Oxygen gradient ektacytometry–derived biomarkers are associated with occurrence of >1 type of acute complications.
Sickling, measured by oxygen gradient ektacytometry, correlates with hemocytometry parameters and can be used to assess novel therapies.
Visual Abstract
We investigated the potential of the point of sickling (PoS; the pO2 tension at which red cells start to sickle), determined by oxygen gradient ektacytometry to serve as a biomarker associated with the incidence of acute sickle cell disease–related complications in 177 children and 50 adults. In the pediatric cohort, for every 10 mmHg increase in PoS reflecting a greater likelihood of sickling, the likelihood of an individual experiencing >1 type of acute complication increased; the adjusted odds ratio (aOR) was 1.65. For every 0.1 increase in minimum elongation index (EImin; reflecting improved red blood cell deformability at hypoxia), the aOR was 0.50. In the adult cohort, for every 10 mmHg increase in PoS, we found an aOR of 3.00, although this was not significant after correcting for multiple testing. There was a trend for an association between higher PoS and greater likelihood of vaso-occlusive episodes (VOEs; children aOR, 1.35; adults aOR, 2.22). In children, only EImin was associated with VOEs (aOR, 0.68). When data of both cohorts were pooled, significant associations with PoS and/or EImin were found for all acute complications, independently and when >1 type of acute complication was assessed. These findings indicate that oxygen gradient ektacytometry generates novel biomarkers and provides a rationale for further development of these biomarkers in the assessment of clinical severity, evaluation of novel therapies, and as surrogate clinical trial end points. These biomarkers may be useful in assessing efficacy of novel therapies like pyruvate kinase activators, voxelotor, and L-glutamine.
Introduction
Sickle cell disease (SCD) is a monogenetic disorder characterized by chronic hemolytic anemia, painful vaso-occlusive episodes (VOEs), and progressive organ failure. The mutation in HBB coding for the β-globin gene results in the formation of hemoglobin S (HbS) that polymerizes under deoxygenation, leading to red blood cell (RBC) sickling.1 Sickled RBCs are poorly deformable, causing occlusion of the microvasculature, initiating a cascade of other immunogenic and inflammatory factors,2 leading to painful ischemic events and progressive organ damage.3 Acute complications that require immediate care include acute chest syndrome (ACS), VOEs, and cerebral infarction.3,4
The rapidly evolving therapeutic options that are now in development for SCD require objective and clinically relevant end points for clinical trials. Hydroxyurea, as part of current standard of care therapy, induces fetal Hb (HbF) production. Together with Hb concentration and mean cell volume (MCV), HbF levels are often used to monitor hydroxyurea efficacy, because HbF is a well-known modifier of SCD clinical severity. On a population level, higher HbF levels correlate with fewer complications, although some individuals with HbF concentration > 20% still report clinical complications.5 This may be due to heterocellular distribution of HbF, which is not accurately reflected by the percentage of HbF measured by high-performance liquid chromatography. Methods to measure F cells or the amount of HbF in RBCs are currently under investigation to determine the level at which a VOE is likely to be prevented.6-8 However, because the ameliorating effects on disease burden are not always associated with increases in total HbF percentage after hydroxyurea treatment, there is an unmet need for robust reproducible biomarkers that can assess RBC function, thereby predicting disease severity and risk of SCD-related complications. The availability of novel biomarkers of red cell function will facilitate treatment selection and monitoring of therapy according to the principles of precision medicine.
RBC deformability is a measure of RBC function and membrane health. In SCD, previous studies investigated the use of RBC deformability during normoxic conditions as a marker for complications and identified decreased deformability in patients with complications such as leg ulcers and nephropathy.9,10 In contrast, individuals with VOEs and osteonecrosis had higher RBC deformability.11-13 However, measuring RBC deformability at normoxic conditions has limited value, because deoxygenation affects RBC deformability both in vivo and in vitro. It is therefore essential to measure RBC deformability under both hypoxia and normoxia to generate the most informative and useful biomarkers of RBC function in SCD.
