blotting
Red cell ghosts were isolated from frozen whole blood samples from patients enrolled under the phase 1 study (NCT04000165) and other participants for ex vivo studies on the effects of AG-348, AG-946, and R406, using hypotonic lysis buffer. The RBC whole cell lysates were obtained by adding radioimmunoprecipitation assay buffer and sonicating. The lysates were mixed with NuPAGE LDS sample buffer (4×) and then subjected to western blotting (see the supplemental Material for details).
ATP assay and calpain activity assay
For ATP assay, HbSS RBCs were treated with DMSO (Control [Con]) or different concentrations of AG-348 for different times, and the ATP level was analyzed using CellTiter-Glo 2.0 Cell Viability Assay kit (Promega, Madison, WI) according to the manufacturer’s instruction.
For calpain activity assay, HbSS RBCs were treated with DMSO (Con) or different concentrations of AG-348 overnight, and the activity of calpain was measured using Calpain Activity Assay Kit (Abcam, Waltham, MA) according to the manufacturer’s instruction (see the supplemental Material for details).
Immunoprecipitation
HbSS RBCs were treated with different concentrations of AG-348 for different times and subjected to ghost preparation as described above. A total of 500-μL lysate was incubated overnight (4°C with mixing) with 5 μL of anti-PTP1B and anti–ankyrin-1 antibodies or control immunoglobulin G (IgG) for another 6 hours, after which 30 μL of Dynabeads Protein G beads (4°C; Invitrogen, Waltham, MA) were added. Beads were then washed 3 times with radioimmunoprecipitation assay buffer before proteins were eluted, separated by 4% to 12% NuPAGE gel (Invitrogen, Waltham, MA).
Ca2+ efflux assay
We used a recently developed fluorescence assay that tracks PMCA activity after ionophore-mediated Ca2+ loading at levels that saturate the calcium pump.20 Upon collection, blood was washed 3 times in RPMI 1640 and stored at 50% hematocrit at 4°C. A total of 1.5 mL each of HbAA and HbSS red cell pellets were washed 3 times in 10-mL 1× PBS, then treated to a 6-hour incubation at 37°C and 300 revolutions per minute with either 30 μM AG-348 or a matched DMSO control in 1× PBS containing 1% glucose, 170 mg/L adenine, and 5.25 g/L mannitol. After incubation, the samples were washed in cold 3-(N-morpholino)propanesulfonic acid (MOPS) Buffer (100-mM KCl + 30-mM MOPS), then loaded with Ca2+ for 20 minutes in a 37°C water bath using MOPS Buffer containing 500-nM A23187 ionophore, 150-μM MgCl2, and 500-μM CaCl2. After this, the sample was washed 3 times in cold MOPS Buffer with AlbuMAX II (Gibco; lipid-rich albumin) to remove the ionophore, then resuspended at 5% hematocrit in final efflux buffer (MOPS Buffer + 0.2-μM Fluo-8 dye and 10-μM EGTA). To initiate Ca2+ efflux, the samples were placed in a 37°C water bath, and aliquots were taken at set time intervals for analysis. For each time point (0, 8, 16, 24, 32, 40, and 60 minutes), 100-μL aliquots were transferred to wells of a 384-well plate in triplicate for each experimental arm, centrifuged to pellet blood cells, and read in a Synergy Neo2 reader (BioTek). Experiments were repeated using 4 pairs of HbAA and HbSS samples.
Statistical analyses
Linear mixed effects regression models were used to evaluate the dose-dependent effects of mitapivat on Tyr-p-bd3, PTP1B, and ankyrin levels using data from 15 participants. The P values given for these models estimate dose level effects for a given outcome measure while adjusting for sex and age. The P value threshold for declaring statistical significance was a 2-sided P value ≤ .05; results were not adjusted for multiple comparisons. For ex vivo experiments, data are mean ± standard error of the mean of values from 3 experiments, with significance defined as P value < .05 using Student t test.