We previously reported that oxygen gradient ektacytometry, a next generation deformability and sickling assay, measures RBC deformability over a gradient of oxygen tensions (pO2), generating the key parameter, the so called point of sickling (PoS) that reflects the patient-specific pO2 at which HbS polymers start to grow and sickling begins.14 Oxygen gradient ektacytometry–derived biomarkers improve with standard of care therapies, hydroxyurea and RBC transfusion.15,16 Individuals with frequent VOEs have less deformability and higher PoS, providing clinical validation for these biomarkers of VOEs.15 Here, we report on further clinical validation of oxygen gradient ektacytometry by investigating the association of its parameters with acute SCD-related complications (VOEs, ACS, and cerebral infarction). Furthermore, we examined hemocytometry, HbF quantification, whole blood viscosity, and dense RBCs as complementary biomarkers in individuals with SCD.
Methods
Subjects
A total of 227 individuals with SCD were included in this multicenter study. We analyzed 2 cohorts of patients with sickle cell anemia (SCA): a pediatric cohort of 177 individuals with SCA enrolled at Texas Children’s Hospital and an adult cohort of 50 individuals with SCA, enrolled at either University Medical Center Utrecht, The Netherlands (n = 26) or Hospital Lyon France (n = 24). Study procedures were approved by local ethical committees/internal review boards in accordance with the Declaration of Helsinki. All patients or legal guardians gave written informed consent during routine visit to the outpatient clinic. The pediatric and adult cohorts were initially analyzed separately, because the pathophysiology of SCD complications change with increasing age,17,18 and differences in RBC deformability between adults and young children have been reported.19 Participants were genotyped as SCA (HbSS or HbS/βo thalassemia). Individuals with HbSC disease or HbS/β+ thalassemia were excluded from this analysis. A substantial number of subjects were on hydroxyurea therapy (89% of children and 64.0% of adults with SCD, Table 1). Subjects who received an RBC transfusion less than 3 months before measurements were excluded from the study. Measurements were performed at steady state, and all subjects on hydroxyurea had been on a consistent dose (ie, stable dose for adults; and for children, a stable dose when adjusted for weight gain: consistent mg/kg dose) for at least 1 year before measurements. Correlations between oxygen gradient ektacytometry–derived parameters and acute complications such as ACS, VOEs, and cerebral infarction (arterial ischemic stroke or silent infarcts) were examined. Silent infarcts were diagnosed by magnetic resonance imaging, performed according to local protocols, only when clinically indicated. Patient characteristics and SCD-related clinical complications experienced in the past were collected from medical charts. VOE was defined as a painful VOE in the last 2 years that required hospital admission or visits to the emergency room or outpatient clinic. ACS was defined as a new pulmonary infiltrate on chest radiography in combination with fever and respiratory symptoms and/or chest pain for which medical treatment was indicated. Occurrence of >1 type of acute complication included any history of ACS and cerebral infarction and/or VOEs in the past 2 years.
Hematologic laboratory parameters
Laboratory parameters, including complete blood count, absolute reticulocyte count (ARC), and HbF levels (by high-performance liquid chromatography), were obtained at each study site’s clinical laboratory during routine visits to the outpatient clinic. Percentage of dense RBCs (%DRBCs), defined as RBCs with mean corpuscular Hb concentration > 41 g/dL and MCV < 120 fL, were measured with an ADVIA 120/2120 hematology analyzer (Siemens, Healthcare GmBH, Erlangen, Germany).