Results
AG-348 decreased tyrosine phosphorylation of RBC band 3 in a dose- and time-dependent manner, in vivo and ex vivo
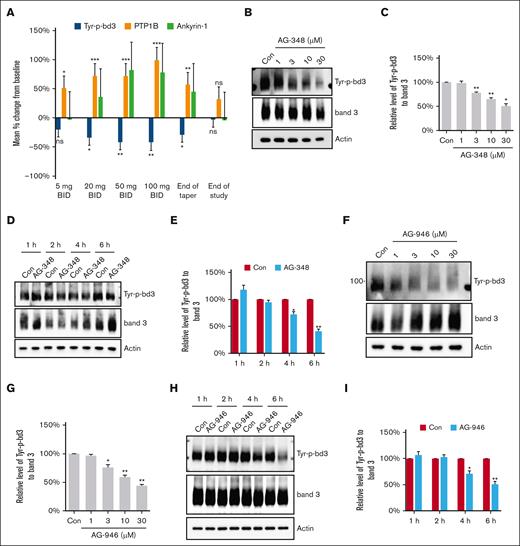
RBC ghosts at the different time points from 15 patients in the phase 1 study (supplemental Figure 1) were isolated and subjected to western blotting using a specific antibody against Tyr-8 and Tyr-21 of band 3. We calculated the change in Tyr-p-bd3 of each patient from baseline and then estimated the averaged change through a regression model adjusting for age and sex (Table 1). AG-348 treatment significantly decreased RBC Tyr-p-bd3 after 20-, 50-, and 100-mg treatment and at the end of taper and returned to near baseline by EOS (Table 1; Figure 1A). To further confirm the effect of PK activators on Tyr-p-bd3, we treated HbSS RBCs with different concentrations of AG-348 and AG-946 for different times ex vivo. Both AG-348 and AG-946 decreased Tyr-p-bd3 in a dose- and time-dependent manner (Figure 1B-I). Three biological replicates (blood samples from 3 different HbSS donors) were used in the ex vivo assays in Figure 1A-G and 4 biological replicates in Figure 1H-I.
Effect of PK activators on Tyr-p-bd3. (A) In vivo effect of AG-348 on Tyr-p-bd3, PTP1B, and ankyrin-1. The levels of Tyr-p-bd3, intact PTP1B, and membrane-associated ankyrin-1 were quantified by western blotting in frozen blood samples from 15 patients (Tyr-p-bd3 and PTP1B) or 11 patients (ankyrin-1) treated twice daily with 5-, 20-, 50-, and 100-mg AG-348. ∗P ˂ .05; ∗∗P ˂ .01; ∗∗∗P ˂ .001. (B-E) Ex vivo effect of AG-348 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 4 hours (B-C) or 30-μM AG-348 for different times (1, 2, 4, and 6 hours) (D-E). The level of Tyr-p-bd3 was analyzed by western blotting and quantified by densitometry analysis. The mean reduction on Tyr-p-bd3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 1%, 22%, 35%, and 50%, respectively, and with 30-μM AG-348 for 2, 4, and 6 hours were 6%, 28%, and 59%, respectively. ∗P ˂ .05; ∗∗P ˂ .01. (F-I) Ex vivo effect of AG-946 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-946 in DMSO for 4 hours (F-G) or 30-μM AG-946 for different times (1, 2, 4, and 6 hours) and the level of Tyr-p-bd3 analyzed by western blotting and quantified by densitometry analysis (H-I). The mean reduction on Tyr-p-bd3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-946 were 3%, 24%, 41%, and 56%, respectively, and with 30-μM AG-946 for 4 and 6 hours were 29% and 49%, respectively. ∗P ˂ .05; ∗∗ P ˂ .01. Three biological replicates (blood samples from 3 different HbSS donors) were used in all ex vivo assays, except in panels H and I, which had 4 biological replicates. ∗P ˂ .05; ∗∗P ˂ .01.
Effect of PK activators on Tyr-p-bd3. (A) In vivo effect of AG-348 on Tyr-p-bd3, PTP1B, and ankyrin-1. The levels of Tyr-p-bd3, intact PTP1B, and membrane-associated ankyrin-1 were quantified by western blotting in frozen blood samples from 15 patients (Tyr-p-bd3 and PTP1B) or 11 patients (ankyrin-1) treated twice daily with 5-, 20-, 50-, and 100-mg AG-348. ∗P ˂ .05; ∗∗P ˂ .01; ∗∗∗P ˂ .001. (B-E) Ex vivo effect of AG-348 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 4 hours (B-C) or 30-μM AG-348 for different times (1, 2, 4, and 6 hours) (D-E). The level of Tyr-p-bd3 was analyzed by western blotting and quantified by densitometry analysis. The mean reduction on Tyr-p-bd3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 1%, 22%, 35%, and 50%, respectively, and with 30-μM AG-348 for 2, 4, and 6 hours were 6%, 28%, and 59%, respectively. ∗P ˂ .05; ∗∗P ˂ .01. (F-I) Ex vivo effect of AG-946 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-946 in DMSO for 4 hours (F-G) or 30-μM AG-946 for different times (1, 2, 4, and 6 hours) and the level of Tyr-p-bd3 analyzed by western blotting and quantified by densitometry analysis (H-I). The mean reduction on Tyr-p-bd3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-946 were 3%, 24%, 41%, and 56%, respectively, and with 30-μM AG-946 for 4 and 6 hours were 29% and 49%, respectively. ∗P ˂ .05; ∗∗ P ˂ .01. Three biological replicates (blood samples from 3 different HbSS donors) were used in all ex vivo assays, except in panels H and I, which had 4 biological replicates. ∗P ˂ .05; ∗∗P ˂ .01.
AG-348 increases the association of ankyrin-1 with RBC membrane in vivo and ex vivo
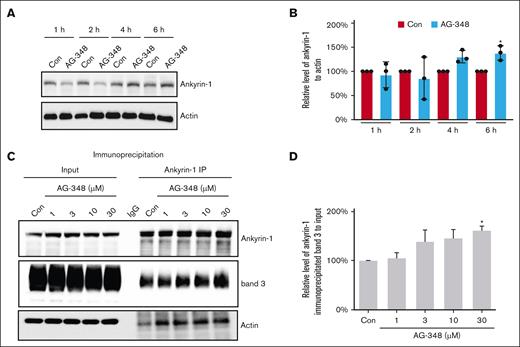
To evaluate the effects of AG-348 on the interaction of band 3 with ankyrin-1, we measured the level of membrane-associated ankyrin-1 using the same patient samples (only 11/15 patient samples available) that were analyzed for Tyr-p-bd3. AG-348 treatment increased the level of membrane-associated ankyrin-1 in a dose-dependent manner by 36.0%, 82.0%, 78.0%, and 45.0% after 20 mg, 50 mg, 100 mg, and the end of taper, respectively, and returned to baseline at –4.0% by EOS (Figure 1A; Table 1). No statistical significance was reached likely due to the limited number of longitudinal patient samples. To further confirm the effect of AG-348 on ankyrin-1, we treated HbSS RBCs with 30-μM AG-348 for different times ex vivo; AG-348 increased the level of membrane-associated ankyrin-1 starting from 4 hours (Figure 2A-B). Because band 3 interacts directly with ankyrin-1,21 and Tyr-p-bd3 interrupts this interaction, we treated HbSS RBCs with different doses of AG-348; AG-348 treatment increased the interaction between band 3 and ankyrin-1 in a dose-dependent manner (Figure 2C-D). We compared the baseline levels of membrane-associated ankyrin-1 in RBCs from 6 pairs of HbAA (AA1-6) and HbSS (SS1-6) patients. Our result showed that the membrane-associated ankyrin-1 in HbSS RBCs is significantly decreased compared with HbAA RBCs (supplemental Figure 2). Using an antibody to full-length ankyrin-1, we compared the protein profiles of ankyrin-1 in paired whole cell lysates vs membranes (ghosts) from HbAA and HbSS RBCs at baseline and showed that there were relatively more cleaved ankyrin-1 peptides in whole cell lysates than its membrane counterpart, in both HbSS and HbAA RBCs. There was also much less full-length (intact) ankyrin-1 in HbSS RBC membranes that showed relatively more Tyr-p-bd3 (supplemental Figure 3).
Effect of AG-348 on the association of band 3 with ankyrin-1. (A-B) HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (1, 2, 4, and 6 hours), and the level of membrane-associated ankyrin-1 was analyzed by western blotting and quantified by densitometry. The mean increase on ankyrin-1 of RBC treated with 30-μM AG-348 for 4 and 6 hours were 31% and 38%, respectively. ∗P ˂ .05. (C-D) HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 8 hours, and the interaction between ankyrin-1 and band 3 was measured by immunoprecipitation and quantified by densitometry analysis. The mean increase on the interaction between ankyrin-1 and band 3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 5%, 38%, 45%, and 61%, respectively. ∗P ˂ .05. Three biological replicates of HbSS donors were used in all ex vivo assays.