Oxygen gradient ektacytometry
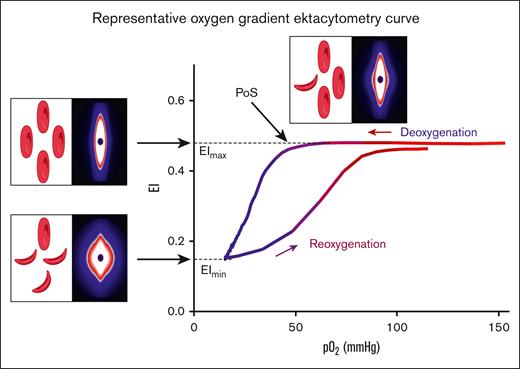
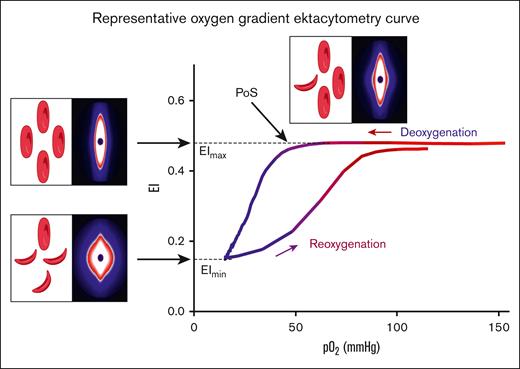
Oxygen gradient ektacytometry generates 3 key parameters: (1) maximum elongation index (EImax), RBC deformability at normoxia, before the deoxygenation cycle is initiated; (2) minimum elongation index (EImin), minimum RBC deformability upon deoxygenation; and (3) PoS, the oxygen tension at which a 5% decrease in deformability is observed during deoxygenation, reflecting the patient-specific pO2 at which polymers further grow and sickling begins (Figure 1).14 Oxygen gradient ektacytometry was carried out using the Laser Optical Rotational Red Cell Analyzer (Lorrca, RR Mechatronics, Zwaag, The Netherlands), as described elsewhere.14,20,21 To carry out an oxygen gradient ektacytometry measurement, whole blood, standardized to a fixed RBC count of 200 × 106, is mixed with 5 mL Oxy-Iso (RR Mechatronics; osmolarity, 282-286 mOsm/kg; pH, 7.35-7.45; viscosity, 27-32 cP at room temperature of 21 ± 1°C). Detailed description of the technique and sample handling, including a video article, is described elsewhere.20,21 Samples were measured at 1 of the 3 different sites located in The Netherlands (Utrecht), France (Lyon), and the United States (Houston). Ektacytometers located in Utrecht and Lyon were harmonized to each other, thereby generating comparable curves when measuring the same blood sample.
Representative oxygen gradient ektacytometry curve. RBC deformability is continuously measured with the laser assisted optical rotational red cell analyzer (Lorrca) while the sample is gradually deoxygenated and reoxygenated. Oxygen gradient ektacytometry generates 3 key parameters: EImax reflects deformability at normoxia; EImin represents minimal deformability due to change in shape; and orientation of RBCs when sickling, upon deoxygenation. PoS embodies the oxygen tension when the first RBCs start to sickle. Diffraction patterns and schematic illustration of RBCs that cause the change in deformability (EI) are shown for each parameter. Parts of this figure have been created with BioRender.com.
Representative oxygen gradient ektacytometry curve. RBC deformability is continuously measured with the laser assisted optical rotational red cell analyzer (Lorrca) while the sample is gradually deoxygenated and reoxygenated. Oxygen gradient ektacytometry generates 3 key parameters: EImax reflects deformability at normoxia; EImin represents minimal deformability due to change in shape; and orientation of RBCs when sickling, upon deoxygenation. PoS embodies the oxygen tension when the first RBCs start to sickle. Diffraction patterns and schematic illustration of RBCs that cause the change in deformability (EI) are shown for each parameter. Parts of this figure have been created with BioRender.com.
Blood viscosity
Whole blood viscosity was measured at a native hematocrit, at 37°C, at shear rates of 45 per second and 225 per second using a Brookfield DVT cone and plate viscometer (Brookfield, MA), respectively, as previously described.22
Statistical analysis
We performed a logistic regression analysis for oxygen gradient ektacytometry parameters, hemocytometry parameters that are known to be associated with SCD, DRBCs, and blood viscosity to calculate odds ratios (OR). To explore how our results would relate to treatment modification of hydroxyurea on PoS, that is, how much PoS changes upon initiation or alteration of hydroxyurea treatment,15 we calculated ORs and adjusted ORs (aORs) for every 10 mmHg increase in PoS in both the pediatric and adult cohort (Table 2). aORs were corrected for age and hydroxyurea therapy. ORs were not corrected for age when the association with occurrence of VOE was analyzed. GraphPad Prism (version 8.3) or Statistical Packages for Social Sciences (SPSS) (version 25.0.0.2 International Business Machines Corporation, IBM) was used in the statistical analysis. A P value < .05 was considered statistically significant. However, because we performed several analysis, exploring different associations, we did correct for multiple testing by using the false discovery rate analysis in SPSS.