Effect of AG-348 on the association of band 3 with ankyrin-1. (A-B) HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (1, 2, 4, and 6 hours), and the level of membrane-associated ankyrin-1 was analyzed by western blotting and quantified by densitometry. The mean increase on ankyrin-1 of RBC treated with 30-μM AG-348 for 4 and 6 hours were 31% and 38%, respectively. ∗P ˂ .05. (C-D) HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 8 hours, and the interaction between ankyrin-1 and band 3 was measured by immunoprecipitation and quantified by densitometry analysis. The mean increase on the interaction between ankyrin-1 and band 3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 5%, 38%, 45%, and 61%, respectively. ∗P ˂ .05. Three biological replicates of HbSS donors were used in all ex vivo assays.
AG-348 increased the activity of protein phosphatase, PTP1B, in vivo and ex vivo
To unravel whether PK activator regulates Tyr-p-bd3 via affecting the activities of either tyrosine kinase or protein phosphatase, we treated HbSS RBCs with different concentrations of AG-348, R406 (a spleen tyrosine kinase [SYK] inhibitor), or a combination of both agents. We observed a mean reduction of 15% and 6% at 10-μM R406 and AG-348 each, respectively, and a reduction of 36% and 31% at 30-μM R406 and AG-348 each, respectively. A combination of R406 and AG-348 at 10 and 30 μM each showed additional Tyr-p-bd3 reduction of 39% and 66%, respectively, compared with single-drug treatment alone, suggesting a potential effect of AG-348 on protein phosphatase activity (Figure 3A-B).
Effect of AG-348 on protein phosphatase, PTP1B. (A-B) Ex vivo effect of AG-348 and R406 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (3, 10, and 30 μM) of R406, AG-348, or a combination of R406 and AG-348 for 6 hours, and the level of Tyr-p-bd3 was analyzed by western blotting and quantified by densitometry analysis. The mean reductions on Tyr-p-bd3 of RBCs treated with 10- and 30-μM R406, AG-348, or a combination compared with vehicle control were 15% and 36%, 6% and 31%, 39% and 66%, respectively. ∗∗P ˂ .01; ∗∗∗P ˂ .001. (C-D) Effect of AG-348 on RBC PTP1B. HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (1, 2, 4, and 6 hours), and the level of membrane-associated intact PTP1B was analyzed by western blotting and quantified by densitometry analysis. The mean increase on PTP1B of RBCs treated with 30-μM AG-348 for 4 and 6 hours were 25% and 43%, respectively. ∗P ˂ .05. (E) Immunoprecipitation with anti-PTP1B antibody clearly showed interaction between PTP1B and band 3. (F-G) HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 for 8 hours, and the interaction between PTP1B and band 3 was measured by immunoprecipitation and quantified by densitometry analysis. The mean increase on the interaction between PTP1B and band 3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 31%, 35%, 70%, and 121%, respectively. ∗P ˂ .05. Three biological replicates of HbSS donors were used in all ex vivo assays. IgG, immunoglobulin G.
Effect of AG-348 on protein phosphatase, PTP1B. (A-B) Ex vivo effect of AG-348 and R406 on Tyr-p-bd3. HbSS RBCs were treated with DMSO (Con) or different concentrations (3, 10, and 30 μM) of R406, AG-348, or a combination of R406 and AG-348 for 6 hours, and the level of Tyr-p-bd3 was analyzed by western blotting and quantified by densitometry analysis. The mean reductions on Tyr-p-bd3 of RBCs treated with 10- and 30-μM R406, AG-348, or a combination compared with vehicle control were 15% and 36%, 6% and 31%, 39% and 66%, respectively. ∗∗P ˂ .01; ∗∗∗P ˂ .001. (C-D) Effect of AG-348 on RBC PTP1B. HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (1, 2, 4, and 6 hours), and the level of membrane-associated intact PTP1B was analyzed by western blotting and quantified by densitometry analysis. The mean increase on PTP1B of RBCs treated with 30-μM AG-348 for 4 and 6 hours were 25% and 43%, respectively. ∗P ˂ .05. (E) Immunoprecipitation with anti-PTP1B antibody clearly showed interaction between PTP1B and band 3. (F-G) HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 for 8 hours, and the interaction between PTP1B and band 3 was measured by immunoprecipitation and quantified by densitometry analysis. The mean increase on the interaction between PTP1B and band 3 of RBC treated with 1-, 3-, 10-, and 30-μM AG-348 were 31%, 35%, 70%, and 121%, respectively. ∗P ˂ .05. Three biological replicates of HbSS donors were used in all ex vivo assays. IgG, immunoglobulin G.
We then evaluated the effect of AG-348 on PTP1B activity in the same phase 1 patient RBC ghost samples used in the analysis of Tyr-p-bd3 and ankyrin-1. We calculated the change in membrane-associated intact PTP1B of each patient from baseline and calculated the mean change across the 15 patients and showed a significant increase in intact PTP1B after 5-, 20-, 50-, and 100-mg AG-348 treatment, which paralleled the degree of reduction in Tyr-p-bd3 (Figure 1A; Table 1). A representative blot of Tyr-p-bd3 with total band 3 as control, ankyrin-1, and PTP1B with actin as control of longitudinal RBC ghosts from a patient enrolled in the phase 1 (NCT04000165) study is shown in supplemental Figure 4. To validate the effect of AG-348 on PTP1B, we treated HbSS RBCs with 30-μM AG-348 for different times ex vivo and further demonstrated that AG-348 increased PTP1B activity in a time-dependent manner (Figure 3C-D). We then confirmed the interaction between PTP1B and band 3 by immunoprecipitation (Figure 3E); AG-348 treatment increased this interaction in a dose-dependent manner (Figure 3F-G). Taken together, our results suggests that AG-348 decreases Tyr-p-bd3 by regulating the activity of PTP1B.
AG-348 increases the production of ATP and decreases the activity of calpain
We first confirmed that HbSS RBCs have lower level of membrane-associated intact PTP1B (active form) compared with HbAA control cells (Figure 4A-B). We posit that once PTP1B is cleaved by calpain, it will be dissociated from band 3 and the RBC membrane. Thus, less cleaved PTP1B will be detected in the ghost. To support our hypothesis, we checked the levels of PTP1B in paired samples of RBC ghosts and whole cell lysates from 5 different HbSS patients. Our results indicated that the percentage of cleaved PTP1B in the ghost samples was significantly lower than that in whole cell lysate samples (supplemental Figure 5). Because PTP1B can be cleaved into an inactive form by calpain, we next examined the effect of AG-348 on calpain activity. Treatment with 10- and 30-μM AG-348 significantly decreased the activity of calpain in HbSS RBCs (Figure 4C). In parallel, AG-348 significantly increased the production of ATP in a time- and dose-dependent manner on HbSS RBCs, consistent with our in vivo phase 1 study results (Figure 4D-E). We then examined PMCA-mediated efflux kinetics in paired samples of HbAA and HbSS RBCs using a recently developed fluorescence assay.20 Ca2+ efflux from HbAA cells was faster than that in HbSS cells (Figure 4F-G), consistent with prior studies using Ca2+ tracer efflux measurements.16 Efflux halftimes, although slower in SS cells (Figure 4G), did not reach statistical significance (Student t test, P = .1; n = 4 donors), possibly because of donor variability or small sample size. We also compared efflux halftimes for both HbAA and HbSS cells after a 6-hour treatment with AG-348 and compared these with matched DMSO-treated controls. Ca2+ efflux was modestly faster after AG-348 treatment for both HbAA and HbSS cells (Figure 4H, faster efflux apparent as a lower efflux halftime). The difference, however, did not reach significance (paired t test, P > .2 for comparisons between matched DMSO and AG-348 treatment for both genotypes). RBCs from 4 pairs of ethnic-matched healthy controls (HbAA) and HbSS patients were used in each assay, and the results represent a mean from the 4 separate assays.