Results
Patient characteristics of both cohorts are depicted in Table 1. In the pediatric cohort, percentage of patients on hydroxyurea therapy was 89% compared with 64% in the adult cohort, which was highlighted by a lower mean HbF of 16.0% in children compared with 13.3% in adults. This was accompanied by a slightly lower mean corpuscular Hb concentration and MCV in the pediatric group than in adults. In contrast, ARC was higher in children with SCA than in adults, indicating that irrespective of hydroxyurea therapy phenotype is different between the cohorts. Oxygen gradient ektacytometry–derived biomarkers EImax and EImin were comparable between the cohorts, whereas PoS was lower in children. Besides the differences in laboratory parameters between the 2 cohorts, we found a lower occurrence of VOE in the past 2 years of 49% in children compared with 66% in adults. In contrast, we observed a higher occurrence of ACS in children, with a lifetime history of 55%, compared with 28% in adults, which is indicative of a different phenotype (Table 1).
Occurrence of >1 type of acute clinical complication is associated with increased sickling and poor RBC deformability
To assess the likelihood of occurrence of acute complications in the past based on oxygen gradient ektacytometry and laboratory parameters, we calculated OR and aORs (Tables 2-5). ORs were not significantly affected by sex or genotype, but age and hydroxyurea therapy did significantly confound results; therefore, aORs were calculated.
In the pediatric cohort, for every 10 mmHg increase in PoS, the likelihood of occurrence of >1 type of acute complication increased; the aOR was 1.65 (P = .006), indicating that sickling at a higher oxygen tension is associated with more types of acute complications. For every 0.1 increase in EImin (reflecting improved RBC deformability at hypoxia), the aOR was 0.50 (P = .001). For every 0.1 EI increase in EImax (reflecting RBC deformability at normoxia), a trend was found with an aOR of 0.70 (P = .052), indicating that lower RBC deformability in normoxic conditions is associated with the occurrence of >1 type of acute complication.
These findings are in accordance with the results of the adult cohort in which the likelihood of >1 type of acute complication increased with an increment in PoS; the aOR for PoS was 3.00 (P = .015). For every 0.1 increase in EImax, the aOR was 0.33 (P = .035), and for EImin, there was a trend found with an aOR of 0.39 (P = .085; Table 2). However, after correcting for multiple testing, the results were not significant. We found no significant association between HbF or blood viscosity and the prior occurrence of >1 type of acute complication, and no other laboratory parameter was found to be associated with occurrences of >1 type of acute complication in both cohorts (Table 2). When the data of both cohorts were pooled, we found significant associations for all 3 oxygen gradient ektacytometry–derived biomarkers and HbF (supplemental Table 1).
HbF is associated with occurrence of VOE
In the pediatric cohort, higher HbF was associated with the reduced likelihood of occurrence of a VOE in the past 2 years (aOR, 0.50; P = .018), which was reproduced in the adult cohort (aOR, 0.32; P = .01). In the pediatric cohort, higher EImin was associated with reduced risk of occurrence of VOEs (aOR, 0.60; P = .004). In the adult cohort, a trend was observed for a higher EImax associated with reduced likelihood of VOEs (aOR, 0.32; P = .011). There was a trend toward an association between higher PoS and greater likelihood of VOEs in both cohorts (children aOR, 1.35; P = .071; adults aOR, 2.22; P = .050). Pooled data of both cohorts showed significant associations for PoS, EImin, EImax, and HbF with the occurrence of VOEs (supplemental Table 2). No other laboratory parameters except elevated ARC in the pediatric cohort (aOR, 1.48; P = .001) were associated with prior occurrence of VOEs (Table 3).
Occurrence of ACS is associated with PoS in children with SCD
We found a trend that a history of ACS was associated with elevated PoS (aOR, 1.55; P = .015) and EImin (aOR, 0.59; P = .007) in children. These results indicate that the likelihood of experiencing an ACS episode is increased when RBCs start to sickle at high oxygen tension. In adults, no oxygen gradient ektacytometry–derived biomarker was associated with the occurrence of ACS, nor were other laboratory parameters (Table 4). This may be due to the fact that only 14 of 50 adults experienced ACS, compared with 97 of 177 children (Table 1). Pooled data of both cohorts showed significant associations for PoS, EImin, EImax, and HbF with the occurrence of ACS (supplemental Table 3).