Effect of AG-348 on calpain activity, ATP production, and Ca2+ efflux. (A-B) PTP1B in HbAA vs HbSS RBCs. The levels of membrane-associated intact PTP1B in RBCs from 6 pairs of healthy controls (AA1-6) and HbSS patients (SS1-6) were analyzed by western blotting and quantified by densitometry. The relative levels of PTP1B to actin in AA1-6 RBCs were 0.42, 0.84, 0.81, 0.63, 0.39, and 0.75, respectively, with an average of 0.64; and in SS1-6 RBCs were 0.28, 0.63, 0.37, 0.21, 0.51, and 0.20, respectively, with an average of 0.37. ∗P ˂ .05. (C) Effect of AG-348 on calpain activity. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO overnight, and the activity of calpain was measured using Calpain Activity Assay Kit. Mean reductions in calpain activity of RBCs treated with 1-, 3-, 10-, and 30-μM AG-348 were 9%, 15%, 24%, and 26%, respectively. ∗P ˂ .05. (D-E) Effect of AG-348 on ATP production. HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (2, 4, 6, and 8 hours) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 8 hours; ATP level was analyzed using CellTiter-Glo 2.0 Cell Viability Assay kit. The mean increase in ATP levels of RBCs treated with 30-μM AG-348 for 2, 4, 6, and 8 hours were 2%, 5%, 11%, and 21%, respectively, and with 1-, 3-, 10-, and 30-μM AG-348 for 8 hours were 2%, 5%, 14%, and 26%, respectively. Three biological replicates of HbSS donors were used in the study here. ∗P ˂ .05. (F-H) Effect of AG-348 on Ca2+ efflux. (F) Ca2+ efflux kinetics were measured using extracellular Fluo-8. Symbols represent mean ± standard error of the mean (SEM) from triplicate measurements from single HbAA and HbSS donors (dark blue and red circles, respectively). Notice the slower efflux with the SS donor. (G) Mean ± SEM efflux halftimes from 4 donors, measured similar to panel F. The slower efflux observed from SS erythrocytes did not reach statistical significance. (H) Mean ± SEM efflux halftimes for indicated cells after a 6-hour treatment with 30-μM AG-348 or matched DMSO control (blue and red bars, respectively, for each genotype). RBCs from 4 pairs of ethnic-matched healthy controls (HbAA) and HbSS patients were used in each assay, and the results represent a mean from the 4 separate assays.
Effect of AG-348 on calpain activity, ATP production, and Ca2+ efflux. (A-B) PTP1B in HbAA vs HbSS RBCs. The levels of membrane-associated intact PTP1B in RBCs from 6 pairs of healthy controls (AA1-6) and HbSS patients (SS1-6) were analyzed by western blotting and quantified by densitometry. The relative levels of PTP1B to actin in AA1-6 RBCs were 0.42, 0.84, 0.81, 0.63, 0.39, and 0.75, respectively, with an average of 0.64; and in SS1-6 RBCs were 0.28, 0.63, 0.37, 0.21, 0.51, and 0.20, respectively, with an average of 0.37. ∗P ˂ .05. (C) Effect of AG-348 on calpain activity. HbSS RBCs were treated with DMSO (Con) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO overnight, and the activity of calpain was measured using Calpain Activity Assay Kit. Mean reductions in calpain activity of RBCs treated with 1-, 3-, 10-, and 30-μM AG-348 were 9%, 15%, 24%, and 26%, respectively. ∗P ˂ .05. (D-E) Effect of AG-348 on ATP production. HbSS RBCs were treated with DMSO (Con) or 30-μM AG-348 in DMSO for different times (2, 4, 6, and 8 hours) or different concentrations (1, 3, 10, and 30 μM) of AG-348 in DMSO for 8 hours; ATP level was analyzed using CellTiter-Glo 2.0 Cell Viability Assay kit. The mean increase in ATP levels of RBCs treated with 30-μM AG-348 for 2, 4, 6, and 8 hours were 2%, 5%, 11%, and 21%, respectively, and with 1-, 3-, 10-, and 30-μM AG-348 for 8 hours were 2%, 5%, 14%, and 26%, respectively. Three biological replicates of HbSS donors were used in the study here. ∗P ˂ .05. (F-H) Effect of AG-348 on Ca2+ efflux. (F) Ca2+ efflux kinetics were measured using extracellular Fluo-8. Symbols represent mean ± standard error of the mean (SEM) from triplicate measurements from single HbAA and HbSS donors (dark blue and red circles, respectively). Notice the slower efflux with the SS donor. (G) Mean ± SEM efflux halftimes from 4 donors, measured similar to panel F. The slower efflux observed from SS erythrocytes did not reach statistical significance. (H) Mean ± SEM efflux halftimes for indicated cells after a 6-hour treatment with 30-μM AG-348 or matched DMSO control (blue and red bars, respectively, for each genotype). RBCs from 4 pairs of ethnic-matched healthy controls (HbAA) and HbSS patients were used in each assay, and the results represent a mean from the 4 separate assays.
Discussion
HbSS patients treated with AG-348 showed significant improvements in both intravascular hemolysis and sickling kinetics, supporting the critical role of 2,3-DPG reduction derived from the increased glycolytic flux.12 ATP has an essential role in the maintenance of RBC hydration through the maintenance of ionic gradients,22,23 RBC deformability,24 and protection from oxidative damage.25 ATP has been shown to play a critical role in the maintenance of RBC membrane cytoskeletal integrity.26 A prime candidate for the improved RBC survival in the patients treated with AG-348 is reduced Tyr-p-bd3 that we confirmed through longitudinal blood samples of 15 patients from the phase 1 study (NCT04000165).12 This finding was further confirmed by treating HbSS RBCs with AG-348 and AG-946 ex vivo. Here, we provide the mechanistic data underlying how increasing ATP availability improves RBC membrane integrity by reducing Tyr-p-bd3 in RBCs.
Band 3, also known as anion exchanger 1, comprises ∼25% of RBC membrane protein and is a transport protein responsible for mediating the exchange of chloride (Cl−) with bicarbonate (HCO3−) across erythrocyte membranes.27 Band 3 is also one of the major components that connect the lipid bilayer to the cytoskeleton. RBC band 3 has a 2-domain structure: the 43 kDa N-terminal is located in the cytosol and is responsible for binding the cytoskeleton to the membrane, whereas the 55 kDa C-terminal is embedded in the lipid bilayer and is responsible for anion transport.28 The N-terminal domain of band 3 contains multiple tyrosine residues, and phosphorylation of these tyrosine residues affects its interaction with ankyrin21; a preferential phosphorylation site has been identified on tyrosine 8 at the N-terminus of band 3.29,30
The process of tyrosine phosphorylation is a balance of activity between 2 groups of opposing enzymes: kinases and phosphatases. For band 3, the kinases and phosphatases are p72 SYK and PTP1B, respectively. Two potential reasons could account for the increased Tyr-p-bd3 in sickle RBCs: first, increased oxidative stress that inhibits the activity of RBC tyrosine phosphatases that normally prevent constitutive band 3 tyrosine phosphorylation31; and second, activation of protein tyrosine kinase p72 SYK by endogenous reactive oxygen intermediates.32 AG-348 has been shown to improve oxidative stress in β-thalassemia mice33 and thus could potentially regulate the activities of both kinase and/or phosphatase. To test the possibilities, we treated HbSS RBCs with AG-348 and R406 (a SYK kinase inhibitor) singly or in combination. We observed additional reduction of Tyr-p-bd3 in RBCs in the group treated with both drugs compared with single-drug treatment alone, suggesting a potential regulatory effect of AG-348 on PTP1B activity. Indeed, we showed that AG-348 significantly increased the membrane-associated intact PTP1B (active form) in a dose-dependent manner in the same patients’ samples who were analyzed for Tyr-p-bd3. Further, the degree of increase in intact PTP1B paralleled the degree of reduction in Tyr-p-bd3 and was accompanied by increase in RBC membrane ankyrin-1 levels, reflecting the increased association of ankyrin-1 with RBC membrane, although no significance was reached (a possible reason could be the small sample size). We also observed an increased interaction between ankyrin-1 and band 3 in HbSS RBCs after ex vivo treatment with AG-348. We compared the baseline levels of ankyrin-1 in RBCs of HbAA and HbSS patients (supplemental Figure 2), which indeed showed that membrane-associated full-length ankyrin-1 in HbSS RBCs was significantly decreased compared with HbAA RBCs, supporting the dissociation of the ankyrin-spectrin complex from the RBC cytoskeleton as a consequence of the increased Tyr-p-bd3 in HbSS RBCs.
PTP1B could be cleaved into an inactive form and dissociated from its substrate by Ca2+-dependent calpain.15,34 Intracellular Ca2+ in RBC is a balance between influx and efflux, which is mediated by PMCA, whose activity relies on the ATP level. In normal healthy RBCs, Ca2+ is maintained at low levels by PMCA.17 Intracellular Ca2+ in sickle RBCs has been reported to be reversibly increased upon deoxygenation due to an increased leak uptake and a possibly decreased PMCA activity.16,17 The resulting increase in intracellular Ca2+ may lead to erythrocyte dehydration because it activates Ca2+-dependent K+ channels. The absolute increase in free intracellular Ca2+ depends not only on the rates of uptake and efflux but also on cellular Ca2+ buffering capacity, which may be altered in oxygenated sickle cells and could be further affected by deoxygenation and/or AG-348 treatment.