Cerebral infarction
We found a trend for an association between lifetime occurrence of cerebral infarction (silent or overt stroke) and RBC deformability at normoxic conditions (EImax) in children (aOR, 0.47; P = .011) but not in adults (aOR, 0.18; P = .126). A trend was observed for an association with occurrence of cerebral infarction and PoS in both cohorts (pediatric cohort aOR, 1.80; P = .058; adult cohort aOR, 3.25; P = .080; Table 5). No other laboratory parameters were found to be significantly associated with the occurrence of cerebral infarction except for elevated DRBCs in children (aOR, 1.19l; P = .038) for which we observed a trend. The strength of the association between DRBCs and stroke risk may be limited by the low occurrence of cerebral infarction in both cohorts. Fourteen of 177 children had a history of cerebral infarction, as did 3 of 50 adults (Table 1). Numbers were further reduced by the necessary exclusion of individuals on exchange transfusion therapy from this study. Accordingly, pooled data of both cohorts showed significant associations for EImin, and EImax, with the occurrence of cerebral infarction (supplemental Table 4).
Correlations of laboratory parameters with oxygen gradient ektacytometry–derived parameters
To explore how oxygen gradient ektacytometry–derived parameters are related to laboratory parameters, we performed several correlation analyses. In both cohorts, strong correlations were found between HbF and biomarkers of sickling (ie, PoS and EImin) and RBC deformability (EImax; supplemental Table 1), indicating that low HbF correlates with high PoS. In the pediatric cohort, strong correlations were found with DRBC and PoS, EImin, and EImax, indicating that a high fraction of DRBCs is associated with high PoS. Both ARC and white blood cell count correlated with oxygen gradient ektacytometry–derived parameters, indicating that both reticulocytosis and leukocytosis are associated with high PoS and reduced RBC deformability, both oxygenated and deoxygenated. In addition, PoS, EImin, and EImax showed a weak correlation with Transcranial Doppler (TCD) velocities, indicating that high sickling tendency was associated with high TCD velocities (supplemental Table 1). Although we found correlations with TCD velocities, oxygen gradient ektacytometry–derived biomarkers explain a minority (only 7%-16%) of the variation in TCD velocities.
Intrapatient variability of PoS
To investigate how variable an individual’s PoS levels were when measured longitudinally, we analyzed PoS values of 11 adults with SCD 2 or 3 times at routine visits during 1- to 2-year follow-up. Patients were at steady state during routine visits and at a stable dose of hydroxyurea during follow-up (mean, 6 months; range, 3-10 months). Coefficient of variance of 6 adults without therapy and 5 adults on hydroxyurea showed mean coefficient of variances of 5.1% (standard deviation, 6.9%) and 7.8% (standard deviation, 6.0%), respectively (supplemental Table 4). These results indicate minimal variability in PoS over time in patients whose clinical situation remains unchanged, which confirms another previous study.23
Discussion
In this study, we report on the association of oxygen gradient ektacytometry–derived biomarkers with the occurrence of acute complications in pediatric and adult cohorts of patients with SCD (Tables 2-5). In both adults and children with SCD, higher ORs for the occurrence of >1 type of acute complication were found with every 10 mmHg increment in PoS (Table 2). The lack of significance after correcting for multiple testing is presumably due to low sample size in the adult cohort. This assumption is strengthened by the finding of significant associations with acute complications for PoS, EImin, and EImax when data of both cohorts were pooled (supplemental Tables 1-4). These results indicate that there is an association between sickling at higher pO2 and the risk of an acute event. Oxygen gradient ektacytometry is a technique that comprehensively assesses RBC function and sickling behavior. Sickling behavior is determined by several different factors within RBCs. Some of these factors such as HbF or HbS levels are easily measured, whereas others such as levels of 2,3-diphosphoglycerate, adenosine triphosphate, or oxidative stress are more challenging and time consuming.14 A limitation of this study is that it is a retrospective analysis of 2 different cohorts investigating how occurrence of complications in the past correlate with recently measured laboratory parameters. Currently a prospective study is ongoing to further analyze to which extent acute complications are associated with oxygen gradient ektacytometry–derived biomarkers.
Red cell sickling is associated with different types of acute complications
Numerous studies have focused on identifying biomarkers that can predict acute and chronic complications or overall clinical severity; however, this goal remains a challenge, and there are no clinically validated biomarkers of global RBC function. HbF is the main biomarker used to predict disease severity because individuals with hereditary persistence of HbF generally present with a mild phenotype and experience less VOEs or ACS.24 The strong association we found between VOEs and HbF is in accordance with prior studies.25 In contrast, we found no association between HbF and ACS (Table 4), although a trend was observed in the pediatric cohort (aOR, 0.57; P = .068). The lack of association between HbF and ACS in children may be largely because of the different types of ACS events in children (compared with in adults), probably more commonly due to prevalent childhood viral respiratory infections that tend to be mild. In addition, no association was found between HbF and cerebral infarction or occurrence of >1 type of acute complication when assessing both cohorts independently (Tables 2 and 5). Our results differ from prior studies that do report associations between HbF and ACS and cerebral infarction, and it could be explained by the small sample size of our study,26-29 which is supported by the finding of a significant association of HbF and ACS when data of both cohorts were pooled (supplemental Table 3).3,4,30 Moreover, ACS and VOE are thought to be associated with increased blood viscosity.3,4,31-33 In contrast to findings of previous studies,31-33 we found no association between the occurrence of ACS or VOE and blood viscosity (Tables 3 and 4). The fact that the majority of children now are on hydroxyurea therapy may change the associations previously reported. A limitation of our viscosity measurements are that they are performed at normoxia.
Differences between cohorts
We observed differences in occurrence of complications between the adult and pediatric cohorts. These findings and the observed differences in PoS (Table 1) between the cohorts could be due to differences in pediatric and adult SCD pathophysiology. Although studies investigating the effect of age on clinical symptoms and signs are scarce, SCD symptoms generally worsen with age, particularly in the transition period between adolescence and adulthood.17,18 This is partly due to differences in treatment, access to care, and differences in guidelines and clinical practice between adult and pediatric care providers.34,35 In our study, fewer adults were on hydroxyurea therapy than the pediatric cohort (64.2% vs 85.2%). Regarding differences found in the occurrence of clinical complications, the adult cohort was substantially smaller, making associations between biomarkers and relatively rare complications difficult to substantiate. Environmental factors such as climate36 could also result in differences between the adult and pediatric cohort; the adult cohort is located in Europe and the pediatric in Houston, Texas. In addition, RBC rheological characteristics, and notably RBC deformability, have been found to be different between adults and young children.19
Emerging treatments and the need for clinically relevant biomarkers
In an era in which therapeutic options are rapidly expanding, several new drugs that do not target HbF induction, such as voxelotor,37 L-glutamine38 and crizanlizumab,39 are recently approved by the US Food and Drug Administration as therapies, whereas several other agents, such as pyruvate kinase activators, are currently in clinical trials on the North American continent and in Europe. Pyruvate kinase activators decrease 2,3-diphosphoglycerate levels, thereby increasing oxygen affinity and subsequently reducing sickling tendency.40-44
Biomarkers that reflect RBC characteristics and, more importantly, RBC cell function, such as those provided by oxygen gradient ektacytometry, are excellent ways to assess curative therapy outcomes. The amount of HbF induction or modified HbA needed to be gained by gene therapy to abrogate the SCD phenotype is still a topic of discussion. In most clinical trials, there is no sickling assay included that measures RBC function under hypoxia, because microscopy based sickling assays are static and functional assays do not report the individual patients sickling behavior.45-48 VOE remains to be the primary end point in ongoing gene therapy trials; however, absence of VOEs does not necessarily mean the patient is cured, because organ damage may still be ongoing. In this article, we show that oxygen gradient ektacytometry–derived biomarkers provide additional information about RBC function beyond that of standard laboratory values such as HbF and HbS levels, MCV, reticulocyte count, and markers of hemolysis. In particular, associations were found between oxygen gradient ektacytometry–derived biomarkers and a history of >1 type of acute complication in the past, whereas for routine laboratory values such an association was absent, underlining the value of these biomarkers in addition to HbF. Therefore, biomarkers such as PoS and blood viscosity can be helpful to assess which treatment and which combination of drugs is suited for the individual patient with SCD, according to the principle of precision medicine. It is reasonable to assume that certain drugs might work synergistically, and patients could benefit from that. However, no patient with SCD is the same in terms of phenotype, which warrants an extended RBC characterization including sickling behavior and evaluation of other factors that contribute to pathophysiology of SCD. Ideally, a single biomarker provides the clinician information on clinical severity and response to a certain therapy. However, regarding the highly complex pathophysiology of SCD, it seems reasonable to assume that >1 biomarker is needed to reflect all aspects contributing to disease pathology.