Our studies revealed that AG-348 increases Ca2+ efflux from both HbAA and HbSS erythrocytes, presumably via increasing cellular ATP, which is functionally deficient in sickle cells.25 Although the accelerated efflux could be minor and transient, such transients could be sufficient to trigger events such as inhibition of calpain activity. Indeed, we observed a decreased activity of calpain after AG-348 treatment. However, we should interpret these findings with caution. First, the donors used in these Ca2+ efflux studies were asymptomatic, possibility limiting the extent of improvement observable in our ex vivo efflux studies. We also restricted our studies to active efflux, recognizing that AG-348 treatment is expected to increase cellular ATP pools. We were unable to establish a statistically significant effect of AG-348 in our transport measurements, likely due to small sample size compounded by donor-to-donor variation. Based on significantly improved sickling parameters in clinical trials of AG-348 in patients with SCD,12,35 we propose that PMCA-mediated Ca2+ efflux may exhibit greater improvements in deoxygenated sickled cells upon treatment with this drug. The Ca2+ assay we used is well suited for the small amounts of blood typically obtained from sickle cell donors; it allows for kinetic measurements in 384-well microplates,20 and it also avoids the use of 45Ca2+ radioactive tracer. At the same time, however, this assay has a few limitations that should be considered when interpreting our results. Most importantly, it requires erythrocyte loading with supraphysiological Ca2+ concentrations, which may trigger various metabolic changes in erythrocytes, including cytoskeletal rigidity,36 altered membrane structure, and phospholipid asymmetry.37
Although we were fortunate to have access to frozen samples that provided conclusive evidence in support of improved RBC integrity in mitapivat-treated patients with SCD, our study was limited by patient numbers and material, accounting for the smaller sample sizes for the in vivo assays. It should also be noted that the significant reduction in tyrosine phosphorylation of RBC band 3 may not be solely explained by the improvement of constitutive PTP1B activity. We propose that an added contribution could be a reduction in SYK activity as an indirect effect of the overall reduction in reactive oxygen species and oxidative stress levels from reduced RBC sickling. Comparison of red cell membrane Tyr-p-bd3, ankyrin-1, and PTP1B in patients treated with the drug and without treatment in a randomized placebo-controlled study would be relevant to understanding the mechanisms of activating PK on survival of sickle RBCs.
Nonetheless, based on a combination of in vivo and ex vivo assays, we suggest that the increase in intracellular ATP from activating PK leads to increased PMCA activity and efflux of intracellular Ca2+. A reduction in intracellular Ca2+ reduced the activity of calpain and thereby suppressed calpain’s cleavage of PTP1B, restoring some of its constitutive activity, resulting in the reduction of Tyr-p-bd3. A reduced calpain activity could also reduce the cleavage of ankyrin. Consequently, there was increased interaction between band 3 and full-length ankyrin-spectrin complex that led to the improvement of RBC membrane integrity and improved RBC survival. We suggest that it is likely that this mechanism of reduced Tyr-p-bd3 also contributes to the Hb improvement observed in AG-348 treatment of patients with thalassemia and PK deficiency.38,39
Acknowledgments
This work formed part of the exploratory end points of the phase 1 mitapivat dose escalation study (NCT04000165). The authors thank William Eaton (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health [NIH]) for reviewing the manuscript and Alan Hoofring (Medical Arts Design Section, NIH) for preparation of the visual abstract.
The study was supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute (S.L.T.) and the National Institute of Allergy and Infectious Diseases (S.A.D.) at the NIH.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: K.L. and S.L.T. designed the study, analyzed data, and wrote the manuscript; K.L., X.W., and M.L. collected and processed blood samples; K.L. and Y.Y.L. performed experiments and analyzed data; A.C. and I.F. recruited and enrolled patients; S.G. and P.A.K. processed blood samples for ATP studies; J.C. performed Ca2+ efflux assays and S.A.D. designed Ca2+ efflux assays and provided input on the data analysis; J.M.H. and P.S.L. provided input on red cell membrane analyses; N.J. analyzed data and performed statistical analyses; K.L., P.S.L., N.J., S.A.D., and S.L.T. edited subsequent versions of the manuscript; and all coauthors reviewed and approved the final version.
Conflict-of-interest disclosure: S.G. and P.A.K. were employees and stockholders at the time the project was conducted but are no longer in Agios Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Correspondence: Swee Lay Thein, National Heart, Lung, and Blood Institute/National Institutes of Health, Building 10-CRC, Room 6S241, 10 Center Dr, Bethesda, MD 20892; email: sl.thein@nih.gov.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
Original data are available on reasonable request from the corresponding author, Swee Lay Thein (sl.thein@nih.gov).
The full-text version of this article contains a data supplement.