Conclusions
Oxygen gradient ektacytometry provides clinically relevant next generation biomarkers that assess RBC function in SCD. We show that these biomarkers are associated with a history of acute complications in individuals with SCD. This study, therefore, further validates the clinical usefulness of these biomarkers, particularly in relation to VOEs, ACS, and cerebral infarction. Because our results merely describe an association and not causality, further validation, including a prospective study using predefined outcomes is warranted to establish how well oxygen gradient ektacytometry can assess disease severity and how well therapeutic modification of these values correlates with clinical improvement. Our results provide a rationale for further development of these biomarkers in the evaluation of novel therapies as part of clinical care or clinical trial end points.
Acknowledgments
The authors thank all patients who donated blood for this study. The authors also thank the technicians of the Central Diagnostic Laboratory, Specialized Hematology, University Medical Center Utrecht. The authors also thank the clinical research coordinators of the department of Pediatric Hematology and Oncology at the Erasmus MC Sophia Children’s Hospital and Erasmus MC, University Medical Center Rotterdam, and the department of Hematology at the Amsterdam University Medical Centers.
M.A.E.R., M.E.H., A.W.R., E.N., M.H.C. and E.J.v.B. are clinical researchers within the Sickle Cell Outcome Research consortium, a multicenter clinical research collaboration in The Netherlands, which gave input on the work described in this manuscript, which the authors value highly. This work has been funded in part by Eurostars grant estar18105 and by an unrestricted grant provided by RR Mechatronics. This work is also funded in part by a K08 grant from the National Institute of Diabetes and Digestive and Kidney Diseases and provided with support from the Department of Pediatrics, Baylor College of Medicine.
Authorship
Contribution: M.A.E.R., R.v.W., P.C., V.A.S., and E.J.v.B. designed the study; M.A.E.R., C.K.K., M.E.H., C.R., P.J., M.B., A.W.R., E. Nur., R.F., M.H.C., P.C., V.A.S., and E.J.v.B. collected clinical and laboratory data; M.A.E.R., C.K.K., J.B., B.A.v.O., E. Nader., and C.B. performed laboratory experiments; M.A.E.R., and R.v.W., analyzed the data; M.A.E.R. wrote the manuscript with input from R.v.W., P.C., V.A.S., and E.J.v.B.; and all authors edited the manuscript and approved the final version.
Conflict-of-interest disclosure: C.B. and J.B. received grant funding from RR Mechatronics. M.A.E.R. and R.v.W. received grant funding from Agios Pharmaceuticals, Axcella Health, and RR Mechatronics. R.v.W. received grant funding from Agios Pharmaceuticals, Global Blood Therapeutics, Axcella Health, and RR Mechatronics. P.C. received grant funding from Agios Pharmaceuticals and RR Mechatronics. M.H.C. received grant funding from Takeda, Shire, Baxter, Bayer, Sobi, CSL Behring, Nordic Pharma, Novo Nordisk, and Pfizer; (institution) has received investigator-initiated research and travel grants as well as speaker fees over the years from The Netherlands Organisation for Scientific Research and Netherlands National research Agenda, The Netherlands Organization for Health Research and Development (ZonMw), the Dutch Innovatiefonds Zorgverzekeraars, Baxter/Baxalta/Shire/Takeda, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, and Nordic Pharma; and has served as a steering board member for Roche, Bayer, and Novartis for which fees go to the Erasmus MC as an institution. V.A.S. received grant funding from National Heart, Lung, and Blood Institute Cure SCD Initiative, Global Blood Therapeutics, Beam Therapeutics, Emmaus, and Novartis for investigator-initiated research projects. E.J.v.B. received grant funding Agios Pharmaceuticals, RR Mechatronics, Novartis, and Pfizer for investigator-initiated research projects. The remaining authors have declared no competing financial interests.
Correspondence: Minke A.E. Rab, Division Laboratories, Pharmacy and Biomedical Genetics, Central Diagnostic Laboratory-Research & Department of Hematology, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; email: m.a.e.rab@umcutrecht.nl.
References
Author notes
∗C.K.K., and C.B. contributed equally to this study.
†V.A.S. and E.J.v.B. contributed equally to this study.
Presented in abstract form at the European Hematology Association annual meeting in Vienna, Austria, in June 2022.
Data are available on request from the corresponding author, Minke A.E. Rab (m.a.e.rab@umcutrecht.nl).
The full-text version of this article contains a data supplement.